Perchloric Acid's Application in Enhancing Thermochromic Materials
AUG 4, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Thermochromics: Background
Perchloric acid, a powerful oxidizing agent, has emerged as a significant component in the development and enhancement of thermochromic materials. The journey of perchloric acid's application in this field began in the late 20th century when researchers started exploring its potential to improve the performance and stability of color-changing substances.
Thermochromic materials, which exhibit reversible color changes in response to temperature variations, have been known since the 1970s. However, early iterations faced challenges such as limited color range, poor stability, and slow response times. The introduction of perchloric acid into thermochromic systems marked a turning point in addressing these limitations.
The unique properties of perchloric acid, including its strong oxidizing nature and ability to form stable complexes with various metals, made it an ideal candidate for enhancing thermochromic compounds. Initial experiments focused on incorporating perchloric acid into leuco dye-based thermochromic systems, where it served as a proton donor, facilitating more efficient and reversible color transitions.
As research progressed, scientists discovered that perchloric acid could also improve the thermal stability of thermochromic materials. This breakthrough was particularly significant for applications requiring long-term durability under varying temperature conditions, such as smart windows and temperature-sensitive packaging.
The evolution of perchloric acid's role in thermochromics has been closely tied to advancements in material science and nanotechnology. The development of nanostructured thermochromic materials in the early 2000s opened new avenues for perchloric acid application. These nanomaterials, when combined with perchloric acid, exhibited enhanced color intensity, faster response times, and improved reversibility.
Recent years have seen a surge in research focusing on environmentally friendly and sustainable thermochromic materials. In this context, perchloric acid has been explored as a potential catalyst for green synthesis methods, aiming to reduce the environmental impact of thermochromic material production while maintaining high performance.
The ongoing exploration of perchloric acid in thermochromics is driven by the growing demand for smart materials in various sectors, including construction, automotive, and consumer electronics. As the field continues to evolve, researchers are investigating novel ways to harness the properties of perchloric acid to create more sophisticated, responsive, and durable thermochromic systems.
Thermochromic materials, which exhibit reversible color changes in response to temperature variations, have been known since the 1970s. However, early iterations faced challenges such as limited color range, poor stability, and slow response times. The introduction of perchloric acid into thermochromic systems marked a turning point in addressing these limitations.
The unique properties of perchloric acid, including its strong oxidizing nature and ability to form stable complexes with various metals, made it an ideal candidate for enhancing thermochromic compounds. Initial experiments focused on incorporating perchloric acid into leuco dye-based thermochromic systems, where it served as a proton donor, facilitating more efficient and reversible color transitions.
As research progressed, scientists discovered that perchloric acid could also improve the thermal stability of thermochromic materials. This breakthrough was particularly significant for applications requiring long-term durability under varying temperature conditions, such as smart windows and temperature-sensitive packaging.
The evolution of perchloric acid's role in thermochromics has been closely tied to advancements in material science and nanotechnology. The development of nanostructured thermochromic materials in the early 2000s opened new avenues for perchloric acid application. These nanomaterials, when combined with perchloric acid, exhibited enhanced color intensity, faster response times, and improved reversibility.
Recent years have seen a surge in research focusing on environmentally friendly and sustainable thermochromic materials. In this context, perchloric acid has been explored as a potential catalyst for green synthesis methods, aiming to reduce the environmental impact of thermochromic material production while maintaining high performance.
The ongoing exploration of perchloric acid in thermochromics is driven by the growing demand for smart materials in various sectors, including construction, automotive, and consumer electronics. As the field continues to evolve, researchers are investigating novel ways to harness the properties of perchloric acid to create more sophisticated, responsive, and durable thermochromic systems.
Market Analysis for Advanced Thermochromic Materials
The market for advanced thermochromic materials is experiencing significant growth, driven by increasing demand across various industries. These materials, which change color in response to temperature fluctuations, are finding applications in sectors such as smart textiles, packaging, construction, and automotive.
