Perchloric Acid's Influence on Electroplating of Non-Ferrous Metals
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Electroplating: Background and Objectives
Perchloric acid has played a significant role in the electroplating industry, particularly in the realm of non-ferrous metal plating. This powerful oxidizing agent has been utilized for decades to enhance the efficiency and quality of electroplating processes. The primary objective of this technical research report is to comprehensively examine the influence of perchloric acid on the electroplating of non-ferrous metals, with a focus on its historical development, current applications, and future potential.
The use of perchloric acid in electroplating can be traced back to the mid-20th century when researchers began exploring its unique properties as an electrolyte additive. Its ability to form stable complexes with metal ions and its high conductivity made it an attractive option for improving plating efficiency and deposit quality. Over time, perchloric acid has become an essential component in various electroplating baths, particularly those involving precious metals and specialized alloys.
The evolution of perchloric acid usage in electroplating has been driven by the increasing demands of industries such as electronics, aerospace, and automotive. These sectors require high-performance coatings with specific properties, such as improved corrosion resistance, enhanced wear resistance, and precise thickness control. Perchloric acid-based electrolytes have demonstrated the ability to meet these stringent requirements, leading to their widespread adoption in advanced plating processes.
One of the key technological trends in this field has been the development of perchloric acid-based electrolytes for the electrodeposition of nanostructured materials. These novel materials exhibit unique properties that can significantly enhance the performance of various components and devices. The use of perchloric acid in these applications has enabled better control over the deposition process, resulting in more uniform and finer-grained coatings.
Despite its advantages, the use of perchloric acid in electroplating also presents several challenges. Safety concerns associated with its strong oxidizing properties have led to the implementation of strict handling and storage protocols. Additionally, environmental regulations have prompted research into alternative electrolytes that can provide similar benefits with reduced environmental impact. These factors have shaped the current research landscape and continue to influence the direction of future developments in the field.
Looking ahead, the technical objectives for perchloric acid in electroplating of non-ferrous metals are multifaceted. Researchers aim to further optimize electrolyte compositions to achieve even greater control over deposit properties and microstructure. There is also a focus on developing more environmentally friendly formulations that maintain the performance benefits of perchloric acid while reducing its potential hazards. Furthermore, the integration of perchloric acid-based electroplating processes with emerging technologies, such as additive manufacturing and smart materials, presents exciting opportunities for innovation in this field.
The use of perchloric acid in electroplating can be traced back to the mid-20th century when researchers began exploring its unique properties as an electrolyte additive. Its ability to form stable complexes with metal ions and its high conductivity made it an attractive option for improving plating efficiency and deposit quality. Over time, perchloric acid has become an essential component in various electroplating baths, particularly those involving precious metals and specialized alloys.
The evolution of perchloric acid usage in electroplating has been driven by the increasing demands of industries such as electronics, aerospace, and automotive. These sectors require high-performance coatings with specific properties, such as improved corrosion resistance, enhanced wear resistance, and precise thickness control. Perchloric acid-based electrolytes have demonstrated the ability to meet these stringent requirements, leading to their widespread adoption in advanced plating processes.
One of the key technological trends in this field has been the development of perchloric acid-based electrolytes for the electrodeposition of nanostructured materials. These novel materials exhibit unique properties that can significantly enhance the performance of various components and devices. The use of perchloric acid in these applications has enabled better control over the deposition process, resulting in more uniform and finer-grained coatings.
Despite its advantages, the use of perchloric acid in electroplating also presents several challenges. Safety concerns associated with its strong oxidizing properties have led to the implementation of strict handling and storage protocols. Additionally, environmental regulations have prompted research into alternative electrolytes that can provide similar benefits with reduced environmental impact. These factors have shaped the current research landscape and continue to influence the direction of future developments in the field.
