Perchloric Acid's Role in Low-Temperature Electrosynthesis Processes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Electrosynthesis Background
Perchloric acid has emerged as a significant player in low-temperature electrosynthesis processes, marking a notable advancement in the field of electrochemistry. This strong oxidizing agent, with its unique properties, has opened new avenues for conducting electrochemical reactions under milder conditions, thereby expanding the scope of sustainable chemical synthesis.
The journey of perchloric acid in electrosynthesis began in the mid-20th century when researchers first recognized its potential as an electrolyte. Its high conductivity and ability to remain stable at low temperatures made it an attractive option for electrochemical applications. As environmental concerns grew in the latter part of the century, the focus shifted towards developing greener chemical processes, propelling the exploration of low-temperature electrosynthesis methods.
The evolution of perchloric acid's role in electrosynthesis has been closely tied to advancements in electrode materials and cell designs. Early experiments primarily utilized platinum electrodes, but the introduction of carbon-based electrodes in the 1980s significantly broadened the range of possible reactions. Concurrently, improvements in electrolytic cell configurations enhanced the efficiency and selectivity of perchloric acid-mediated processes.
A key milestone in this field was the discovery of perchloric acid's ability to facilitate electron transfer at lower temperatures compared to traditional electrolytes. This breakthrough, reported in the late 1990s, paved the way for conducting sensitive organic transformations that were previously challenging or impossible under conventional conditions. The lower temperature requirements not only improved the energy efficiency of these processes but also allowed for better control over reaction kinetics and product selectivity.
The 21st century has witnessed a surge in research focused on leveraging perchloric acid for green chemistry applications. Its role in enabling metal-free oxidations and C-H functionalizations at ambient temperatures has garnered significant attention. These developments align with the principles of atom economy and step efficiency, crucial aspects of sustainable chemical synthesis.
Recent years have seen an increasing emphasis on understanding the mechanistic aspects of perchloric acid-mediated electrosynthesis. Advanced spectroscopic and computational studies have shed light on the intricate interplay between perchloric acid, electrode surfaces, and substrate molecules. This deeper understanding has led to the design of more efficient and selective electrosynthetic protocols, further cementing perchloric acid's position in modern synthetic chemistry.
As we look towards the future, the role of perchloric acid in low-temperature electrosynthesis continues to evolve. Ongoing research is exploring its potential in emerging areas such as flow electrochemistry and electrochemical CO2 reduction. These developments promise to further expand the utility of perchloric acid, potentially revolutionizing industrial-scale chemical production processes.
The journey of perchloric acid in electrosynthesis began in the mid-20th century when researchers first recognized its potential as an electrolyte. Its high conductivity and ability to remain stable at low temperatures made it an attractive option for electrochemical applications. As environmental concerns grew in the latter part of the century, the focus shifted towards developing greener chemical processes, propelling the exploration of low-temperature electrosynthesis methods.
The evolution of perchloric acid's role in electrosynthesis has been closely tied to advancements in electrode materials and cell designs. Early experiments primarily utilized platinum electrodes, but the introduction of carbon-based electrodes in the 1980s significantly broadened the range of possible reactions. Concurrently, improvements in electrolytic cell configurations enhanced the efficiency and selectivity of perchloric acid-mediated processes.
A key milestone in this field was the discovery of perchloric acid's ability to facilitate electron transfer at lower temperatures compared to traditional electrolytes. This breakthrough, reported in the late 1990s, paved the way for conducting sensitive organic transformations that were previously challenging or impossible under conventional conditions. The lower temperature requirements not only improved the energy efficiency of these processes but also allowed for better control over reaction kinetics and product selectivity.
The 21st century has witnessed a surge in research focused on leveraging perchloric acid for green chemistry applications. Its role in enabling metal-free oxidations and C-H functionalizations at ambient temperatures has garnered significant attention. These developments align with the principles of atom economy and step efficiency, crucial aspects of sustainable chemical synthesis.
Recent years have seen an increasing emphasis on understanding the mechanistic aspects of perchloric acid-mediated electrosynthesis. Advanced spectroscopic and computational studies have shed light on the intricate interplay between perchloric acid, electrode surfaces, and substrate molecules. This deeper understanding has led to the design of more efficient and selective electrosynthetic protocols, further cementing perchloric acid's position in modern synthetic chemistry.
