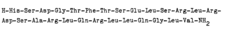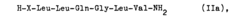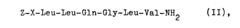Perchloric Acid and Its Role in the Formation of Solvated Ions
AUG 4, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Background and Objectives
Perchloric acid, a powerful oxidizing agent and one of the strongest acids known, has been a subject of significant interest in the field of chemistry for decades. Its unique properties and behavior in solution have made it a crucial component in various industrial and scientific applications. The study of perchloric acid and its role in the formation of solvated ions is essential for understanding complex chemical processes and developing new technologies.
The history of perchloric acid dates back to the early 19th century when it was first discovered by German chemist G. S. Serullas in 1831. Since then, research into its properties and applications has steadily progressed, with significant advancements made in the latter half of the 20th century. The acid's exceptional ability to form stable solvated ions has been a focal point of numerous studies, leading to its widespread use in analytical chemistry, electrochemistry, and materials science.
In recent years, the importance of perchloric acid in the formation of solvated ions has gained renewed attention due to its potential applications in emerging technologies. The acid's unique characteristics, such as its strong oxidizing power and ability to form stable perchlorate salts, have made it invaluable in fields ranging from rocket propellants to advanced battery technologies.
The primary objective of studying perchloric acid and its role in solvated ion formation is to gain a deeper understanding of the fundamental chemical processes involved. This knowledge is crucial for developing more efficient and sustainable chemical processes, improving analytical techniques, and creating novel materials with enhanced properties. Additionally, research in this area aims to address safety concerns associated with the handling and use of perchloric acid, given its highly reactive nature.
Another key goal is to explore the potential of perchloric acid in emerging technologies. As the demand for high-performance materials and energy storage solutions continues to grow, understanding the behavior of perchloric acid and its solvated ions could lead to breakthroughs in areas such as advanced batteries, supercapacitors, and fuel cells. Furthermore, investigating the acid's role in solvation processes may provide insights into developing more environmentally friendly alternatives to traditional chemical processes.
The study of perchloric acid also aims to elucidate its impact on biological systems and the environment. While the acid itself is not commonly found in nature, perchlorate ions are present in some environments and can affect various ecosystems. Understanding the formation and behavior of these ions is crucial for developing effective remediation strategies and assessing potential health risks.
The history of perchloric acid dates back to the early 19th century when it was first discovered by German chemist G. S. Serullas in 1831. Since then, research into its properties and applications has steadily progressed, with significant advancements made in the latter half of the 20th century. The acid's exceptional ability to form stable solvated ions has been a focal point of numerous studies, leading to its widespread use in analytical chemistry, electrochemistry, and materials science.
In recent years, the importance of perchloric acid in the formation of solvated ions has gained renewed attention due to its potential applications in emerging technologies. The acid's unique characteristics, such as its strong oxidizing power and ability to form stable perchlorate salts, have made it invaluable in fields ranging from rocket propellants to advanced battery technologies.
The primary objective of studying perchloric acid and its role in solvated ion formation is to gain a deeper understanding of the fundamental chemical processes involved. This knowledge is crucial for developing more efficient and sustainable chemical processes, improving analytical techniques, and creating novel materials with enhanced properties. Additionally, research in this area aims to address safety concerns associated with the handling and use of perchloric acid, given its highly reactive nature.
Another key goal is to explore the potential of perchloric acid in emerging technologies. As the demand for high-performance materials and energy storage solutions continues to grow, understanding the behavior of perchloric acid and its solvated ions could lead to breakthroughs in areas such as advanced batteries, supercapacitors, and fuel cells. Furthermore, investigating the acid's role in solvation processes may provide insights into developing more environmentally friendly alternatives to traditional chemical processes.
The study of perchloric acid also aims to elucidate its impact on biological systems and the environment. While the acid itself is not commonly found in nature, perchlorate ions are present in some environments and can affect various ecosystems. Understanding the formation and behavior of these ions is crucial for developing effective remediation strategies and assessing potential health risks.
Industrial Applications and Market Analysis
Perchloric acid and its role in the formation of solvated ions have significant industrial applications and market potential. The use of perchloric acid in various sectors has been steadily growing due to its unique properties and versatility in chemical processes.
In the electronics industry, perchloric acid plays a crucial role in the production of printed circuit boards and semiconductors. Its strong oxidizing properties make it an excellent etching agent for copper and other metals used in electronic components. The increasing demand for smaller and more powerful electronic devices has driven the growth of this market segment.
The aerospace and defense sectors also rely heavily on perchloric acid for the manufacture of solid rocket propellants. Its ability to form stable perchlorate salts with high oxygen content makes it an essential ingredient in these applications. As space exploration and satellite launches continue to expand, the demand for perchloric acid in this sector is expected to grow.
