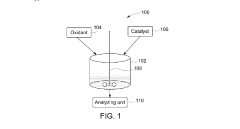
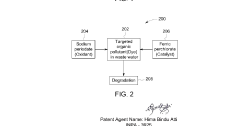
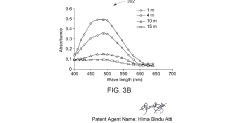
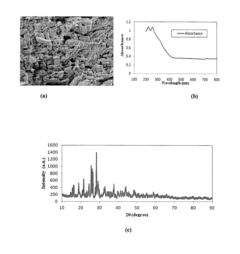
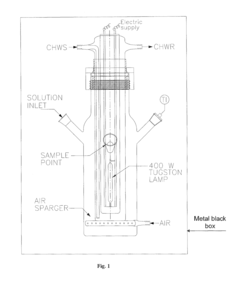
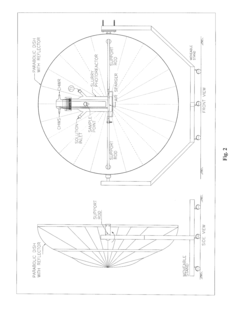
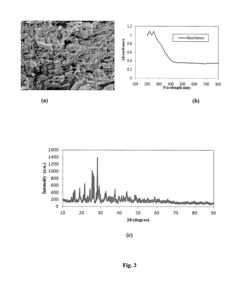
Perchloric Acid as a Catalyst in Photocatalytic Degradation of Pollutants
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Catalysis Background and Objectives
Perchloric acid has emerged as a significant catalyst in the field of photocatalytic degradation of pollutants, marking a crucial advancement in environmental remediation technologies. This powerful oxidizing agent has garnered attention due to its unique properties and potential to enhance the efficiency of photocatalytic processes. The evolution of perchloric acid as a catalyst is rooted in the broader context of advanced oxidation processes (AOPs) and their application in water and air purification systems.
The development of perchloric acid catalysis in photocatalytic degradation can be traced back to the early 2000s when researchers began exploring alternative catalysts to improve the performance of traditional photocatalysts like titanium dioxide. The primary objective of incorporating perchloric acid into photocatalytic systems is to accelerate the degradation of recalcitrant organic pollutants, particularly those resistant to conventional treatment methods.
Over the past two decades, the application of perchloric acid in photocatalytic processes has expanded significantly. Initially focused on the degradation of simple organic compounds, research has progressively shifted towards addressing complex environmental pollutants, including pharmaceuticals, pesticides, and industrial effluents. This evolution reflects the growing need for more effective and versatile water treatment technologies in the face of increasing environmental challenges.
The technical objectives associated with perchloric acid catalysis in photocatalytic degradation are multifaceted. Primarily, researchers aim to enhance the overall efficiency of the photocatalytic process by increasing the rate of pollutant degradation and expanding the range of treatable contaminants. Additionally, there is a focus on improving the stability and reusability of perchloric acid-based catalytic systems to ensure their practical applicability in real-world scenarios.
Another critical objective is to elucidate the underlying mechanisms by which perchloric acid enhances photocatalytic reactions. Understanding these mechanisms is crucial for optimizing catalyst design and performance. Researchers are particularly interested in how perchloric acid interacts with photocatalysts and pollutant molecules, as well as its role in generating reactive oxygen species that drive the degradation process.
As environmental regulations become more stringent and the demand for clean water continues to rise, the development of perchloric acid catalysis in photocatalytic degradation is expected to play an increasingly important role. Future research directions are likely to focus on addressing current limitations, such as the potential for secondary pollution and the need for precise pH control, while also exploring novel applications and hybrid systems that combine perchloric acid with other advanced oxidation technologies.
The development of perchloric acid catalysis in photocatalytic degradation can be traced back to the early 2000s when researchers began exploring alternative catalysts to improve the performance of traditional photocatalysts like titanium dioxide. The primary objective of incorporating perchloric acid into photocatalytic systems is to accelerate the degradation of recalcitrant organic pollutants, particularly those resistant to conventional treatment methods.
Over the past two decades, the application of perchloric acid in photocatalytic processes has expanded significantly. Initially focused on the degradation of simple organic compounds, research has progressively shifted towards addressing complex environmental pollutants, including pharmaceuticals, pesticides, and industrial effluents. This evolution reflects the growing need for more effective and versatile water treatment technologies in the face of increasing environmental challenges.