As we look towards the future, the role of perchloric acid in low-temperature electrosynthesis continues to evolve. Ongoing research is exploring its potential in emerging areas such as flow electrochemistry and electrochemical CO2 reduction. These developments promise to further expand the utility of perchloric acid, potentially revolutionizing industrial-scale chemical production processes.
Market Analysis for Low-Temperature Electrosynthesis
The market for low-temperature electrosynthesis processes is experiencing significant growth, driven by increasing demand for sustainable and energy-efficient chemical production methods. This technology offers numerous advantages over traditional high-temperature synthesis, including reduced energy consumption, improved selectivity, and the ability to produce sensitive compounds that may degrade at higher temperatures.
The pharmaceutical industry represents a major market segment for low-temperature electrosynthesis, as it enables the production of complex drug molecules with greater precision and fewer side reactions. The fine chemicals sector is another key market, where electrosynthesis allows for the creation of high-value specialty chemicals with improved yields and purity.
Environmental concerns and stringent regulations are also fueling market growth, as low-temperature electrosynthesis processes generally have a lower environmental impact compared to conventional methods. This aligns with the growing trend towards green chemistry and sustainable manufacturing practices across various industries.
The market for low-temperature electrosynthesis equipment and services is expected to expand rapidly in the coming years. Factors contributing to this growth include advancements in electrode materials, improved reactor designs, and the development of more efficient electrolytes. Additionally, the integration of artificial intelligence and machine learning for process optimization is opening up new possibilities for increased efficiency and product quality.
Geographically, North America and Europe currently lead the market due to their strong pharmaceutical and chemical industries, as well as supportive regulatory environments for green technologies. However, the Asia-Pacific region is anticipated to show the fastest growth rate, driven by expanding manufacturing sectors and increasing investments in sustainable technologies.
Challenges in the market include the need for specialized equipment and expertise, which can present barriers to entry for smaller companies. Additionally, scaling up low-temperature electrosynthesis processes from laboratory to industrial scale remains a significant hurdle that requires ongoing research and development efforts.
Despite these challenges, the overall market outlook for low-temperature electrosynthesis is highly positive. As industries continue to prioritize sustainability and seek more efficient production methods, the demand for this technology is expected to grow substantially. This presents significant opportunities for both established players and innovative startups in the chemical and electrochemical sectors.
The pharmaceutical industry represents a major market segment for low-temperature electrosynthesis, as it enables the production of complex drug molecules with greater precision and fewer side reactions. The fine chemicals sector is another key market, where electrosynthesis allows for the creation of high-value specialty chemicals with improved yields and purity.
Environmental concerns and stringent regulations are also fueling market growth, as low-temperature electrosynthesis processes generally have a lower environmental impact compared to conventional methods. This aligns with the growing trend towards green chemistry and sustainable manufacturing practices across various industries.
The market for low-temperature electrosynthesis equipment and services is expected to expand rapidly in the coming years. Factors contributing to this growth include advancements in electrode materials, improved reactor designs, and the development of more efficient electrolytes. Additionally, the integration of artificial intelligence and machine learning for process optimization is opening up new possibilities for increased efficiency and product quality.
Geographically, North America and Europe currently lead the market due to their strong pharmaceutical and chemical industries, as well as supportive regulatory environments for green technologies. However, the Asia-Pacific region is anticipated to show the fastest growth rate, driven by expanding manufacturing sectors and increasing investments in sustainable technologies.
Challenges in the market include the need for specialized equipment and expertise, which can present barriers to entry for smaller companies. Additionally, scaling up low-temperature electrosynthesis processes from laboratory to industrial scale remains a significant hurdle that requires ongoing research and development efforts.
Despite these challenges, the overall market outlook for low-temperature electrosynthesis is highly positive. As industries continue to prioritize sustainability and seek more efficient production methods, the demand for this technology is expected to grow substantially. This presents significant opportunities for both established players and innovative startups in the chemical and electrochemical sectors.
Current Challenges in Perchloric Acid Electrosynthesis
The current challenges in perchloric acid electrosynthesis primarily revolve around safety concerns, environmental impact, and process optimization. Perchloric acid's highly oxidizing nature poses significant risks in handling and storage, requiring stringent safety protocols and specialized equipment. This inherent reactivity also contributes to potential environmental hazards if not properly managed, necessitating robust containment and disposal procedures.
From a process perspective, achieving consistent and controlled reactions at low temperatures remains a significant hurdle. The high reactivity of perchloric acid can lead to unwanted side reactions or rapid decomposition, particularly when temperature control is inadequate. This challenge is exacerbated in scaled-up industrial applications, where maintaining uniform low temperatures across larger reaction volumes becomes increasingly difficult.