In analytical chemistry, perchloric acid is widely used as a reagent for various laboratory procedures, including sample digestion and extraction. Its strong oxidizing properties make it valuable in the analysis of complex organic compounds and metal alloys. The pharmaceutical and environmental testing industries are major consumers of perchloric acid for these purposes.
The automotive industry utilizes perchloric acid in the production of airbag inflators, where its role in forming solvated ions is crucial for the rapid deployment of safety systems. As vehicle safety regulations become more stringent worldwide, the demand for perchloric acid in this application is projected to increase.
The global market for perchloric acid is expected to experience steady growth in the coming years. Factors driving this growth include the expansion of the electronics industry, increased investment in space exploration, and the growing emphasis on safety in automotive manufacturing. However, the market faces challenges related to the handling and transportation of perchloric acid due to its corrosive and potentially explosive nature.
Geographically, Asia-Pacific is the largest consumer of perchloric acid, primarily due to the region's dominant position in electronics manufacturing. North America and Europe follow, with significant demand from the aerospace and defense sectors. Emerging economies in South America and Africa are also showing increased interest in perchloric acid applications, particularly in mining and metallurgy.
In the electronics industry, perchloric acid plays a crucial role in the production of printed circuit boards and semiconductors. Its strong oxidizing properties make it an excellent etching agent for copper and other metals used in electronic components. The increasing demand for smaller and more powerful electronic devices has driven the growth of this market segment.
The aerospace and defense sectors also rely heavily on perchloric acid for the manufacture of solid rocket propellants. Its ability to form stable perchlorate salts with high oxygen content makes it an essential ingredient in these applications. As space exploration and satellite launches continue to expand, the demand for perchloric acid in this sector is expected to grow.
In analytical chemistry, perchloric acid is widely used as a reagent for various laboratory procedures, including sample digestion and extraction. Its strong oxidizing properties make it valuable in the analysis of complex organic compounds and metal alloys. The pharmaceutical and environmental testing industries are major consumers of perchloric acid for these purposes.
The automotive industry utilizes perchloric acid in the production of airbag inflators, where its role in forming solvated ions is crucial for the rapid deployment of safety systems. As vehicle safety regulations become more stringent worldwide, the demand for perchloric acid in this application is projected to increase.
The global market for perchloric acid is expected to experience steady growth in the coming years. Factors driving this growth include the expansion of the electronics industry, increased investment in space exploration, and the growing emphasis on safety in automotive manufacturing. However, the market faces challenges related to the handling and transportation of perchloric acid due to its corrosive and potentially explosive nature.
Geographically, Asia-Pacific is the largest consumer of perchloric acid, primarily due to the region's dominant position in electronics manufacturing. North America and Europe follow, with significant demand from the aerospace and defense sectors. Emerging economies in South America and Africa are also showing increased interest in perchloric acid applications, particularly in mining and metallurgy.
Current Challenges in Perchloric Acid Research
Despite significant advancements in perchloric acid research, several challenges persist in understanding its role in the formation of solvated ions. One of the primary obstacles is the highly reactive and oxidizing nature of perchloric acid, which makes it difficult to study under controlled laboratory conditions. This reactivity poses safety concerns and requires specialized handling procedures, limiting the scope of experiments that can be conducted.
Another challenge lies in the complex behavior of perchloric acid in solution. The acid's strong dissociation and its ability to form various solvated species complicate the analysis of ion formation processes. Researchers struggle to accurately model and predict the interactions between perchloric acid, solvent molecules, and other ionic species in solution, particularly at high concentrations or under extreme conditions.
The formation of perchlorate ions and their subsequent solvation mechanisms are not fully understood. The strong oxidizing power of perchloric acid can lead to unexpected side reactions, making it challenging to isolate and study specific solvated ion species. This complexity hinders the development of comprehensive theoretical models that can accurately describe the solvation process across different solvent systems and concentration ranges.
Furthermore, the role of perchloric acid in promoting specific ion-pairing phenomena and its impact on solution structure remain areas of active research. The acid's unique properties can lead to unusual solvation behaviors that are not observed with other strong acids, making it difficult to generalize findings across different chemical systems.
Analytical techniques for studying solvated ions formed by perchloric acid also present challenges. Traditional spectroscopic methods may be limited in their ability to distinguish between different solvated species or to capture the dynamic nature of ion-solvent interactions. Advanced techniques such as time-resolved spectroscopy or in-situ NMR studies are being developed but still face limitations in resolution and applicability to highly reactive systems.