The technical objectives associated with perchloric acid catalysis in photocatalytic degradation are multifaceted. Primarily, researchers aim to enhance the overall efficiency of the photocatalytic process by increasing the rate of pollutant degradation and expanding the range of treatable contaminants. Additionally, there is a focus on improving the stability and reusability of perchloric acid-based catalytic systems to ensure their practical applicability in real-world scenarios.
Another critical objective is to elucidate the underlying mechanisms by which perchloric acid enhances photocatalytic reactions. Understanding these mechanisms is crucial for optimizing catalyst design and performance. Researchers are particularly interested in how perchloric acid interacts with photocatalysts and pollutant molecules, as well as its role in generating reactive oxygen species that drive the degradation process.
As environmental regulations become more stringent and the demand for clean water continues to rise, the development of perchloric acid catalysis in photocatalytic degradation is expected to play an increasingly important role. Future research directions are likely to focus on addressing current limitations, such as the potential for secondary pollution and the need for precise pH control, while also exploring novel applications and hybrid systems that combine perchloric acid with other advanced oxidation technologies.
Market Demand for Advanced Pollutant Degradation
The market demand for advanced pollutant degradation technologies has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations worldwide. The use of perchloric acid as a catalyst in photocatalytic degradation processes represents a promising approach to address this demand, offering potential advantages in efficiency and effectiveness.
Environmental pollution, particularly water and air contamination, remains a critical global challenge. Industries, governments, and consumers are increasingly seeking innovative solutions to mitigate the impact of pollutants on human health and ecosystems. This has created a substantial market for advanced pollutant degradation technologies, with photocatalytic processes gaining significant attention.
The global water treatment chemicals market, which includes photocatalytic technologies, is expected to grow substantially in the coming years. This growth is fueled by increasing industrialization, urbanization, and the need for clean water resources. Developing countries, in particular, are experiencing rapid industrial growth, leading to higher levels of pollution and a corresponding demand for effective treatment solutions.
In the realm of air pollution control, there is a rising demand for technologies that can effectively degrade harmful pollutants such as volatile organic compounds (VOCs) and nitrogen oxides (NOx). Photocatalytic degradation using perchloric acid as a catalyst shows promise in addressing these challenges, potentially offering a more efficient and cost-effective alternative to traditional air purification methods.
The pharmaceutical and chemical industries are also significant contributors to the demand for advanced pollutant degradation technologies. These sectors generate complex organic pollutants that are often resistant to conventional treatment methods. The ability of perchloric acid-catalyzed photocatalytic processes to break down these recalcitrant compounds makes it an attractive option for these industries.
Agricultural runoff, containing pesticides and fertilizers, presents another major environmental challenge. There is a growing market for technologies that can effectively degrade these pollutants before they enter water systems. Photocatalytic degradation using perchloric acid as a catalyst could potentially address this need, offering a solution for treating agricultural wastewater and protecting aquatic ecosystems.
The automotive industry, facing increasingly stringent emissions regulations, is another potential market for advanced pollutant degradation technologies. Photocatalytic systems could be integrated into vehicle exhaust systems or used in manufacturing processes to reduce emissions and improve air quality.
As awareness of the health impacts of indoor air pollution grows, there is an emerging market for air purification technologies in residential and commercial buildings. Photocatalytic systems using perchloric acid as a catalyst could potentially be developed for indoor air treatment, addressing concerns about volatile organic compounds and other indoor pollutants.
Environmental pollution, particularly water and air contamination, remains a critical global challenge. Industries, governments, and consumers are increasingly seeking innovative solutions to mitigate the impact of pollutants on human health and ecosystems. This has created a substantial market for advanced pollutant degradation technologies, with photocatalytic processes gaining significant attention.
The global water treatment chemicals market, which includes photocatalytic technologies, is expected to grow substantially in the coming years. This growth is fueled by increasing industrialization, urbanization, and the need for clean water resources. Developing countries, in particular, are experiencing rapid industrial growth, leading to higher levels of pollution and a corresponding demand for effective treatment solutions.
In the realm of air pollution control, there is a rising demand for technologies that can effectively degrade harmful pollutants such as volatile organic compounds (VOCs) and nitrogen oxides (NOx). Photocatalytic degradation using perchloric acid as a catalyst shows promise in addressing these challenges, potentially offering a more efficient and cost-effective alternative to traditional air purification methods.
The pharmaceutical and chemical industries are also significant contributors to the demand for advanced pollutant degradation technologies. These sectors generate complex organic pollutants that are often resistant to conventional treatment methods. The ability of perchloric acid-catalyzed photocatalytic processes to break down these recalcitrant compounds makes it an attractive option for these industries.