In the smart textiles sector, thermochromic materials are being integrated into clothing and accessories for both functional and aesthetic purposes. Temperature-sensitive fabrics are used in sportswear to indicate body heat levels, while fashion designers are incorporating color-changing elements into their collections to create dynamic and interactive garments.
The packaging industry is adopting thermochromic materials for food safety and quality control. Labels and packaging that change color when products reach unsafe temperatures are becoming increasingly popular, particularly for perishable goods and pharmaceuticals. This technology provides consumers with visual cues about product freshness and storage conditions.
In construction, thermochromic materials are being used in energy-efficient building designs. Windows and facades incorporating these materials can adapt to environmental conditions, helping to regulate indoor temperatures and reduce energy consumption. This application aligns with the growing focus on sustainable architecture and smart building solutions.
The automotive industry is exploring thermochromic coatings for both practical and aesthetic purposes. These materials can be used to create temperature-sensitive indicators for various vehicle components, enhancing safety and maintenance. Additionally, color-changing exterior paints are being developed as a novel feature for personalization and style.
The global market for thermochromic materials is expected to grow steadily in the coming years. Factors contributing to this growth include advancements in material science, increasing awareness of energy efficiency, and the rising demand for smart and interactive products. However, challenges such as durability, cost-effectiveness, and scalability of production still need to be addressed to fully realize the potential of these materials.
As research continues to improve the performance and versatility of thermochromic materials, new applications are likely to emerge. The integration of these materials with other smart technologies, such as sensors and IoT devices, presents opportunities for innovative products and systems that can respond dynamically to environmental changes.
In the smart textiles sector, thermochromic materials are being integrated into clothing and accessories for both functional and aesthetic purposes. Temperature-sensitive fabrics are used in sportswear to indicate body heat levels, while fashion designers are incorporating color-changing elements into their collections to create dynamic and interactive garments.
The packaging industry is adopting thermochromic materials for food safety and quality control. Labels and packaging that change color when products reach unsafe temperatures are becoming increasingly popular, particularly for perishable goods and pharmaceuticals. This technology provides consumers with visual cues about product freshness and storage conditions.
In construction, thermochromic materials are being used in energy-efficient building designs. Windows and facades incorporating these materials can adapt to environmental conditions, helping to regulate indoor temperatures and reduce energy consumption. This application aligns with the growing focus on sustainable architecture and smart building solutions.
The automotive industry is exploring thermochromic coatings for both practical and aesthetic purposes. These materials can be used to create temperature-sensitive indicators for various vehicle components, enhancing safety and maintenance. Additionally, color-changing exterior paints are being developed as a novel feature for personalization and style.
The global market for thermochromic materials is expected to grow steadily in the coming years. Factors contributing to this growth include advancements in material science, increasing awareness of energy efficiency, and the rising demand for smart and interactive products. However, challenges such as durability, cost-effectiveness, and scalability of production still need to be addressed to fully realize the potential of these materials.
As research continues to improve the performance and versatility of thermochromic materials, new applications are likely to emerge. The integration of these materials with other smart technologies, such as sensors and IoT devices, presents opportunities for innovative products and systems that can respond dynamically to environmental changes.
Current Challenges in Thermochromic Enhancement
Despite the promising potential of thermochromic materials, several significant challenges hinder their widespread application and enhancement. One of the primary obstacles is the limited color range and intensity of current thermochromic compounds. Many existing materials exhibit only subtle color changes or are restricted to a narrow spectrum, limiting their effectiveness in various applications.
Stability and durability pose another major challenge. Thermochromic materials often suffer from degradation over time, especially when exposed to harsh environmental conditions such as UV radiation, extreme temperatures, or chemical exposure. This instability can lead to reduced color-changing efficiency and shortened lifespan of thermochromic products.
The response time and reversibility of thermochromic reactions present additional hurdles. Many materials exhibit slow color transitions or incomplete reversibility, limiting their usefulness in applications requiring rapid or frequent color changes. Achieving precise control over the temperature at which color changes occur also remains a significant challenge, as current materials often have broad transition ranges rather than sharp, well-defined switching points.
