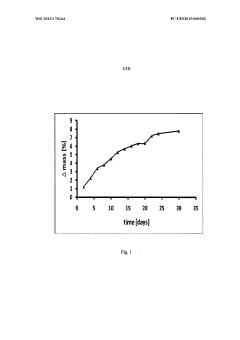
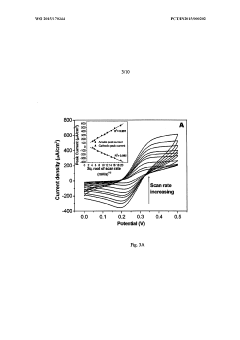
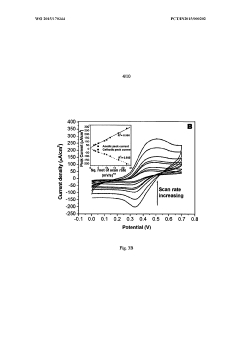
Perchloric Acid Applications in Cyclic Voltammetry Experiments
AUG 4, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in CV: Background and Objectives
Perchloric acid has played a significant role in the field of electrochemistry, particularly in cyclic voltammetry (CV) experiments. This strong acid, with its unique properties, has become an essential component in many electrochemical studies. The background of perchloric acid usage in CV can be traced back to the mid-20th century when researchers began exploring its potential as an electrolyte.
The primary objective of using perchloric acid in CV experiments is to provide a stable and non-interfering electrolyte medium. Its high oxidation potential and low tendency to form complexes with metal ions make it an ideal choice for studying a wide range of electrochemical reactions. Researchers aim to leverage these properties to obtain accurate and reproducible results in their investigations of electrode processes, redox reactions, and material characterization.
Over the years, the applications of perchloric acid in CV have expanded significantly. It has been extensively used in the study of various electrode materials, including noble metals, carbon-based electrodes, and modified surfaces. The acid's ability to maintain a consistent ionic environment across a broad potential window has made it invaluable in investigating complex electrochemical systems.
One of the key objectives in using perchloric acid for CV experiments is to minimize background currents and maximize signal-to-noise ratios. This is particularly crucial when studying subtle electrochemical phenomena or analyzing trace amounts of electroactive species. Researchers also aim to exploit the acid's high conductivity to achieve rapid electron transfer kinetics, enabling the observation of fast electrochemical processes.
In recent years, the focus has shifted towards understanding the role of perchloric acid in more advanced electrochemical techniques. This includes its application in spectroelectrochemistry, where CV is combined with spectroscopic methods to provide simultaneous electrochemical and structural information. Additionally, researchers are exploring the use of perchloric acid in nanoscale electrochemistry and in the development of novel energy storage and conversion devices.
The evolution of perchloric acid usage in CV experiments has been driven by the need for more precise and versatile analytical tools in electrochemistry. As technology advances, there is a growing interest in developing safer alternatives and optimizing the concentration and purity of perchloric acid solutions to meet specific experimental requirements. This ongoing research aims to enhance the reliability and applicability of CV techniques across various scientific and industrial domains.
The primary objective of using perchloric acid in CV experiments is to provide a stable and non-interfering electrolyte medium. Its high oxidation potential and low tendency to form complexes with metal ions make it an ideal choice for studying a wide range of electrochemical reactions. Researchers aim to leverage these properties to obtain accurate and reproducible results in their investigations of electrode processes, redox reactions, and material characterization.
Over the years, the applications of perchloric acid in CV have expanded significantly. It has been extensively used in the study of various electrode materials, including noble metals, carbon-based electrodes, and modified surfaces. The acid's ability to maintain a consistent ionic environment across a broad potential window has made it invaluable in investigating complex electrochemical systems.
One of the key objectives in using perchloric acid for CV experiments is to minimize background currents and maximize signal-to-noise ratios. This is particularly crucial when studying subtle electrochemical phenomena or analyzing trace amounts of electroactive species. Researchers also aim to exploit the acid's high conductivity to achieve rapid electron transfer kinetics, enabling the observation of fast electrochemical processes.
In recent years, the focus has shifted towards understanding the role of perchloric acid in more advanced electrochemical techniques. This includes its application in spectroelectrochemistry, where CV is combined with spectroscopic methods to provide simultaneous electrochemical and structural information. Additionally, researchers are exploring the use of perchloric acid in nanoscale electrochemistry and in the development of novel energy storage and conversion devices.