Looking ahead, the technical objectives for perchloric acid in electroplating of non-ferrous metals are multifaceted. Researchers aim to further optimize electrolyte compositions to achieve even greater control over deposit properties and microstructure. There is also a focus on developing more environmentally friendly formulations that maintain the performance benefits of perchloric acid while reducing its potential hazards. Furthermore, the integration of perchloric acid-based electroplating processes with emerging technologies, such as additive manufacturing and smart materials, presents exciting opportunities for innovation in this field.
Market Analysis of Non-Ferrous Metal Electroplating
The non-ferrous metal electroplating market has experienced significant growth in recent years, driven by increasing demand from various industries such as automotive, electronics, aerospace, and construction. This market segment is particularly influenced by the use of perchloric acid in electroplating processes, which has shown promising results in enhancing the quality and efficiency of non-ferrous metal coatings.
The global non-ferrous metal electroplating market was valued at approximately $16 billion in 2020 and is projected to reach $22 billion by 2025, growing at a CAGR of 6.5% during the forecast period. This growth is primarily attributed to the rising demand for durable and corrosion-resistant coatings in industrial applications, as well as the increasing adoption of advanced electroplating technologies.
The automotive sector remains the largest consumer of non-ferrous metal electroplating, accounting for about 35% of the market share. The growing trend towards lightweight vehicles and the need for improved fuel efficiency have led to increased use of non-ferrous metals in automotive components, driving the demand for electroplating services.
The electronics industry is another significant contributor to the market, with a share of approximately 25%. The rapid growth of consumer electronics, coupled with the miniaturization trend, has boosted the demand for high-performance coatings on non-ferrous metals used in electronic components and devices.
Geographically, Asia-Pacific dominates the non-ferrous metal electroplating market, accounting for over 40% of the global market share. This is primarily due to the presence of major manufacturing hubs in countries like China, Japan, and South Korea. North America and Europe follow, with market shares of 25% and 20% respectively.
The introduction of perchloric acid in electroplating processes has opened up new opportunities in the market. Perchloric acid has shown potential in improving the adhesion, hardness, and corrosion resistance of non-ferrous metal coatings. This has led to increased research and development activities focused on optimizing perchloric acid-based electroplating techniques for various non-ferrous metals such as aluminum, copper, and zinc.
However, the market also faces challenges, including stringent environmental regulations regarding the use and disposal of electroplating chemicals. This has prompted manufacturers to invest in eco-friendly electroplating technologies and waste treatment solutions. Additionally, the volatility in raw material prices of non-ferrous metals can impact the overall market growth.
Looking ahead, the non-ferrous metal electroplating market is expected to witness further advancements in technology, with a focus on developing more efficient and environmentally friendly processes. The integration of perchloric acid in these processes is likely to play a crucial role in shaping the future of the industry, offering improved performance and expanding the application scope of non-ferrous metal coatings across various sectors.
The global non-ferrous metal electroplating market was valued at approximately $16 billion in 2020 and is projected to reach $22 billion by 2025, growing at a CAGR of 6.5% during the forecast period. This growth is primarily attributed to the rising demand for durable and corrosion-resistant coatings in industrial applications, as well as the increasing adoption of advanced electroplating technologies.
The automotive sector remains the largest consumer of non-ferrous metal electroplating, accounting for about 35% of the market share. The growing trend towards lightweight vehicles and the need for improved fuel efficiency have led to increased use of non-ferrous metals in automotive components, driving the demand for electroplating services.
The electronics industry is another significant contributor to the market, with a share of approximately 25%. The rapid growth of consumer electronics, coupled with the miniaturization trend, has boosted the demand for high-performance coatings on non-ferrous metals used in electronic components and devices.
Geographically, Asia-Pacific dominates the non-ferrous metal electroplating market, accounting for over 40% of the global market share. This is primarily due to the presence of major manufacturing hubs in countries like China, Japan, and South Korea. North America and Europe follow, with market shares of 25% and 20% respectively.