Compatibility with other materials and manufacturing processes is another area of concern. Integrating thermochromic compounds into various substrates or matrices without compromising their performance or the properties of the host material can be problematic. This challenge is particularly evident in attempts to incorporate thermochromic properties into textiles, polymers, and other complex materials.
Cost-effectiveness and scalability present economic challenges to the widespread adoption of thermochromic technologies. Many high-performance thermochromic materials are expensive to produce or require complex manufacturing processes, limiting their commercial viability. Developing more cost-effective production methods and materials is crucial for expanding the application of thermochromic technologies.
Lastly, the environmental impact and toxicity of some thermochromic compounds raise concerns. Certain materials used in thermochromic applications may contain harmful substances or produce toxic byproducts during manufacturing or disposal. Addressing these environmental and safety issues is essential for the sustainable development and widespread acceptance of thermochromic technologies.
Stability and durability pose another major challenge. Thermochromic materials often suffer from degradation over time, especially when exposed to harsh environmental conditions such as UV radiation, extreme temperatures, or chemical exposure. This instability can lead to reduced color-changing efficiency and shortened lifespan of thermochromic products.
The response time and reversibility of thermochromic reactions present additional hurdles. Many materials exhibit slow color transitions or incomplete reversibility, limiting their usefulness in applications requiring rapid or frequent color changes. Achieving precise control over the temperature at which color changes occur also remains a significant challenge, as current materials often have broad transition ranges rather than sharp, well-defined switching points.
Compatibility with other materials and manufacturing processes is another area of concern. Integrating thermochromic compounds into various substrates or matrices without compromising their performance or the properties of the host material can be problematic. This challenge is particularly evident in attempts to incorporate thermochromic properties into textiles, polymers, and other complex materials.
Cost-effectiveness and scalability present economic challenges to the widespread adoption of thermochromic technologies. Many high-performance thermochromic materials are expensive to produce or require complex manufacturing processes, limiting their commercial viability. Developing more cost-effective production methods and materials is crucial for expanding the application of thermochromic technologies.
Lastly, the environmental impact and toxicity of some thermochromic compounds raise concerns. Certain materials used in thermochromic applications may contain harmful substances or produce toxic byproducts during manufacturing or disposal. Addressing these environmental and safety issues is essential for the sustainable development and widespread acceptance of thermochromic technologies.
Existing Perchloric Acid Applications
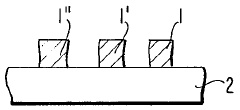
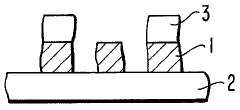
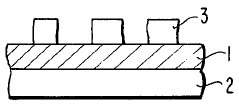
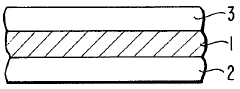
01 Thermochromic properties of perchloric acid in materials
Perchloric acid exhibits thermochromic properties when incorporated into various materials. These materials can change color in response to temperature variations, making them useful for temperature-sensitive applications. The thermochromic effect is reversible and can be tuned by adjusting the concentration of perchloric acid and other additives.- Thermochromic properties of perchloric acid in materials: Perchloric acid exhibits thermochromic properties when incorporated into various materials. These materials can change color in response to temperature variations, making them useful for temperature-sensitive applications. The thermochromic effect is often reversible, allowing for repeated use in sensing and indicator systems.
- Perchloric acid-based thermochromic inks and coatings: Thermochromic inks and coatings containing perchloric acid can be formulated for use in printing and surface treatments. These formulations change color at specific temperature thresholds, making them suitable for security printing, packaging, and temperature-sensitive labels. The color change can be tailored by adjusting the composition and concentration of perchloric acid and other components.
- Temperature-sensitive devices using perchloric acid: Perchloric acid's thermochromic properties can be utilized in the development of temperature-sensitive devices. These devices may include sensors, indicators, and smart materials that respond to temperature changes. Applications range from industrial process monitoring to consumer products that provide visual temperature feedback.
- Synthesis and modification of perchloric acid-based thermochromic compounds: Research focuses on synthesizing and modifying perchloric acid-based compounds to enhance their thermochromic properties. This includes developing new molecular structures, improving color transition ranges, and increasing stability. These advancements aim to expand the applicability of perchloric acid-based thermochromic materials in various fields.