The environmental implications of perchloric acid and its solvated ions pose another significant challenge. As perchlorate contamination becomes an increasing concern in water sources, there is a pressing need to understand the behavior of these ions in environmental systems. However, the complexity of natural matrices and the low concentrations typically encountered make it difficult to study the fate and transport of perchlorate ions under realistic conditions.
Lastly, the development of safer alternatives or mitigation strategies for perchloric acid use in industrial and research applications remains an ongoing challenge. While efforts are being made to find substitutes, the unique properties of perchloric acid often make it irreplaceable in certain processes, necessitating continued research into safer handling and disposal methods.
Another challenge lies in the complex behavior of perchloric acid in solution. The acid's strong dissociation and its ability to form various solvated species complicate the analysis of ion formation processes. Researchers struggle to accurately model and predict the interactions between perchloric acid, solvent molecules, and other ionic species in solution, particularly at high concentrations or under extreme conditions.
The formation of perchlorate ions and their subsequent solvation mechanisms are not fully understood. The strong oxidizing power of perchloric acid can lead to unexpected side reactions, making it challenging to isolate and study specific solvated ion species. This complexity hinders the development of comprehensive theoretical models that can accurately describe the solvation process across different solvent systems and concentration ranges.
Furthermore, the role of perchloric acid in promoting specific ion-pairing phenomena and its impact on solution structure remain areas of active research. The acid's unique properties can lead to unusual solvation behaviors that are not observed with other strong acids, making it difficult to generalize findings across different chemical systems.
Analytical techniques for studying solvated ions formed by perchloric acid also present challenges. Traditional spectroscopic methods may be limited in their ability to distinguish between different solvated species or to capture the dynamic nature of ion-solvent interactions. Advanced techniques such as time-resolved spectroscopy or in-situ NMR studies are being developed but still face limitations in resolution and applicability to highly reactive systems.
The environmental implications of perchloric acid and its solvated ions pose another significant challenge. As perchlorate contamination becomes an increasing concern in water sources, there is a pressing need to understand the behavior of these ions in environmental systems. However, the complexity of natural matrices and the low concentrations typically encountered make it difficult to study the fate and transport of perchlorate ions under realistic conditions.
Lastly, the development of safer alternatives or mitigation strategies for perchloric acid use in industrial and research applications remains an ongoing challenge. While efforts are being made to find substitutes, the unique properties of perchloric acid often make it irreplaceable in certain processes, necessitating continued research into safer handling and disposal methods.
Solvated Ion Formation Mechanisms
01 Electrolyte solutions containing perchloric acid solvated ions
Perchloric acid solvated ions are used in electrolyte solutions for various applications, including batteries and electrochemical cells. These solutions often contain other components to enhance their performance and stability.- Electrolyte solutions containing perchloric acid solvated ions: Perchloric acid solvated ions are used in electrolyte solutions for various applications, including batteries and electrochemical cells. These solutions often contain other components to enhance their performance and stability.
- Synthesis and characterization of perchloric acid solvated ion complexes: Research focuses on the synthesis and characterization of perchloric acid solvated ion complexes, studying their structure, properties, and potential applications in various fields of chemistry and materials science.
- Analytical methods for detecting perchloric acid solvated ions: Various analytical techniques and methods are developed to detect and quantify perchloric acid solvated ions in different matrices, including spectroscopic and chromatographic approaches.
- Applications of perchloric acid solvated ions in energy storage devices: Perchloric acid solvated ions are utilized in the development of advanced energy storage devices, such as supercapacitors and high-performance batteries, due to their unique electrochemical properties.
- Safety considerations and handling of perchloric acid solvated ions: Due to the reactive nature of perchloric acid, special safety measures and handling procedures are required when working with perchloric acid solvated ions. This includes the use of specialized equipment and protective gear.
02 Synthesis and characterization of perchloric acid solvated ion complexes
Research focuses on the synthesis and characterization of perchloric acid solvated ion complexes, studying their structure, properties, and potential applications in various fields such as materials science and catalysis.Expand Specific Solutions03 Perchloric acid solvated ions in analytical chemistry
Perchloric acid solvated ions play a role in analytical chemistry techniques, including spectroscopy and chromatography. They are used as reagents or components in analytical methods for various substances.Expand Specific Solutions04 Safety considerations and handling of perchloric acid solvated ions
Due to the reactive nature of perchloric acid and its solvated ions, special safety measures and handling procedures are required. This includes proper storage, disposal, and use of specialized equipment to prevent accidents and ensure safe laboratory practices.Expand Specific Solutions05 Applications of perchloric acid solvated ions in material science
Perchloric acid solvated ions find applications in material science, including the synthesis of novel materials, surface treatments, and modification of existing materials to enhance their properties or functionalities.Expand Specific Solutions
Key Players in Perchloric Acid Industry
The competitive landscape for perchloric acid and its role in solvated ion formation is characterized by a mature market with established players. The technology's development stage appears advanced, with applications spanning across academic research, chemical manufacturing, and industrial processes. Key players include Shizuoka University, Cumberland Pharmaceuticals, and Liuyang City Chemical Factory, each contributing to the field's advancement. The market size is moderate, driven by demand in sectors such as pharmaceuticals, electrochemistry, and materials science. Technological maturity is evident, with ongoing research focusing on optimizing solvated ion formation processes and exploring new applications in energy storage and catalysis.