The introduction of perchloric acid in electroplating processes has opened up new opportunities in the market. Perchloric acid has shown potential in improving the adhesion, hardness, and corrosion resistance of non-ferrous metal coatings. This has led to increased research and development activities focused on optimizing perchloric acid-based electroplating techniques for various non-ferrous metals such as aluminum, copper, and zinc.
However, the market also faces challenges, including stringent environmental regulations regarding the use and disposal of electroplating chemicals. This has prompted manufacturers to invest in eco-friendly electroplating technologies and waste treatment solutions. Additionally, the volatility in raw material prices of non-ferrous metals can impact the overall market growth.
Looking ahead, the non-ferrous metal electroplating market is expected to witness further advancements in technology, with a focus on developing more efficient and environmentally friendly processes. The integration of perchloric acid in these processes is likely to play a crucial role in shaping the future of the industry, offering improved performance and expanding the application scope of non-ferrous metal coatings across various sectors.
Current Challenges in Perchloric Acid Electroplating
The use of perchloric acid in electroplating non-ferrous metals presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary concerns is the inherent safety risks associated with perchloric acid. Its strong oxidizing properties make it highly reactive and potentially explosive, especially when in contact with organic compounds or at elevated temperatures. This necessitates stringent safety protocols and specialized handling equipment, which can increase operational costs and complexity.
Another challenge lies in the environmental impact of perchloric acid usage. The disposal of perchlorate-containing waste poses a significant environmental hazard, as perchlorates can persist in soil and water systems, potentially affecting ecosystems and human health. Regulatory bodies worldwide are imposing increasingly strict guidelines on perchlorate emissions, forcing industries to seek alternative solutions or invest heavily in treatment technologies.
The corrosive nature of perchloric acid also presents material compatibility issues. It can rapidly degrade many common materials used in electroplating equipment, leading to increased maintenance costs and potential contamination of the plating bath. This necessitates the use of specialized, corrosion-resistant materials, which can be expensive and may have limited availability.
Control of the electroplating process itself is another area of difficulty. Perchloric acid-based electrolytes can be highly sensitive to changes in operating conditions, such as temperature and current density. Maintaining consistent plating quality across large surface areas or complex geometries can be challenging, often requiring sophisticated process control systems and extensive operator expertise.
Furthermore, the high conductivity of perchloric acid solutions, while beneficial for some applications, can lead to issues with current distribution and throwing power. This can result in uneven plating thickness and quality, particularly on parts with complex shapes or recessed areas. Overcoming these limitations often requires careful bath formulation and specialized electrode designs.
The long-term stability of perchloric acid electrolytes is also a concern. These baths can degrade over time, leading to changes in plating performance and potentially introducing contaminants that affect the quality of the deposited metal. Regular monitoring and maintenance of the electrolyte composition are necessary, adding to the operational complexity and cost.
Lastly, there are ongoing challenges related to the optimization of perchloric acid-based plating processes for specific non-ferrous metals and alloys. Each metal or alloy combination may require a unique set of operating parameters and electrolyte compositions to achieve desired properties such as hardness, ductility, and corrosion resistance. This necessitates extensive research and development efforts to fine-tune processes for different applications.
Another challenge lies in the environmental impact of perchloric acid usage. The disposal of perchlorate-containing waste poses a significant environmental hazard, as perchlorates can persist in soil and water systems, potentially affecting ecosystems and human health. Regulatory bodies worldwide are imposing increasingly strict guidelines on perchlorate emissions, forcing industries to seek alternative solutions or invest heavily in treatment technologies.
The corrosive nature of perchloric acid also presents material compatibility issues. It can rapidly degrade many common materials used in electroplating equipment, leading to increased maintenance costs and potential contamination of the plating bath. This necessitates the use of specialized, corrosion-resistant materials, which can be expensive and may have limited availability.