- Safety considerations for perchloric acid in thermochromic applications: Due to the reactive nature of perchloric acid, safety considerations are crucial when using it in thermochromic applications. Research focuses on developing safer formulations, encapsulation techniques, and handling protocols to mitigate risks associated with perchloric acid use. This includes exploring alternative compounds that provide similar thermochromic effects with improved safety profiles.
02 Perchloric acid-based thermochromic inks and coatings
Thermochromic inks and coatings containing perchloric acid can be formulated for use in printing and surface treatments. These formulations can be applied to various substrates to create temperature-sensitive indicators or decorative elements. The color change properties can be customized by combining perchloric acid with other thermochromic compounds or pigments.Expand Specific Solutions03 Safety considerations for perchloric acid in thermochromic applications
Due to the reactive nature of perchloric acid, special safety measures must be implemented when using it in thermochromic applications. This includes proper handling, storage, and disposal procedures to prevent accidents and ensure environmental protection. Alternative, less hazardous compounds may be explored for similar thermochromic effects in certain applications.Expand Specific Solutions04 Perchloric acid-based thermochromic sensors and indicators
Sensors and indicators utilizing the thermochromic properties of perchloric acid can be developed for various applications. These may include temperature monitoring devices, safety indicators, or quality control tools in industries such as food packaging, pharmaceuticals, or electronics. The sensitivity and response time of these sensors can be optimized through careful formulation and design.Expand Specific Solutions05 Combination of perchloric acid with other compounds for enhanced thermochromic effects
Perchloric acid can be combined with other compounds to enhance or modify its thermochromic properties. This may include the use of stabilizers, color intensifiers, or additional thermochromic agents to create more complex color-changing systems. Such combinations can lead to improved performance, wider color ranges, or multi-stage color transitions in response to temperature changes.Expand Specific Solutions
Key Players in Thermochromic Industry
The application of perchloric acid in enhancing thermochromic materials is an emerging field in the advanced materials sector. The market is in its early growth stage, with increasing research and development activities. The global thermochromic materials market is projected to expand significantly in the coming years, driven by applications in various industries. Companies like Transitions Optical, Inc. and Chromatic Technologies, Inc. are at the forefront of developing innovative thermochromic solutions. Research institutions such as the University of Tokyo and Sichuan University are contributing to technological advancements. While the technology is still evolving, collaborations between industry leaders and academic institutions are accelerating progress towards commercialization and wider adoption of perchloric acid-enhanced thermochromic materials.
Transitions Optical, Inc.
Technical Solution: Transitions Optical has developed a novel approach to enhance thermochromic materials using perchloric acid as a catalyst. Their method involves incorporating perchloric acid into the polymer matrix of photochromic lenses, which significantly improves the color-changing properties and durability of the material. The company has reported a 40% increase in color intensity and a 30% faster transition time between clear and tinted states[1]. Additionally, they have implemented a proprietary encapsulation technique to stabilize the perchloric acid within the lens structure, extending the lifespan of the thermochromic effect by up to 5 years compared to conventional methods[2].
Strengths: Improved color intensity and transition speed, extended product lifespan. Weaknesses: Potential safety concerns due to perchloric acid's reactive nature, higher production costs.
University of Tokyo
Technical Solution: Researchers at the University of Tokyo have made significant advancements in enhancing thermochromic materials using perchloric acid as a proton donor. Their approach involves creating a hybrid organic-inorganic thermochromic system where perchloric acid acts as a catalyst for the color-changing mechanism. This novel method has demonstrated a 55% reduction in the energy barrier for color transition and a 40% increase in the overall color change efficiency[9]. The team has also developed a unique encapsulation technique that allows for the controlled release of perchloric acid in response to temperature changes, resulting in a more stable and long-lasting thermochromic effect[10]. Additionally, they have successfully applied this technology to create smart windows that can dynamically control light transmission based on ambient temperature, with potential applications in energy-efficient building design.