Another critical issue is the corrosive nature of perchloric acid, which limits the choice of materials for reaction vessels, electrodes, and other equipment. Finding materials that can withstand prolonged exposure to perchloric acid while maintaining their structural integrity and electrochemical properties is an ongoing challenge. This constraint often leads to increased production costs and potential compromises in process efficiency.
The selectivity of perchloric acid-based electrosynthesis processes also presents challenges, especially when targeting specific products in complex reaction mixtures. Achieving high yields of desired compounds while minimizing unwanted by-products requires precise control over reaction conditions, including temperature, concentration, and electrode potentials. This becomes particularly challenging in low-temperature environments, where reaction kinetics are significantly altered.
Furthermore, the recovery and recycling of perchloric acid from reaction mixtures pose technical and economic challenges. Efficient separation and purification methods are crucial for sustainable and cost-effective processes, but current techniques often struggle to achieve high recovery rates without compromising the purity of the recovered acid.
Lastly, the regulatory landscape surrounding perchloric acid usage continues to evolve, with increasingly stringent safety and environmental regulations. Compliance with these regulations while maintaining process efficiency and economic viability represents a significant challenge for industries relying on perchloric acid electrosynthesis. Developing alternative methodologies or finding less hazardous substitutes that can match the performance of perchloric acid in low-temperature electrosynthesis processes remains an active area of research and development.
From a process perspective, achieving consistent and controlled reactions at low temperatures remains a significant hurdle. The high reactivity of perchloric acid can lead to unwanted side reactions or rapid decomposition, particularly when temperature control is inadequate. This challenge is exacerbated in scaled-up industrial applications, where maintaining uniform low temperatures across larger reaction volumes becomes increasingly difficult.
Another critical issue is the corrosive nature of perchloric acid, which limits the choice of materials for reaction vessels, electrodes, and other equipment. Finding materials that can withstand prolonged exposure to perchloric acid while maintaining their structural integrity and electrochemical properties is an ongoing challenge. This constraint often leads to increased production costs and potential compromises in process efficiency.
The selectivity of perchloric acid-based electrosynthesis processes also presents challenges, especially when targeting specific products in complex reaction mixtures. Achieving high yields of desired compounds while minimizing unwanted by-products requires precise control over reaction conditions, including temperature, concentration, and electrode potentials. This becomes particularly challenging in low-temperature environments, where reaction kinetics are significantly altered.
Furthermore, the recovery and recycling of perchloric acid from reaction mixtures pose technical and economic challenges. Efficient separation and purification methods are crucial for sustainable and cost-effective processes, but current techniques often struggle to achieve high recovery rates without compromising the purity of the recovered acid.
Lastly, the regulatory landscape surrounding perchloric acid usage continues to evolve, with increasingly stringent safety and environmental regulations. Compliance with these regulations while maintaining process efficiency and economic viability represents a significant challenge for industries relying on perchloric acid electrosynthesis. Developing alternative methodologies or finding less hazardous substitutes that can match the performance of perchloric acid in low-temperature electrosynthesis processes remains an active area of research and development.
Existing Low-Temperature Electrosynthesis Solutions
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical processes and reactions. This may involve the use of specific catalysts, reactants, or equipment to optimize the production process and ensure high purity of the final product.- Synthesis and purification of perchloric acid: Methods for synthesizing and purifying perchloric acid, including various chemical reactions and distillation processes. These techniques aim to produce high-purity perchloric acid for industrial and laboratory applications.
- Safety measures and handling of perchloric acid: Development of safety protocols, protective equipment, and specialized containers for handling and storing perchloric acid. These measures are crucial due to the highly corrosive and potentially explosive nature of the compound.
- Applications of perchloric acid in chemical analysis: Use of perchloric acid as a strong oxidizing agent in various analytical techniques, including spectroscopy, chromatography, and electrochemistry. Its unique properties make it valuable for dissolving and analyzing complex materials.
- Perchloric acid in battery technology: Incorporation of perchloric acid or its derivatives in battery electrolytes to enhance performance, conductivity, and energy density. This application is particularly relevant for advanced lithium-ion batteries and other energy storage systems.