Control of the electroplating process itself is another area of difficulty. Perchloric acid-based electrolytes can be highly sensitive to changes in operating conditions, such as temperature and current density. Maintaining consistent plating quality across large surface areas or complex geometries can be challenging, often requiring sophisticated process control systems and extensive operator expertise.
Furthermore, the high conductivity of perchloric acid solutions, while beneficial for some applications, can lead to issues with current distribution and throwing power. This can result in uneven plating thickness and quality, particularly on parts with complex shapes or recessed areas. Overcoming these limitations often requires careful bath formulation and specialized electrode designs.
The long-term stability of perchloric acid electrolytes is also a concern. These baths can degrade over time, leading to changes in plating performance and potentially introducing contaminants that affect the quality of the deposited metal. Regular monitoring and maintenance of the electrolyte composition are necessary, adding to the operational complexity and cost.
Lastly, there are ongoing challenges related to the optimization of perchloric acid-based plating processes for specific non-ferrous metals and alloys. Each metal or alloy combination may require a unique set of operating parameters and electrolyte compositions to achieve desired properties such as hardness, ductility, and corrosion resistance. This necessitates extensive research and development efforts to fine-tune processes for different applications.
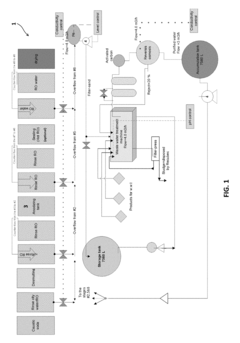
Existing Perchloric Acid Electroplating Techniques
01 Electroplating bath composition
Perchloric acid is used as a key component in electroplating bath compositions. These baths are formulated to achieve specific metal deposition characteristics, such as improved adhesion, uniformity, and surface finish. The concentration of perchloric acid and other additives in the bath can be optimized to control the electroplating process and enhance the quality of the deposited metal layer.- Electroplating bath composition: Perchloric acid is used as a key component in electroplating bath compositions. These baths are designed to improve the quality and efficiency of the electroplating process, often incorporating other additives to enhance specific properties of the deposited metal layer.
- Electroplating equipment and apparatus: Specialized equipment and apparatus are developed for perchloric acid electroplating processes. These include modified electroplating tanks, electrode configurations, and circulation systems designed to handle the unique properties of perchloric acid-based electrolytes safely and efficiently.
- Surface preparation techniques: Specific surface preparation methods are employed before perchloric acid electroplating to ensure optimal adhesion and quality of the deposited layer. These techniques may include chemical cleaning, mechanical abrasion, or electrochemical pretreatment of the substrate.
- Control and monitoring systems: Advanced control and monitoring systems are implemented in perchloric acid electroplating processes to maintain precise bath conditions, current density, and temperature. These systems help ensure consistent plating quality and enhance safety measures when working with perchloric acid.
- Waste treatment and recycling: Specialized waste treatment and recycling methods are developed for perchloric acid electroplating processes. These techniques aim to minimize environmental impact, recover valuable materials, and ensure safe disposal of spent electrolytes and byproducts.
02 Electroplating of specific metals
Perchloric acid electroplating is particularly effective for depositing certain metals and alloys. This technique can be applied to various substrates and is used in industries such as electronics, aerospace, and automotive. The process parameters, including current density, temperature, and plating time, are adjusted to achieve the desired properties of the electroplated layer.Expand Specific Solutions03 Pretreatment and surface preparation
Proper surface preparation is crucial for successful perchloric acid electroplating. This may involve cleaning, degreasing, and activation of the substrate surface. Pretreatment processes can include chemical etching, mechanical abrasion, or electrochemical treatments to ensure optimal adhesion and uniformity of the electroplated layer.Expand Specific Solutions04 Post-treatment and finishing
After perchloric acid electroplating, various post-treatment processes may be employed to enhance the properties of the deposited layer. These can include heat treatments, passivation, or application of protective coatings. Post-treatment steps aim to improve corrosion resistance, hardness, and other functional characteristics of the electroplated surface.Expand Specific Solutions05 Safety and environmental considerations
Perchloric acid electroplating requires strict safety measures due to the hazardous nature of the chemicals involved. Proper handling, storage, and disposal procedures must be followed to minimize risks. Additionally, environmental considerations are addressed through the development of waste treatment methods and the exploration of more environmentally friendly alternatives to traditional perchloric acid electroplating processes.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The competitive landscape for perchloric acid's influence on electroplating of non-ferrous metals is in a mature stage, with a stable market size and well-established technologies. Key players like MacDermid Enthone, Atotech Deutschland, and C. Uyemura & Co. dominate the industry, leveraging their extensive experience and R&D capabilities. The market is characterized by incremental innovations rather than disruptive changes, focusing on improving efficiency, environmental sustainability, and product quality. Technological maturity is high, with companies continuously refining their processes and formulations to meet evolving industry standards and customer demands.