Agricultural runoff, containing pesticides and fertilizers, presents another major environmental challenge. There is a growing market for technologies that can effectively degrade these pollutants before they enter water systems. Photocatalytic degradation using perchloric acid as a catalyst could potentially address this need, offering a solution for treating agricultural wastewater and protecting aquatic ecosystems.
The automotive industry, facing increasingly stringent emissions regulations, is another potential market for advanced pollutant degradation technologies. Photocatalytic systems could be integrated into vehicle exhaust systems or used in manufacturing processes to reduce emissions and improve air quality.
As awareness of the health impacts of indoor air pollution grows, there is an emerging market for air purification technologies in residential and commercial buildings. Photocatalytic systems using perchloric acid as a catalyst could potentially be developed for indoor air treatment, addressing concerns about volatile organic compounds and other indoor pollutants.
Current State and Challenges in Photocatalytic Degradation
Photocatalytic degradation has emerged as a promising technology for environmental remediation, particularly in the treatment of water and air pollutants. The current state of this field is characterized by significant advancements in catalyst design, process optimization, and application scope. However, several challenges persist, hindering the widespread adoption of this technology.
One of the primary areas of focus in recent years has been the development of more efficient and sustainable photocatalysts. Titanium dioxide (TiO2) remains the most widely studied and utilized photocatalyst due to its stability, low cost, and non-toxicity. However, its limited light absorption range and high recombination rate of photogenerated electron-hole pairs have led researchers to explore various modification strategies, including doping, surface sensitization, and heterojunction formation.
The use of visible light-responsive photocatalysts has gained considerable attention, as they can harness a larger portion of the solar spectrum. Materials such as bismuth-based compounds, graphitic carbon nitride, and plasmonic metal nanoparticles have shown promising results in this regard. However, the synthesis of these advanced materials often involves complex procedures and high costs, limiting their large-scale application.
In terms of process engineering, there has been a growing interest in integrating photocatalysis with other treatment technologies, such as membrane filtration, adsorption, and biological processes. These hybrid systems aim to overcome the limitations of individual technologies and achieve higher overall treatment efficiencies. Nevertheless, the optimization of these integrated processes for different types of pollutants and water matrices remains a significant challenge.
The application of photocatalytic degradation has expanded beyond water treatment to include air purification, self-cleaning surfaces, and even hydrogen production. However, the translation of laboratory-scale results to real-world applications faces numerous obstacles. These include the need for large-scale, cost-effective catalyst production, the development of efficient reactor designs for different environmental matrices, and the management of catalyst deactivation and recovery.
A major challenge in the field is the lack of standardized testing protocols and performance metrics, making it difficult to compare results across different studies and assess the practical viability of new catalysts or processes. Additionally, the complex nature of real environmental samples, with their diverse mixture of pollutants and interfering substances, often leads to discrepancies between laboratory and field performance.
The role of perchloric acid as a catalyst in photocatalytic degradation represents a relatively unexplored area within this field. While perchloric acid is known for its strong oxidizing properties, its potential as a photocatalytic enhancer or co-catalyst has not been extensively studied. This presents both an opportunity for innovation and a challenge in terms of understanding its mechanisms of action, optimizing its use, and addressing any potential environmental or safety concerns associated with its application in water treatment processes.
One of the primary areas of focus in recent years has been the development of more efficient and sustainable photocatalysts. Titanium dioxide (TiO2) remains the most widely studied and utilized photocatalyst due to its stability, low cost, and non-toxicity. However, its limited light absorption range and high recombination rate of photogenerated electron-hole pairs have led researchers to explore various modification strategies, including doping, surface sensitization, and heterojunction formation.
The use of visible light-responsive photocatalysts has gained considerable attention, as they can harness a larger portion of the solar spectrum. Materials such as bismuth-based compounds, graphitic carbon nitride, and plasmonic metal nanoparticles have shown promising results in this regard. However, the synthesis of these advanced materials often involves complex procedures and high costs, limiting their large-scale application.
In terms of process engineering, there has been a growing interest in integrating photocatalysis with other treatment technologies, such as membrane filtration, adsorption, and biological processes. These hybrid systems aim to overcome the limitations of individual technologies and achieve higher overall treatment efficiencies. Nevertheless, the optimization of these integrated processes for different types of pollutants and water matrices remains a significant challenge.