The evolution of perchloric acid usage in CV experiments has been driven by the need for more precise and versatile analytical tools in electrochemistry. As technology advances, there is a growing interest in developing safer alternatives and optimizing the concentration and purity of perchloric acid solutions to meet specific experimental requirements. This ongoing research aims to enhance the reliability and applicability of CV techniques across various scientific and industrial domains.
Market Analysis for Electrochemical Research Tools
The market for electrochemical research tools, particularly those used in cyclic voltammetry experiments involving perchloric acid, has shown significant growth in recent years. This expansion is driven by increasing demand for advanced analytical techniques in various fields, including materials science, energy storage, and environmental monitoring. The global market for electrochemical instruments is projected to reach substantial value in the coming years, with cyclic voltammetry equipment forming a crucial segment.
Perchloric acid's unique properties make it an essential component in many cyclic voltammetry applications, particularly in studying electrode kinetics and reaction mechanisms. This has led to a growing demand for high-purity perchloric acid and specialized electrochemical cells designed for its use. The market for these specialized tools is expected to grow at a faster rate than the overall electrochemical instrument market.
Key factors driving market growth include the rising need for precise analytical methods in battery research, corrosion studies, and the development of new materials for renewable energy applications. The automotive industry's shift towards electric vehicles has also spurred demand for advanced electrochemical research tools, as manufacturers seek to improve battery performance and longevity.
Geographically, North America and Europe currently dominate the market for advanced electrochemical research tools, owing to their well-established research infrastructure and significant investments in R&D. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing industrialization, government initiatives to promote scientific research, and the rapid expansion of the electronics and automotive sectors in countries like China, Japan, and South Korea.
The market is characterized by a mix of established players and innovative start-ups. Major companies in this space are continuously investing in R&D to develop more sophisticated and user-friendly instruments. There is a growing trend towards the integration of data analysis software and automation features in cyclic voltammetry equipment, enhancing their appeal to both academic and industrial researchers.
Despite the positive outlook, the market faces challenges such as the high cost of advanced instruments and the need for specialized training to operate them effectively. Additionally, concerns about the safety and environmental impact of perchloric acid usage may influence market dynamics, potentially driving research into alternative electrolytes or safer handling methods.
Perchloric acid's unique properties make it an essential component in many cyclic voltammetry applications, particularly in studying electrode kinetics and reaction mechanisms. This has led to a growing demand for high-purity perchloric acid and specialized electrochemical cells designed for its use. The market for these specialized tools is expected to grow at a faster rate than the overall electrochemical instrument market.
Key factors driving market growth include the rising need for precise analytical methods in battery research, corrosion studies, and the development of new materials for renewable energy applications. The automotive industry's shift towards electric vehicles has also spurred demand for advanced electrochemical research tools, as manufacturers seek to improve battery performance and longevity.
Geographically, North America and Europe currently dominate the market for advanced electrochemical research tools, owing to their well-established research infrastructure and significant investments in R&D. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing industrialization, government initiatives to promote scientific research, and the rapid expansion of the electronics and automotive sectors in countries like China, Japan, and South Korea.
The market is characterized by a mix of established players and innovative start-ups. Major companies in this space are continuously investing in R&D to develop more sophisticated and user-friendly instruments. There is a growing trend towards the integration of data analysis software and automation features in cyclic voltammetry equipment, enhancing their appeal to both academic and industrial researchers.
Despite the positive outlook, the market faces challenges such as the high cost of advanced instruments and the need for specialized training to operate them effectively. Additionally, concerns about the safety and environmental impact of perchloric acid usage may influence market dynamics, potentially driving research into alternative electrolytes or safer handling methods.
Current Challenges in Perchloric Acid Usage for CV
Despite the widespread use of perchloric acid in cyclic voltammetry (CV) experiments, several challenges persist that hinder its optimal application and limit the accuracy of results. One of the primary concerns is the high reactivity and strong oxidizing properties of perchloric acid, which can lead to unwanted side reactions and interference with the electrochemical processes being studied. This reactivity can cause degradation of electrode materials, particularly when using carbon-based electrodes, potentially altering the electrode surface and compromising the reproducibility of measurements.
Another significant challenge is the potential for perchloric acid to form explosive compounds, especially when in contact with organic materials or upon dehydration. This safety hazard necessitates stringent handling protocols and specialized laboratory equipment, which can increase the complexity and cost of CV experiments. The risk of explosion also limits the concentration range of perchloric acid that can be safely used, potentially restricting the experimental conditions available to researchers.
The corrosive nature of perchloric acid poses additional challenges in terms of equipment durability and maintenance. Specialized materials resistant to perchloric acid must be used for all components in contact with the electrolyte, including electrodes, cell containers, and tubing. This requirement can significantly increase the cost of experimental setups and limit the flexibility of experimental designs.