- Environmental and waste management of perchloric acid: Development of methods for treating and disposing of perchloric acid waste, including neutralization techniques, recycling processes, and environmental remediation strategies to minimize its impact on ecosystems.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of organic materials, and as a component in analytical reagents for specific chemical analyses.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Development of safety protocols and specialized equipment for handling perchloric acid due to its highly corrosive and potentially explosive nature. This includes the design of storage containers, ventilation systems, and personal protective equipment to minimize risks associated with its use.Expand Specific Solutions04 Perchloric acid in battery technology
Use of perchloric acid in the development and improvement of battery technologies. This may involve its application in electrolyte formulations, electrode materials, or other components to enhance battery performance, capacity, or longevity.Expand Specific Solutions05 Purification and concentration of perchloric acid
Techniques and methods for purifying and concentrating perchloric acid to achieve high-purity grades suitable for various industrial and laboratory applications. This may include distillation processes, membrane separation, or other advanced purification technologies.Expand Specific Solutions
Key Players in Electrosynthesis Industry
The field of low-temperature electrosynthesis using perchloric acid is in an early development stage, with a growing market driven by increasing demand for sustainable chemical processes. The technology's maturity is still evolving, with key players like Samsung Electro-Mechanics, UOP LLC, and Henkel AG & Co. KGaA leading research efforts. Academic institutions such as California Institute of Technology and South China University of Technology are also contributing significantly to advancements in this area. The market size is expected to expand as industries seek more efficient and environmentally friendly synthesis methods, though widespread adoption is still in progress due to the need for further optimization and scale-up of these processes.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The Commissariat à l'énergie atomique et aux énergies alternatives (CEA) has conducted extensive research on the role of perchloric acid in low-temperature electrosynthesis processes, particularly focusing on its applications in nuclear fuel processing and advanced materials synthesis. CEA researchers have developed a novel electrochemical method for the selective oxidation of actinides using perchloric acid as an electrolyte at temperatures below 0°C, improving the efficiency and safety of nuclear fuel reprocessing [13]. They have also investigated the use of perchloric acid in low-temperature electrodeposition processes for creating nanostructured materials with unique properties [14]. Additionally, CEA scientists have explored the potential of perchloric acid-based electrolytes in the development of next-generation energy storage devices, such as supercapacitors and batteries, that can operate efficiently at low temperatures [15].
Strengths: Unique expertise in nuclear-related applications, access to specialized facilities, and strong government support. Weaknesses: Potential limitations in addressing broader commercial applications outside the nuclear and energy sectors.
California Institute of Technology
Technical Solution: Caltech researchers have made significant contributions to understanding the role of perchloric acid in low-temperature electrosynthesis processes. They have developed a novel approach using perchloric acid as both an electrolyte and a reactant in electrochemical fluorination reactions at temperatures as low as -40°C [4]. This method allows for the selective fluorination of organic compounds with high yields and minimal side products. Caltech scientists have also investigated the influence of perchloric acid concentration on the kinetics and mechanism of electron transfer reactions at low temperatures, providing valuable insights for optimizing electrosynthesis conditions [5]. Furthermore, they have explored the use of perchloric acid in conjunction with ionic liquids to create highly conductive electrolyte systems for low-temperature electrochemical applications, enabling the synthesis of temperature-sensitive compounds [6].
Strengths: Cutting-edge research facilities, interdisciplinary approach combining chemistry, materials science, and engineering. Weaknesses: Limited focus on large-scale industrial applications, potential challenges in technology transfer and commercialization.