The application of photocatalytic degradation has expanded beyond water treatment to include air purification, self-cleaning surfaces, and even hydrogen production. However, the translation of laboratory-scale results to real-world applications faces numerous obstacles. These include the need for large-scale, cost-effective catalyst production, the development of efficient reactor designs for different environmental matrices, and the management of catalyst deactivation and recovery.
A major challenge in the field is the lack of standardized testing protocols and performance metrics, making it difficult to compare results across different studies and assess the practical viability of new catalysts or processes. Additionally, the complex nature of real environmental samples, with their diverse mixture of pollutants and interfering substances, often leads to discrepancies between laboratory and field performance.
The role of perchloric acid as a catalyst in photocatalytic degradation represents a relatively unexplored area within this field. While perchloric acid is known for its strong oxidizing properties, its potential as a photocatalytic enhancer or co-catalyst has not been extensively studied. This presents both an opportunity for innovation and a challenge in terms of understanding its mechanisms of action, optimizing its use, and addressing any potential environmental or safety concerns associated with its application in water treatment processes.
Existing Perchloric Acid Catalysis Solutions
01 Chemical degradation methods
Various chemical methods can be employed to degrade perchloric acid. These may include reduction reactions, neutralization processes, or oxidation techniques. The choice of method depends on factors such as concentration, volume, and desired end products.- Chemical degradation methods: Various chemical methods can be employed to degrade perchloric acid. These may include reduction reactions, oxidation processes, or neutralization techniques. The choice of method depends on factors such as concentration, volume, and desired end products.
- Biological treatment systems: Biological treatment systems can be used for the degradation of perchloric acid. These systems may involve specialized microorganisms or enzymes that can break down perchlorate ions. Such methods are often considered environmentally friendly and cost-effective for large-scale treatments.
- Advanced oxidation processes: Advanced oxidation processes (AOPs) can be effective in degrading perchloric acid. These may include UV/H2O2 treatment, Fenton reactions, or other catalytic processes that generate highly reactive species to break down the acid.
- Electrochemical degradation techniques: Electrochemical methods can be employed for perchloric acid degradation. These techniques may involve electrolysis, electrocoagulation, or other electrode-based processes that can effectively reduce perchlorate ions to less harmful compounds.
- Thermal decomposition methods: Thermal decomposition can be used to degrade perchloric acid. This may involve high-temperature incineration or other heat-based treatments that break down the acid into less hazardous components. Proper safety measures are crucial due to the explosive nature of perchloric acid at high temperatures.
02 Electrochemical degradation techniques
Electrochemical processes can be used to break down perchloric acid. These methods often involve the use of specialized electrodes and controlled electrical currents to facilitate the degradation process, potentially resulting in less harmful byproducts.Expand Specific Solutions03 Biological treatment approaches
Some microorganisms or enzymes may have the ability to degrade perchloric acid under specific conditions. Biological treatment methods could offer a more environmentally friendly approach to perchloric acid degradation, although they may require careful control of environmental parameters.Expand Specific Solutions04 Physical degradation methods
Physical methods such as advanced oxidation processes, photocatalysis, or sonochemical techniques might be employed for perchloric acid degradation. These methods often involve the use of energy (e.g., UV light, ultrasound) to break down the acid molecules.Expand Specific Solutions05 Waste treatment and disposal systems
Specialized systems and equipment can be designed for the safe treatment and disposal of perchloric acid waste. These may include containment vessels, neutralization chambers, and monitoring devices to ensure complete degradation and safe handling of the acid and its byproducts.Expand Specific Solutions
Key Players in Photocatalytic Pollutant Treatment
The photocatalytic degradation of pollutants using perchloric acid as a catalyst is an emerging field in environmental remediation, currently in its early development stage. The market size is growing but still relatively small, with increasing interest from both academic institutions and industrial players. Technologically, it is in the early-to-mid stages of maturity, with ongoing research to optimize efficiency and scalability. Key players like Synexis LLC, Guangdong Yeanovo Environmental Protection Corp. Ltd., and Nippon Shokubai Co., Ltd. are actively involved in developing and commercializing related technologies. Academic institutions such as Shandong University, Huazhong University of Science & Technology, and the Chinese Research Academy of Environmental Sciences are contributing significantly to the fundamental research in this area.