Furthermore, the high ionic strength of perchloric acid solutions can lead to increased background currents and reduced sensitivity in CV measurements, particularly when studying redox processes with low current signals. This effect can obscure subtle electrochemical features and complicate data interpretation, especially in the analysis of complex systems or trace-level analytes.
The environmental impact and disposal of perchloric acid waste present additional challenges. Strict regulations govern the handling and disposal of perchloric acid due to its hazardous nature, requiring specialized waste management procedures that can be both costly and time-consuming for research laboratories.
Lastly, the variability in the quality and purity of commercially available perchloric acid can introduce inconsistencies in experimental results across different batches or suppliers. Trace impurities, particularly metal ions, can significantly affect the electrochemical behavior of the system under study, necessitating rigorous purification steps or the use of high-purity, but more expensive, reagents.
Another significant challenge is the potential for perchloric acid to form explosive compounds, especially when in contact with organic materials or upon dehydration. This safety hazard necessitates stringent handling protocols and specialized laboratory equipment, which can increase the complexity and cost of CV experiments. The risk of explosion also limits the concentration range of perchloric acid that can be safely used, potentially restricting the experimental conditions available to researchers.
The corrosive nature of perchloric acid poses additional challenges in terms of equipment durability and maintenance. Specialized materials resistant to perchloric acid must be used for all components in contact with the electrolyte, including electrodes, cell containers, and tubing. This requirement can significantly increase the cost of experimental setups and limit the flexibility of experimental designs.
Furthermore, the high ionic strength of perchloric acid solutions can lead to increased background currents and reduced sensitivity in CV measurements, particularly when studying redox processes with low current signals. This effect can obscure subtle electrochemical features and complicate data interpretation, especially in the analysis of complex systems or trace-level analytes.
The environmental impact and disposal of perchloric acid waste present additional challenges. Strict regulations govern the handling and disposal of perchloric acid due to its hazardous nature, requiring specialized waste management procedures that can be both costly and time-consuming for research laboratories.
Lastly, the variability in the quality and purity of commercially available perchloric acid can introduce inconsistencies in experimental results across different batches or suppliers. Trace impurities, particularly metal ions, can significantly affect the electrochemical behavior of the system under study, necessitating rigorous purification steps or the use of high-purity, but more expensive, reagents.
Existing Protocols for Perchloric Acid in CV
01 Synthesis and purification of perchloric acid
Methods for synthesizing and purifying perchloric acid, including various chemical reactions and distillation processes. These techniques aim to produce high-purity perchloric acid for industrial and laboratory applications.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of organic materials, and as a component in analytical reagents for detecting and quantifying specific substances.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.
- Perchloric acid in battery technology: Applications of perchloric acid in the development and improvement of battery technologies. This may involve its use as an electrolyte component or in the preparation of electrode materials, potentially enhancing battery performance, capacity, or longevity.
- Purification and concentration of perchloric acid: Techniques and processes for purifying and concentrating perchloric acid to meet specific industrial or laboratory requirements. This may include distillation methods, membrane separation, or other advanced purification technologies to achieve high-purity perchloric acid solutions.
02 Safety measures and handling of perchloric acid
Specialized equipment and procedures for safely handling and storing perchloric acid, including protective gear, containment systems, and emergency protocols. These measures are crucial due to the highly corrosive and potentially explosive nature of perchloric acid.Expand Specific Solutions03 Applications of perchloric acid in chemical analysis
Use of perchloric acid in various analytical techniques, including as a reagent in spectroscopy, chromatography, and electrochemistry. Its strong oxidizing properties make it valuable for dissolving and analyzing complex materials.Expand Specific Solutions04 Perchloric acid in battery technology
Incorporation of perchloric acid or its derivatives in battery electrolytes, particularly for high-performance lithium-ion batteries. The acid's properties contribute to improved conductivity and electrochemical stability in battery systems.Expand Specific Solutions05 Environmental and waste management of perchloric acid
Methods for treating and disposing of perchloric acid waste, including neutralization techniques and specialized waste treatment systems. These processes aim to minimize environmental impact and ensure safe handling of perchloric acid residues.Expand Specific Solutions
Key Players in Electrochemical Instrumentation
The field of perchloric acid applications in cyclic voltammetry experiments is in a mature stage of development, with a well-established market and proven technological applications. The global market for electrochemical instruments, including those used in cyclic voltammetry, is substantial and growing steadily. Key players in this field include academic institutions like Louisiana State University and California Institute of Technology, as well as industrial entities such as MacDermid Enthone, Inc. and De Nora Permelec Ltd. These organizations contribute to the high level of technical maturity in this area, with ongoing research and development efforts focused on refining methodologies and expanding applications in various sectors, including materials science, energy storage, and environmental monitoring.