MacDermid Enthone, Inc.
Technical Solution: MacDermid Enthone has developed advanced electroplating processes utilizing perchloric acid for non-ferrous metals. Their technology involves a proprietary electrolyte formulation containing optimized concentrations of perchloric acid, which enhances the deposition rate and improves the quality of the plated layer. The process employs pulse plating techniques with carefully controlled current densities to achieve uniform metal distribution[1]. Additionally, they have implemented a closed-loop system for electrolyte monitoring and replenishment, ensuring consistent perchloric acid levels throughout the plating process[3]. This approach has resulted in significant improvements in deposit hardness, wear resistance, and corrosion protection for various non-ferrous metals, including copper, nickel, and zinc alloys[5].
Strengths: Enhanced deposition rates, improved deposit quality, and superior mechanical properties. Weaknesses: Higher operational costs due to specialized equipment and safety measures required for handling perchloric acid.
Atotech Deutschland GmbH & Co. KG
Technical Solution: Atotech has pioneered a novel electroplating technique for non-ferrous metals using perchloric acid as a key component. Their approach involves a multi-layer plating process where perchloric acid is used in conjunction with other additives to create a highly conductive electrolyte bath[2]. This method allows for precise control over the metal deposition process, resulting in exceptionally smooth and uniform coatings. Atotech's technology also incorporates advanced current modulation techniques, which, when combined with the perchloric acid electrolyte, enable the formation of nanostructured deposits with enhanced physical and chemical properties[4]. The company has further developed a proprietary waste treatment system to handle the perchloric acid-containing effluents, ensuring environmental compliance and sustainability[6].
Strengths: Exceptional coating uniformity, ability to create nanostructured deposits, and environmentally responsible waste management. Weaknesses: Complex process control requirements and potential safety concerns associated with perchloric acid handling.
Innovations in Perchloric Acid-Based Electroplating
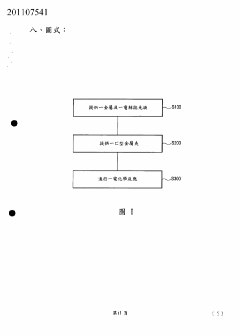
which do not produce defects of pores and traces on the metal surface
PatentInactiveTW201107541A
Innovation
- The use of a combination of perchloric acid solution and an alcohol functional group containing additive for electroplated polishing.
- Specific volume ratio (1:5 to 1:10) of alcohol additive to perchloric acid solution for optimal polishing results.
- Selection of specific alcohol additives (methyl, ethyl, propyl, butyl alcohol) for effective surface flattening.
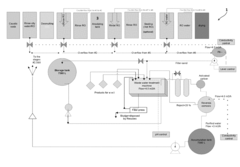
Electrolytic process and apparatus for the surface treatment of non-ferrous metals
PatentActiveUS20180245231A1
Innovation
- An electrolytic process using an alkaline solution with a pH of 9 to 12 and organic acids, applying alternating electric currents to achieve a uniform anodized coating without preliminary chemical treatments, reducing current density and eliminating toxic substances, thus enabling efficient and cost-effective surface treatment.