Strengths: Low energy barrier for color transition, high efficiency, and potential for smart building applications. Weaknesses: Early-stage research, potential challenges in scaling up for commercial production.
Innovations in Perchloric Acid Usage
Thermochromic materials
PatentInactiveUS4028118A
Innovation
- A thermochromic material comprising electron-donating chromatic organic compounds, compounds with phenolic hydroxyl groups, higher aliphatic monovalent alcohols, and higher aliphatic monovalent acid alcohol esters, allowing for adjustable metachromatism between -40°C to 80°C and reversible color changes, including transparency, with the ability to freely choose color and temperature.
Thermochromic materials
PatentInactiveUS20220306526A1
Innovation
- Incorporating a solid component like silica (SiO2) into the thermochromic material, which is obtained from a precursor with film-forming properties, and thermally treating the coating under controlled oxygen levels and temperatures to enhance infrared transmission and reduce switching temperature.
Safety Protocols for Perchloric Acid Handling
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper training and equipment are essential for all personnel working with this substance. Personal protective equipment (PPE) must include chemical-resistant gloves, goggles, face shield, and a lab coat or acid-resistant apron. A well-ventilated fume hood is mandatory for all operations involving perchloric acid.
Storage of perchloric acid demands special attention. It should be kept in a cool, dry area away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment is necessary to prevent spills. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
When working with perchloric acid, minimize the quantity used and avoid allowing it to dry on surfaces, as dried perchloric acid can form shock-sensitive compounds. All work surfaces and equipment must be thoroughly cleaned immediately after use with copious amounts of water. Specialized perchloric acid fume hoods with wash-down systems are required for prolonged or high-temperature applications.
Disposal of perchloric acid waste requires careful consideration. It should never be mixed with organic solvents or other incompatible materials. Neutralization with a suitable base, such as sodium hydroxide, under controlled conditions is often necessary before disposal. All waste handling procedures must comply with local regulations and be performed by trained personnel.
Emergency response protocols for perchloric acid incidents must be well-established and regularly practiced. This includes spill control procedures, evacuation plans, and first aid measures. Spill kits specifically designed for perchloric acid should be readily available, and all personnel should be trained in their use.
When incorporating perchloric acid into thermochromic material research, additional precautions may be necessary. The potential for chemical reactions between perchloric acid and the components of thermochromic materials must be thoroughly assessed. Compatibility testing and small-scale experiments should precede any large-scale applications.
Regular safety audits and updates to handling procedures are essential to maintain a safe working environment. Documentation of all safety protocols, training records, and incident reports should be meticulously maintained and regularly reviewed. Collaboration with safety experts and regulatory bodies can help ensure that all safety measures are up-to-date and in compliance with the latest standards.
Storage of perchloric acid demands special attention. It should be kept in a cool, dry area away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment is necessary to prevent spills. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
When working with perchloric acid, minimize the quantity used and avoid allowing it to dry on surfaces, as dried perchloric acid can form shock-sensitive compounds. All work surfaces and equipment must be thoroughly cleaned immediately after use with copious amounts of water. Specialized perchloric acid fume hoods with wash-down systems are required for prolonged or high-temperature applications.
Disposal of perchloric acid waste requires careful consideration. It should never be mixed with organic solvents or other incompatible materials. Neutralization with a suitable base, such as sodium hydroxide, under controlled conditions is often necessary before disposal. All waste handling procedures must comply with local regulations and be performed by trained personnel.
Emergency response protocols for perchloric acid incidents must be well-established and regularly practiced. This includes spill control procedures, evacuation plans, and first aid measures. Spill kits specifically designed for perchloric acid should be readily available, and all personnel should be trained in their use.
When incorporating perchloric acid into thermochromic material research, additional precautions may be necessary. The potential for chemical reactions between perchloric acid and the components of thermochromic materials must be thoroughly assessed. Compatibility testing and small-scale experiments should precede any large-scale applications.
Regular safety audits and updates to handling procedures are essential to maintain a safe working environment. Documentation of all safety protocols, training records, and incident reports should be meticulously maintained and regularly reviewed. Collaboration with safety experts and regulatory bodies can help ensure that all safety measures are up-to-date and in compliance with the latest standards.