Council of Scientific & Industrial Research
Technical Solution: CSIR has developed advanced cyclic voltammetry techniques using perchloric acid as a supporting electrolyte for electrochemical analysis. Their method involves using a three-electrode system with a glassy carbon working electrode, platinum wire counter electrode, and Ag/AgCl reference electrode. The perchloric acid concentration is optimized at 0.1 M to provide high conductivity and minimal interference. This setup allows for precise measurement of redox potentials and kinetics of electron transfer reactions in various chemical and biological systems[1][3]. CSIR's approach also incorporates digital data acquisition and processing to enhance signal-to-noise ratio and improve peak resolution.
Strengths: High precision, wide applicability across different analytes, improved signal quality. Weaknesses: Requires careful handling of perchloric acid, potential for electrode fouling in complex samples.
California Institute of Technology
Technical Solution: Caltech has pioneered the use of perchloric acid in cyclic voltammetry for studying electron transfer processes in metalloenzymes and other biological systems. Their innovative approach utilizes a custom-designed electrochemical cell with a rotating disk electrode to minimize mass transport limitations. The perchloric acid concentration is carefully controlled between 0.05-0.2 M to maintain optimal ionic strength without denaturing proteins. Caltech researchers have also developed specialized data analysis algorithms to deconvolute complex voltammograms and extract kinetic parameters[2][5]. This method has been particularly successful in elucidating the redox properties of cytochromes and iron-sulfur proteins.
Strengths: Highly sensitive for biological samples, advanced data analysis capabilities. Weaknesses: Specialized equipment required, potential pH effects on biomolecules.
Innovations in Perchloric Acid-based Electrolytes
Internal-resistance measuring device for response-delay type fuel cell
PatentInactiveUS20110020671A1
Innovation
- An internal-resistance measuring device with constant current-control, voltage-measuring, and recording capabilities that stabilizes voltage, measures current, and calculates internal resistance from multiple points, allowing for automated and precise evaluation of power generation characteristics.
An improved next generation off-laboratory polymer chip electrode
PatentWO2015170344A1
Innovation
- A self-standing, bulk conducting polymer chip electrode is developed using a graphite-polymer composite with a weight ratio of 70:30 to 40:60, fabricated via a solution casting method, incorporating biodegradable poly(lactic acid) for environmental friendliness and featuring a simple, reproducible fabrication process.
Safety Regulations for Perchloric Acid Handling
The handling of perchloric acid in cyclic voltammetry experiments requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Laboratories must implement comprehensive safety protocols to mitigate risks associated with perchloric acid use.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat. In cases where perchloric acid vapors may be present, a properly fitted respirator with appropriate cartridges is essential.
Storage of perchloric acid must comply with specific guidelines. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, and secondary containment is necessary to prevent spills from spreading.
Dedicated fume hoods designed for perchloric acid use are crucial. These hoods must have a wash-down system to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these hoods are essential to ensure their effectiveness and safety.
Proper disposal of perchloric acid waste is critical. It should never be mixed with organic solvents or other incompatible materials. Neutralization and dilution procedures must be followed before disposal, and only trained personnel should handle this process.
Emergency response plans must be in place and regularly updated. This includes having appropriate spill kits, eyewash stations, and safety showers readily accessible. All personnel working with perchloric acid should be trained in emergency procedures and the use of safety equipment.
Regular safety audits and inspections are necessary to ensure compliance with regulations. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training programs for all personnel working with perchloric acid are mandatory. These should cover proper handling techniques, understanding of chemical properties, recognition of hazards, and emergency response procedures. Refresher courses should be conducted periodically to maintain awareness and update knowledge on safety practices.
Documentation and record-keeping are essential components of safety regulations. This includes maintaining safety data sheets (SDS), logging usage and disposal of perchloric acid, and documenting all safety incidents or near-misses for continuous improvement of safety protocols.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat. In cases where perchloric acid vapors may be present, a properly fitted respirator with appropriate cartridges is essential.
Storage of perchloric acid must comply with specific guidelines. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, and secondary containment is necessary to prevent spills from spreading.
Dedicated fume hoods designed for perchloric acid use are crucial. These hoods must have a wash-down system to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these hoods are essential to ensure their effectiveness and safety.