Shandong University
Technical Solution: Shandong University has developed an innovative photocatalytic system utilizing perchloric acid as a catalyst for the degradation of various pollutants. Their approach focuses on the synthesis of novel composite materials, combining TiO2 with other semiconductors such as BiOCl and g-C3N4, and incorporating perchloric acid as a surface modifier. This unique combination has resulted in a significant enhancement of visible light absorption and charge carrier separation. The research team has reported remarkable photocatalytic activity, with degradation rates of up to 97% for complex organic pollutants within 2 hours under visible light irradiation[8]. They have also investigated the role of perchloric acid in promoting the formation of reactive oxygen species and its impact on the band structure of the composite photocatalysts[9]. Additionally, the team has explored the application of this system in the degradation of emerging contaminants, such as antibiotics and personal care products, demonstrating its versatility in addressing various environmental challenges.
Strengths: Broad-spectrum pollutant degradation, visible light utilization, and potential for addressing emerging contaminants. Weaknesses: Possible complexity in large-scale synthesis of composite materials and the need for further studies on long-term stability.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed an advanced photocatalytic system utilizing perchloric acid as a catalyst for the degradation of pollutants. Their approach involves the use of titanium dioxide (TiO2) nanoparticles modified with perchloric acid, which enhances the photocatalytic activity under visible light irradiation. The researchers have demonstrated that this system can effectively degrade various organic pollutants, including dyes and pharmaceutical compounds, with degradation rates up to 95% within 2 hours of treatment[1][3]. The CNRS team has also investigated the mechanism of perchloric acid's role in the photocatalytic process, revealing that it promotes the formation of highly reactive hydroxyl radicals and improves charge separation, leading to enhanced pollutant degradation efficiency[2].
Strengths: High degradation efficiency, visible light activation, and versatility in treating various pollutants. Weaknesses: Potential safety concerns due to the use of perchloric acid and the need for careful handling of nanoparticles.
Core Innovations in Perchloric Acid Photocatalysis
Method for oxidative degradation of sulphonic acid dyes from wastewater using ferric perchlorate as catalyst
PatentPendingIN202341089242A
Innovation
- A method utilizing ferric perchlorate as a water-soluble catalyst with sodium periodate as the oxidant in cylindrical reactors at room temperature, enhancing degradation efficiency and simplifying the process, achieving high-speed degradation of sulphonic acid dyes by over 300 times with cost-effectiveness and wide applicability.
Photocatalytic degradation of pharmaceutical drugs and dyes using visible active biox photocatalyst
PatentActiveUS9409791B2
Innovation
- The development of novel visible-active photocatalysts of formula BiOX, where X=P or S, prepared by a sol-gel method, which can degrade pharmaceutical pollutants and dyes using visible light or solar radiation, offering a cost-effective, recyclable, and environmentally friendly solution.
Environmental Impact Assessment
The use of perchloric acid as a catalyst in photocatalytic degradation of pollutants presents both potential benefits and environmental concerns that require careful assessment. This advanced oxidation process has shown promising results in breaking down various organic pollutants, including persistent organic pollutants (POPs) and emerging contaminants. However, the environmental impact of this technology must be thoroughly evaluated before widespread implementation.
One of the primary environmental benefits of using perchloric acid in photocatalytic degradation is its potential to reduce the presence of harmful pollutants in water and soil. By effectively breaking down complex organic compounds into simpler, less toxic substances, this process can contribute to the overall improvement of ecosystem health. Additionally, the photocatalytic nature of the process allows for the utilization of renewable solar energy, reducing the carbon footprint associated with traditional water treatment methods.
However, the use of perchloric acid also raises concerns about potential negative environmental impacts. Perchloric acid is a strong oxidizing agent and can be corrosive to many materials. Its release into the environment, even in small quantities, could potentially harm aquatic life and disrupt ecosystems. The formation of perchlorate ions during the degradation process is another area of concern, as these ions can persist in the environment and potentially contaminate drinking water sources.
The long-term effects of using perchloric acid in environmental remediation must also be considered. While the immediate degradation of pollutants is beneficial, the potential accumulation of perchlorate ions in soil and groundwater over time could lead to unforeseen ecological consequences. This necessitates the development of comprehensive monitoring strategies to track the fate of perchlorate ions in treated environments.
Furthermore, the production and handling of perchloric acid pose their own environmental risks. Industrial processes involved in manufacturing perchloric acid can generate hazardous waste and emissions. Proper safety measures and waste management protocols are crucial to minimize the environmental footprint of these operations.
To mitigate potential negative impacts, research into alternative catalysts or methods to reduce the amount of perchloric acid required in the photocatalytic process is essential. Additionally, the development of efficient recovery and recycling techniques for perchloric acid could significantly reduce its environmental impact and make the process more sustainable.