Safety Regulations for Perchloric Acid Handling
The handling of perchloric acid in electroplating processes involving non-ferrous metals requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. These regulations are designed to protect workers, facilities, and the environment from the hazards associated with perchloric acid use.
Personal protective equipment (PPE) is a critical component of safety protocols. Workers must wear chemical-resistant gloves, face shields or goggles, and protective clothing when handling perchloric acid. Respiratory protection may also be necessary, depending on the concentration and potential for vapor exposure.
Storage and transportation of perchloric acid must follow specific guidelines. The acid should be stored in a cool, well-ventilated area, away from combustible materials and other chemicals. Containers must be properly labeled and sealed. Transportation should be carried out in accordance with local and international regulations for hazardous materials.
Workplace design and engineering controls play a crucial role in safety. Dedicated fume hoods with wash-down systems are required for perchloric acid use. These hoods should be constructed of corrosion-resistant materials and equipped with a water spray system to prevent the accumulation of explosive perchlorates.
Emergency response procedures must be in place and regularly practiced. This includes having readily accessible eyewash stations and safety showers, as well as spill containment and neutralization materials. Workers should be trained in proper spill response and evacuation procedures.
Waste management is another critical aspect of perchloric acid safety. Spent solutions and contaminated materials must be properly neutralized and disposed of according to hazardous waste regulations. Dilution and neutralization should be performed carefully, as heat generation can lead to dangerous situations.
Regular safety audits and inspections are necessary to ensure compliance with regulations and identify potential hazards. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training and education are fundamental to maintaining a safe working environment. All personnel involved in handling perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be regularly updated and documented.
Monitoring and exposure limits must be established and enforced. Air quality monitoring may be necessary to ensure that exposure levels remain below permissible limits. Medical surveillance programs may also be required for workers regularly exposed to perchloric acid.
Documentation and record-keeping are essential components of safety compliance. This includes maintaining safety data sheets, training records, incident reports, and equipment maintenance logs. These records should be readily accessible and regularly reviewed to ensure ongoing compliance and identify areas for improvement.
Personal protective equipment (PPE) is a critical component of safety protocols. Workers must wear chemical-resistant gloves, face shields or goggles, and protective clothing when handling perchloric acid. Respiratory protection may also be necessary, depending on the concentration and potential for vapor exposure.
Storage and transportation of perchloric acid must follow specific guidelines. The acid should be stored in a cool, well-ventilated area, away from combustible materials and other chemicals. Containers must be properly labeled and sealed. Transportation should be carried out in accordance with local and international regulations for hazardous materials.
Workplace design and engineering controls play a crucial role in safety. Dedicated fume hoods with wash-down systems are required for perchloric acid use. These hoods should be constructed of corrosion-resistant materials and equipped with a water spray system to prevent the accumulation of explosive perchlorates.
Emergency response procedures must be in place and regularly practiced. This includes having readily accessible eyewash stations and safety showers, as well as spill containment and neutralization materials. Workers should be trained in proper spill response and evacuation procedures.
Waste management is another critical aspect of perchloric acid safety. Spent solutions and contaminated materials must be properly neutralized and disposed of according to hazardous waste regulations. Dilution and neutralization should be performed carefully, as heat generation can lead to dangerous situations.
Regular safety audits and inspections are necessary to ensure compliance with regulations and identify potential hazards. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training and education are fundamental to maintaining a safe working environment. All personnel involved in handling perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be regularly updated and documented.
Monitoring and exposure limits must be established and enforced. Air quality monitoring may be necessary to ensure that exposure levels remain below permissible limits. Medical surveillance programs may also be required for workers regularly exposed to perchloric acid.
Documentation and record-keeping are essential components of safety compliance. This includes maintaining safety data sheets, training records, incident reports, and equipment maintenance logs. These records should be readily accessible and regularly reviewed to ensure ongoing compliance and identify areas for improvement.