Proper disposal of perchloric acid waste is critical. It should never be mixed with organic solvents or other incompatible materials. Neutralization and dilution procedures must be followed before disposal, and only trained personnel should handle this process.
Emergency response plans must be in place and regularly updated. This includes having appropriate spill kits, eyewash stations, and safety showers readily accessible. All personnel working with perchloric acid should be trained in emergency procedures and the use of safety equipment.
Regular safety audits and inspections are necessary to ensure compliance with regulations. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training programs for all personnel working with perchloric acid are mandatory. These should cover proper handling techniques, understanding of chemical properties, recognition of hazards, and emergency response procedures. Refresher courses should be conducted periodically to maintain awareness and update knowledge on safety practices.
Documentation and record-keeping are essential components of safety regulations. This includes maintaining safety data sheets (SDS), logging usage and disposal of perchloric acid, and documenting all safety incidents or near-misses for continuous improvement of safety protocols.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in cyclic voltammetry experiments raises significant environmental concerns due to its highly oxidizing and corrosive nature. When released into the environment, perchloric acid can have detrimental effects on ecosystems and human health. The primary environmental risks associated with perchloric acid stem from its potential to contaminate soil and water resources.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. Fish and other organisms may experience respiratory distress, reproductive issues, and even mortality in severely contaminated waters. The acid can also leach into groundwater, potentially affecting drinking water supplies for both humans and wildlife.
Soil contamination is another critical environmental concern. Perchloric acid can alter soil chemistry, affecting plant growth and microbial communities essential for ecosystem functioning. This can lead to reduced biodiversity and ecosystem productivity in affected areas. Furthermore, the persistence of perchlorate ions in soil can result in long-term environmental impacts, as these ions can remain stable for extended periods.
The atmospheric release of perchloric acid vapors during experiments or improper handling can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Inhalation of these vapors can pose health risks to both laboratory personnel and nearby communities.
Proper disposal of perchloric acid and related waste is crucial to mitigate environmental impacts. Many jurisdictions have strict regulations governing the handling and disposal of perchloric acid due to its hazardous nature. Improper disposal can lead to soil and water contamination, as well as potential explosions if the acid comes into contact with organic materials.
To address these environmental concerns, researchers and laboratories using perchloric acid in cyclic voltammetry experiments must implement rigorous safety protocols and waste management practices. This includes using fume hoods with specialized wash-down systems, proper neutralization techniques before disposal, and adherence to local and national environmental regulations.
Efforts to develop alternative electrolytes or methodologies that can replace perchloric acid in cyclic voltammetry experiments are ongoing. These research initiatives aim to reduce the environmental footprint of electrochemical experiments while maintaining analytical accuracy and efficiency. As environmental awareness grows, the scientific community continues to explore more sustainable practices in laboratory settings, balancing the need for advanced analytical techniques with environmental stewardship.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. Fish and other organisms may experience respiratory distress, reproductive issues, and even mortality in severely contaminated waters. The acid can also leach into groundwater, potentially affecting drinking water supplies for both humans and wildlife.
Soil contamination is another critical environmental concern. Perchloric acid can alter soil chemistry, affecting plant growth and microbial communities essential for ecosystem functioning. This can lead to reduced biodiversity and ecosystem productivity in affected areas. Furthermore, the persistence of perchlorate ions in soil can result in long-term environmental impacts, as these ions can remain stable for extended periods.
The atmospheric release of perchloric acid vapors during experiments or improper handling can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Inhalation of these vapors can pose health risks to both laboratory personnel and nearby communities.
Proper disposal of perchloric acid and related waste is crucial to mitigate environmental impacts. Many jurisdictions have strict regulations governing the handling and disposal of perchloric acid due to its hazardous nature. Improper disposal can lead to soil and water contamination, as well as potential explosions if the acid comes into contact with organic materials.
To address these environmental concerns, researchers and laboratories using perchloric acid in cyclic voltammetry experiments must implement rigorous safety protocols and waste management practices. This includes using fume hoods with specialized wash-down systems, proper neutralization techniques before disposal, and adherence to local and national environmental regulations.
Efforts to develop alternative electrolytes or methodologies that can replace perchloric acid in cyclic voltammetry experiments are ongoing. These research initiatives aim to reduce the environmental footprint of electrochemical experiments while maintaining analytical accuracy and efficiency. As environmental awareness grows, the scientific community continues to explore more sustainable practices in laboratory settings, balancing the need for advanced analytical techniques with environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!