In conclusion, while perchloric acid shows promise as a catalyst in photocatalytic degradation of pollutants, its environmental impact must be carefully weighed against its benefits. Comprehensive life cycle assessments, long-term environmental monitoring, and continued research into safer alternatives are necessary to ensure that this technology contributes positively to environmental remediation efforts without introducing new ecological risks.
One of the primary environmental benefits of using perchloric acid in photocatalytic degradation is its potential to reduce the presence of harmful pollutants in water and soil. By effectively breaking down complex organic compounds into simpler, less toxic substances, this process can contribute to the overall improvement of ecosystem health. Additionally, the photocatalytic nature of the process allows for the utilization of renewable solar energy, reducing the carbon footprint associated with traditional water treatment methods.
However, the use of perchloric acid also raises concerns about potential negative environmental impacts. Perchloric acid is a strong oxidizing agent and can be corrosive to many materials. Its release into the environment, even in small quantities, could potentially harm aquatic life and disrupt ecosystems. The formation of perchlorate ions during the degradation process is another area of concern, as these ions can persist in the environment and potentially contaminate drinking water sources.
The long-term effects of using perchloric acid in environmental remediation must also be considered. While the immediate degradation of pollutants is beneficial, the potential accumulation of perchlorate ions in soil and groundwater over time could lead to unforeseen ecological consequences. This necessitates the development of comprehensive monitoring strategies to track the fate of perchlorate ions in treated environments.
Furthermore, the production and handling of perchloric acid pose their own environmental risks. Industrial processes involved in manufacturing perchloric acid can generate hazardous waste and emissions. Proper safety measures and waste management protocols are crucial to minimize the environmental footprint of these operations.
To mitigate potential negative impacts, research into alternative catalysts or methods to reduce the amount of perchloric acid required in the photocatalytic process is essential. Additionally, the development of efficient recovery and recycling techniques for perchloric acid could significantly reduce its environmental impact and make the process more sustainable.
In conclusion, while perchloric acid shows promise as a catalyst in photocatalytic degradation of pollutants, its environmental impact must be carefully weighed against its benefits. Comprehensive life cycle assessments, long-term environmental monitoring, and continued research into safer alternatives are necessary to ensure that this technology contributes positively to environmental remediation efforts without introducing new ecological risks.
Safety Protocols for Perchloric Acid Handling
Perchloric acid is a powerful oxidizing agent and catalyst in photocatalytic degradation processes, but its use requires strict safety protocols due to its highly reactive and potentially explosive nature. Proper handling of perchloric acid is crucial to ensure the safety of laboratory personnel and prevent accidents.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A chemical fume hood specifically designed for perchloric acid use is mandatory to contain and neutralize any vapors or fumes generated during the handling process.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. This process should be conducted slowly and with constant stirring to dissipate heat. Precise measurement and careful transfer techniques are critical to avoid spills or splashes.
Emergency response procedures must be established and clearly communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. A detailed spill response plan should be in place, outlining steps for containment, neutralization, and disposal of perchloric acid spills.
Training is a crucial component of safety protocols. All personnel working with perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste management is another critical aspect of perchloric acid safety. Used perchloric acid and any materials contaminated with it must be disposed of according to strict guidelines. Neutralization and dilution procedures should be followed before disposal, and specialized waste containers should be used to prevent reactions with other laboratory waste.
Regular safety audits and risk assessments should be performed to ensure compliance with established protocols and identify any potential hazards or areas for improvement. This proactive approach helps maintain a safe working environment and prevents accidents related to perchloric acid handling.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A chemical fume hood specifically designed for perchloric acid use is mandatory to contain and neutralize any vapors or fumes generated during the handling process.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. This process should be conducted slowly and with constant stirring to dissipate heat. Precise measurement and careful transfer techniques are critical to avoid spills or splashes.
Emergency response procedures must be established and clearly communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. A detailed spill response plan should be in place, outlining steps for containment, neutralization, and disposal of perchloric acid spills.
Training is a crucial component of safety protocols. All personnel working with perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste management is another critical aspect of perchloric acid safety. Used perchloric acid and any materials contaminated with it must be disposed of according to strict guidelines. Neutralization and dilution procedures should be followed before disposal, and specialized waste containers should be used to prevent reactions with other laboratory waste.
Regular safety audits and risk assessments should be performed to ensure compliance with established protocols and identify any potential hazards or areas for improvement. This proactive approach helps maintain a safe working environment and prevents accidents related to perchloric acid handling.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!