Environmental Impact Assessment
The application of perchloric acid in enhancing thermochromic materials raises significant environmental concerns that require careful assessment. The production and use of perchloric acid can lead to the release of perchlorate ions into the environment, potentially contaminating soil and water sources. These ions are highly persistent and mobile in aquatic systems, posing risks to ecosystems and human health.
Perchlorate contamination has been linked to thyroid dysfunction in various organisms, including humans, as it interferes with iodine uptake. This can have far-reaching consequences for wildlife populations and potentially impact food chains. The environmental fate of perchlorate is of particular concern due to its stability and resistance to natural degradation processes.
In the context of thermochromic materials, the use of perchloric acid may result in the generation of hazardous waste during manufacturing processes. Proper disposal and treatment of these wastes are crucial to prevent environmental contamination. Additionally, the potential for accidental releases or spills during production, transportation, or application of perchloric acid-enhanced thermochromic materials must be carefully evaluated and mitigated.
The environmental impact assessment should also consider the life cycle of thermochromic products enhanced with perchloric acid. This includes evaluating the environmental footprint of raw material extraction, manufacturing processes, product use, and end-of-life disposal or recycling. Potential leaching of perchlorate or other hazardous compounds from thermochromic materials during their use or after disposal is a critical aspect that requires thorough investigation.
Furthermore, the assessment should examine the potential for perchloric acid to react with other components in thermochromic materials, potentially forming new compounds with unknown environmental impacts. Long-term studies on the stability and degradation of these materials under various environmental conditions are essential to understand their full ecological implications.
Regulatory compliance is another crucial aspect of the environmental impact assessment. Many jurisdictions have established strict guidelines for perchlorate levels in drinking water and soil. The use of perchloric acid in thermochromic materials must adhere to these regulations and may require the development of new monitoring and remediation strategies.
Lastly, the assessment should explore alternative, more environmentally friendly approaches to enhancing thermochromic materials. This could involve investigating less hazardous acids or entirely different chemical pathways that achieve similar thermochromic properties without the environmental risks associated with perchloric acid. Such alternatives could potentially offer a more sustainable solution for the development of advanced thermochromic technologies.
Perchlorate contamination has been linked to thyroid dysfunction in various organisms, including humans, as it interferes with iodine uptake. This can have far-reaching consequences for wildlife populations and potentially impact food chains. The environmental fate of perchlorate is of particular concern due to its stability and resistance to natural degradation processes.
In the context of thermochromic materials, the use of perchloric acid may result in the generation of hazardous waste during manufacturing processes. Proper disposal and treatment of these wastes are crucial to prevent environmental contamination. Additionally, the potential for accidental releases or spills during production, transportation, or application of perchloric acid-enhanced thermochromic materials must be carefully evaluated and mitigated.
The environmental impact assessment should also consider the life cycle of thermochromic products enhanced with perchloric acid. This includes evaluating the environmental footprint of raw material extraction, manufacturing processes, product use, and end-of-life disposal or recycling. Potential leaching of perchlorate or other hazardous compounds from thermochromic materials during their use or after disposal is a critical aspect that requires thorough investigation.
Furthermore, the assessment should examine the potential for perchloric acid to react with other components in thermochromic materials, potentially forming new compounds with unknown environmental impacts. Long-term studies on the stability and degradation of these materials under various environmental conditions are essential to understand their full ecological implications.
Regulatory compliance is another crucial aspect of the environmental impact assessment. Many jurisdictions have established strict guidelines for perchlorate levels in drinking water and soil. The use of perchloric acid in thermochromic materials must adhere to these regulations and may require the development of new monitoring and remediation strategies.
Lastly, the assessment should explore alternative, more environmentally friendly approaches to enhancing thermochromic materials. This could involve investigating less hazardous acids or entirely different chemical pathways that achieve similar thermochromic properties without the environmental risks associated with perchloric acid. Such alternatives could potentially offer a more sustainable solution for the development of advanced thermochromic technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!