Shizuoka University
Technical Solution: Shizuoka University has conducted extensive research on the role of perchloric acid in the formation of solvated ions, particularly in non-aqueous systems. Their team has developed novel analytical techniques to study the solvation behavior of perchlorate ions in organic solvents, including the use of electrospray ionization mass spectrometry (ESI-MS) to characterize solvated ion clusters[7]. They have also investigated the influence of perchloric acid on the formation of supramolecular structures in solution, leading to new insights into the self-assembly processes of ionic species. The university's research has contributed to the understanding of perchloric acid's role in promoting unusual oxidation states of transition metals in solution, with potential applications in catalysis and materials synthesis[8].
Strengths: Expertise in non-aqueous systems and advanced analytical techniques. Weaknesses: Limited focus on large-scale industrial applications.
University of Delaware
Technical Solution: The University of Delaware has made significant contributions to understanding the role of perchloric acid in solvated ion formation, particularly in the context of environmental chemistry and remediation. Their research team has developed innovative methods for studying the behavior of perchlorate ions in natural aquatic systems, including the use of isotope fractionation techniques to trace the sources and fate of perchlorate contamination[9]. They have also investigated the role of perchloric acid in the formation of complex ion pairs and clusters in highly concentrated electrolyte solutions, providing insights into the behavior of ions under extreme conditions. Additionally, the university has pioneered the use of advanced computational methods to model the solvation dynamics of perchlorate ions in various environments, contributing to the development of more accurate predictive tools for environmental risk assessment[10].
Strengths: Strong focus on environmental applications and advanced computational modeling. Weaknesses: Limited research on industrial uses of perchloric acid.
Innovative Techniques in Ion Solvation Research
Process for producing peptides by the use of perchlorates
PatentInactiveEP0224844A2
Innovation
- The use of perchlorate ions, specifically pyridinium perchlorate, enhances the solubility of arginine-containing peptides in polar solvents like dimethylformamide or dimethylacetamide, facilitating fragment coupling and hydrogenation reactions by binding to the guanidino group and protecting it from acylation, thereby improving reaction efficiency and yield.
Safety Regulations for Perchloric Acid Handling
The handling of perchloric acid requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Regulatory bodies such as OSHA, EPA, and NFPA have established comprehensive guidelines for the safe use, storage, and disposal of perchloric acid in laboratory and industrial settings.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and acid-resistant aprons or lab coats. Proper ventilation is crucial, with all operations involving perchloric acid to be conducted in a designated fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Storage regulations stipulate that perchloric acid must be kept in dedicated, properly labeled containers made of glass or other inert materials. These containers should be stored in a cool, well-ventilated area, away from organic materials, dehydrating agents, and other incompatible substances. Secondary containment is required to prevent spills from spreading.
Workplace design considerations are essential for facilities using perchloric acid. Floors, work surfaces, and fume hoods must be constructed of acid-resistant materials. Emergency eyewash stations and safety showers should be readily accessible in areas where perchloric acid is handled.
Training is a critical component of safety regulations. All personnel working with or around perchloric acid must receive comprehensive training on its hazards, proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses are typically mandated to ensure ongoing compliance and safety awareness.
Waste disposal regulations for perchloric acid are stringent. Neutralization and dilution procedures must be followed carefully, and disposal should only be carried out by trained professionals. Many jurisdictions require special permits for the disposal of perchloric acid waste.
Emergency response plans must be in place and regularly updated. These plans should outline specific procedures for dealing with spills, fires, or explosions involving perchloric acid. Coordination with local emergency services is often required to ensure an effective response in case of a major incident.
Regular inspections and audits of facilities using perchloric acid are typically required by regulatory agencies. These assessments help ensure ongoing compliance with safety regulations and identify areas for improvement in handling practices.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and acid-resistant aprons or lab coats. Proper ventilation is crucial, with all operations involving perchloric acid to be conducted in a designated fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Storage regulations stipulate that perchloric acid must be kept in dedicated, properly labeled containers made of glass or other inert materials. These containers should be stored in a cool, well-ventilated area, away from organic materials, dehydrating agents, and other incompatible substances. Secondary containment is required to prevent spills from spreading.