Environmental Impact of Perchloric Acid Electroplating
The environmental impact of perchloric acid electroplating is a critical concern in the non-ferrous metal industry. Perchloric acid, while effective in electroplating processes, poses significant risks to the environment if not properly managed.
One of the primary environmental concerns is the potential for perchlorate contamination in water sources. Perchlorate ions are highly soluble and mobile in aqueous environments, making them difficult to contain and remove once released. This contamination can persist in groundwater and surface water for extended periods, potentially affecting drinking water supplies and aquatic ecosystems.
The release of perchloric acid into the environment can also lead to soil contamination. Soil exposed to perchloric acid may experience changes in pH levels, potentially affecting plant growth and soil microbial communities. This alteration of soil chemistry can have long-lasting effects on local ecosystems and agricultural productivity.
Air pollution is another environmental concern associated with perchloric acid electroplating. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the electroplating process, contributing to poor air quality and potential health risks for workers and nearby communities.
The disposal of waste products from perchloric acid electroplating processes presents additional environmental challenges. Improper disposal of spent electrolytes, sludges, and other waste materials can lead to the release of toxic substances into the environment, potentially contaminating soil and water resources.
To mitigate these environmental impacts, strict regulations and best practices have been implemented in many jurisdictions. These include the use of closed-loop systems to minimize waste generation, advanced wastewater treatment technologies to remove perchlorate ions, and air pollution control measures to reduce emissions of hazardous substances.
The development of alternative electroplating technologies and less hazardous electrolytes is an ongoing area of research aimed at reducing the environmental footprint of non-ferrous metal electroplating. These efforts focus on finding more environmentally friendly substitutes that maintain or improve the quality of electroplated products while minimizing negative impacts on ecosystems and human health.
In conclusion, while perchloric acid electroplating offers significant benefits in terms of metal finishing quality, its environmental impact remains a serious concern. Balancing the industrial benefits with environmental protection requires ongoing research, technological innovation, and strict adherence to environmental regulations and best practices in the electroplating industry.
One of the primary environmental concerns is the potential for perchlorate contamination in water sources. Perchlorate ions are highly soluble and mobile in aqueous environments, making them difficult to contain and remove once released. This contamination can persist in groundwater and surface water for extended periods, potentially affecting drinking water supplies and aquatic ecosystems.
The release of perchloric acid into the environment can also lead to soil contamination. Soil exposed to perchloric acid may experience changes in pH levels, potentially affecting plant growth and soil microbial communities. This alteration of soil chemistry can have long-lasting effects on local ecosystems and agricultural productivity.
Air pollution is another environmental concern associated with perchloric acid electroplating. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the electroplating process, contributing to poor air quality and potential health risks for workers and nearby communities.
The disposal of waste products from perchloric acid electroplating processes presents additional environmental challenges. Improper disposal of spent electrolytes, sludges, and other waste materials can lead to the release of toxic substances into the environment, potentially contaminating soil and water resources.
To mitigate these environmental impacts, strict regulations and best practices have been implemented in many jurisdictions. These include the use of closed-loop systems to minimize waste generation, advanced wastewater treatment technologies to remove perchlorate ions, and air pollution control measures to reduce emissions of hazardous substances.
The development of alternative electroplating technologies and less hazardous electrolytes is an ongoing area of research aimed at reducing the environmental footprint of non-ferrous metal electroplating. These efforts focus on finding more environmentally friendly substitutes that maintain or improve the quality of electroplated products while minimizing negative impacts on ecosystems and human health.
In conclusion, while perchloric acid electroplating offers significant benefits in terms of metal finishing quality, its environmental impact remains a serious concern. Balancing the industrial benefits with environmental protection requires ongoing research, technological innovation, and strict adherence to environmental regulations and best practices in the electroplating industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!