Workplace design considerations are essential for facilities using perchloric acid. Floors, work surfaces, and fume hoods must be constructed of acid-resistant materials. Emergency eyewash stations and safety showers should be readily accessible in areas where perchloric acid is handled.
Training is a critical component of safety regulations. All personnel working with or around perchloric acid must receive comprehensive training on its hazards, proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses are typically mandated to ensure ongoing compliance and safety awareness.
Waste disposal regulations for perchloric acid are stringent. Neutralization and dilution procedures must be followed carefully, and disposal should only be carried out by trained professionals. Many jurisdictions require special permits for the disposal of perchloric acid waste.
Emergency response plans must be in place and regularly updated. These plans should outline specific procedures for dealing with spills, fires, or explosions involving perchloric acid. Coordination with local emergency services is often required to ensure an effective response in case of a major incident.
Regular inspections and audits of facilities using perchloric acid are typically required by regulatory agencies. These assessments help ensure ongoing compliance with safety regulations and identify areas for improvement in handling practices.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in various industrial and research applications has raised significant environmental concerns due to its potential impact on ecosystems and human health. When released into the environment, perchloric acid can contaminate soil and water sources, leading to long-term ecological consequences. The high oxidizing power of perchloric acid can disrupt natural chemical processes in soil and aquatic environments, affecting the balance of microbial communities and potentially harming plant and animal life.
One of the primary environmental risks associated with perchloric acid is its persistence in groundwater. Once it enters aquifers, perchloric acid can remain stable for extended periods, posing a long-term threat to drinking water supplies. This contamination can lead to the formation of perchlorate ions, which are known to interfere with iodine uptake in the thyroid gland, potentially causing developmental issues in humans and wildlife.
In industrial settings, the improper handling or disposal of perchloric acid can result in air pollution. Volatile perchlorates can be released into the atmosphere, contributing to the formation of smog and potentially affecting air quality over wide areas. Additionally, accidental spills or leaks of perchloric acid can cause immediate damage to local ecosystems, with the potential for rapid soil acidification and the destruction of vegetation.
The environmental impact of perchloric acid extends to its role in the formation of solvated ions. When perchloric acid dissociates in aqueous environments, it produces perchlorate ions that can readily form complexes with various metal cations. These solvated ion complexes can alter the mobility and bioavailability of metals in the environment, potentially leading to increased toxicity or unexpected transport of contaminants through soil and water systems.
To mitigate the environmental risks associated with perchloric acid use, stringent regulations and best practices have been implemented in many jurisdictions. These include strict guidelines for storage, handling, and disposal of perchloric acid and its waste products. Advanced treatment technologies, such as ion exchange and membrane filtration, are being employed to remove perchlorate contamination from water sources. Furthermore, research is ongoing to develop more environmentally friendly alternatives to perchloric acid in various applications, aiming to reduce its overall usage and potential for environmental impact.
One of the primary environmental risks associated with perchloric acid is its persistence in groundwater. Once it enters aquifers, perchloric acid can remain stable for extended periods, posing a long-term threat to drinking water supplies. This contamination can lead to the formation of perchlorate ions, which are known to interfere with iodine uptake in the thyroid gland, potentially causing developmental issues in humans and wildlife.
In industrial settings, the improper handling or disposal of perchloric acid can result in air pollution. Volatile perchlorates can be released into the atmosphere, contributing to the formation of smog and potentially affecting air quality over wide areas. Additionally, accidental spills or leaks of perchloric acid can cause immediate damage to local ecosystems, with the potential for rapid soil acidification and the destruction of vegetation.
The environmental impact of perchloric acid extends to its role in the formation of solvated ions. When perchloric acid dissociates in aqueous environments, it produces perchlorate ions that can readily form complexes with various metal cations. These solvated ion complexes can alter the mobility and bioavailability of metals in the environment, potentially leading to increased toxicity or unexpected transport of contaminants through soil and water systems.
To mitigate the environmental risks associated with perchloric acid use, stringent regulations and best practices have been implemented in many jurisdictions. These include strict guidelines for storage, handling, and disposal of perchloric acid and its waste products. Advanced treatment technologies, such as ion exchange and membrane filtration, are being employed to remove perchlorate contamination from water sources. Furthermore, research is ongoing to develop more environmentally friendly alternatives to perchloric acid in various applications, aiming to reduce its overall usage and potential for environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!