Core Innovations in Perchloric Acid Utilization
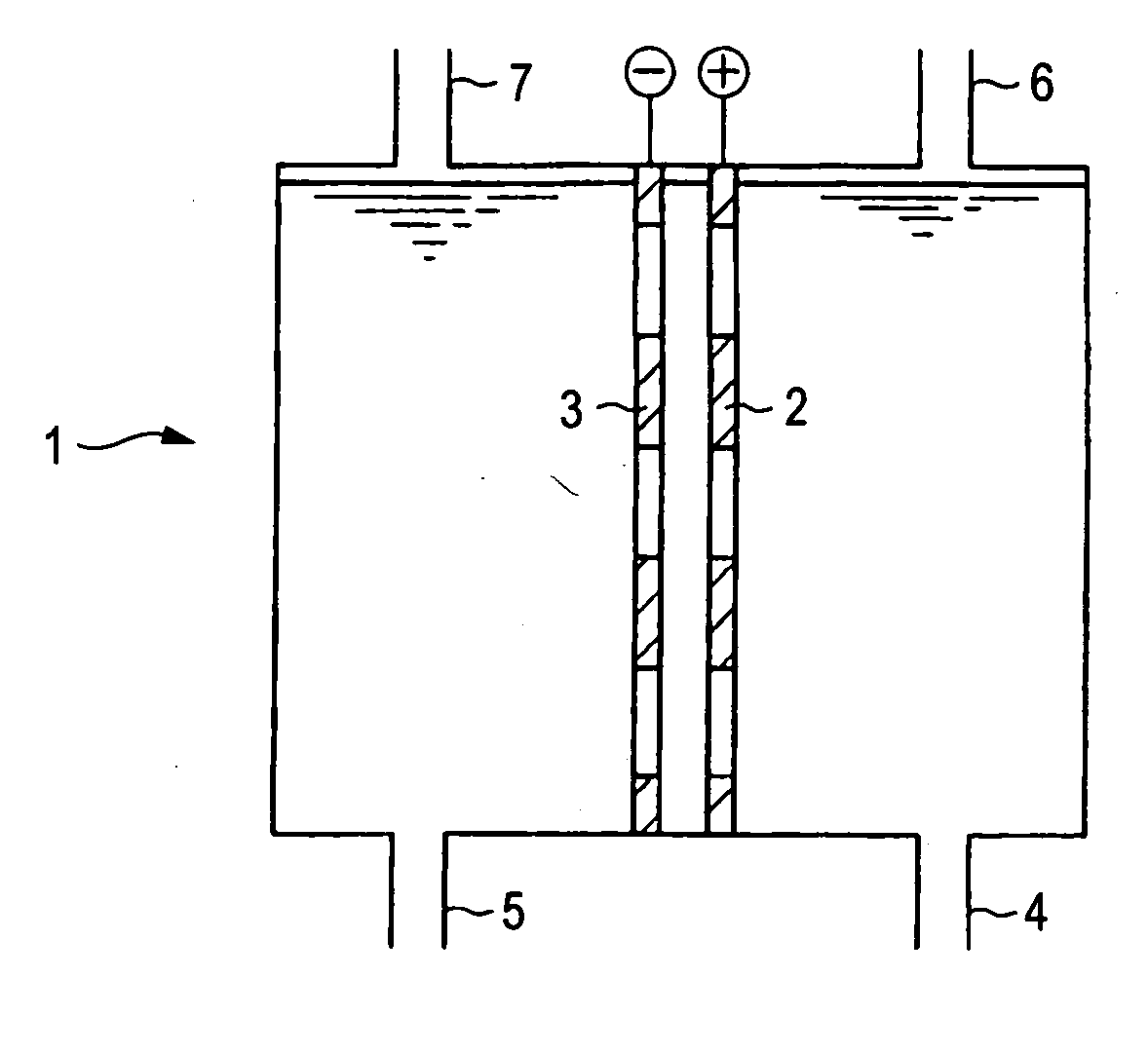
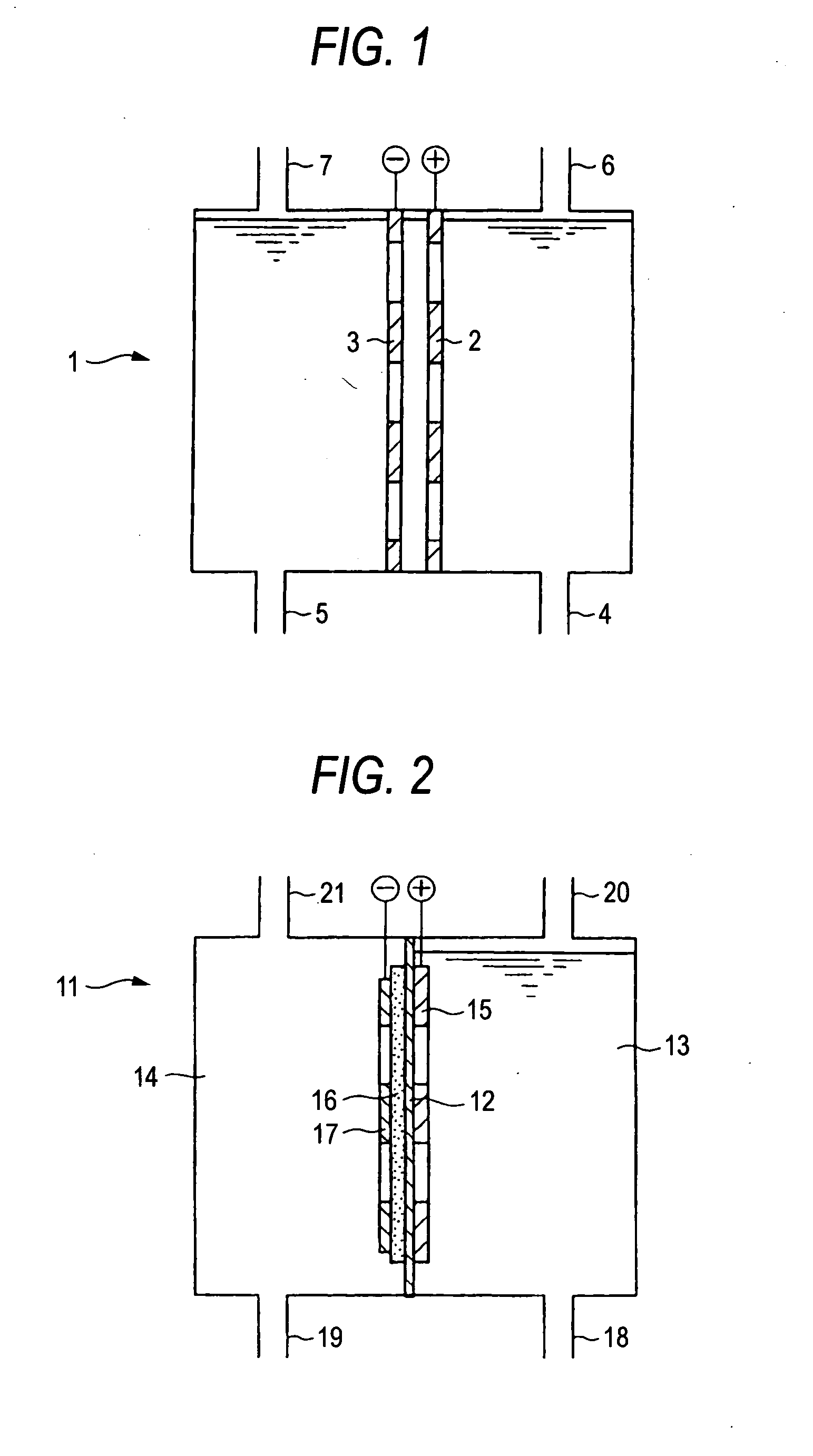
Electrolysis cell for synthesizing perchloric acid compound and method for electrolytically synthesizing perchloric acid compound
PatentInactiveUS20070170070A1
Innovation
- The use of an electroconductive diamond anode in an electrolysis cell with a cation exchange membrane to partition the anode and cathode chambers, allowing for one-stage electrolysis of chloride or chloric acid compounds, preventing ion transfer and achieving high purity perchloric acid synthesis with reduced electrode consumption and energy requirements.
Safety Regulations for Perchloric Acid Handling
The handling of perchloric acid in low-temperature electrosynthesis processes requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Proper storage, handling, and disposal protocols are essential to mitigate risks associated with this powerful oxidizing agent.
Storage of perchloric acid must be conducted in dedicated, well-ventilated areas away from combustible materials and other chemicals. Containers should be made of compatible materials such as glass or high-density polyethylene, and secondary containment is mandatory to prevent spills. Temperature control is crucial, as perchloric acid becomes unstable at elevated temperatures.
Personal protective equipment (PPE) is paramount when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and acid-resistant aprons or suits. Proper training on the use of PPE and emergency procedures is essential for all personnel involved in handling perchloric acid.
Workplace design plays a critical role in safety. Fume hoods specifically designed for perchloric acid use, equipped with wash-down systems, are required to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these systems are vital to ensure their effectiveness.
Strict protocols must be in place for the dilution and mixing of perchloric acid. Adding water to concentrated perchloric acid can result in violent reactions, so the acid should always be added to water slowly and with constant stirring. Mixing with organic compounds or reducing agents must be avoided due to the risk of explosive reactions.
Emergency response plans specific to perchloric acid incidents must be developed and regularly practiced. This includes spill containment procedures, evacuation protocols, and first aid measures. Specialized fire suppression equipment suitable for oxidizer fires should be readily available.
Disposal of perchloric acid and its waste products requires careful consideration. Neutralization and dilution procedures must be followed, and disposal should be conducted through licensed hazardous waste management facilities. Any equipment or materials that have come into contact with perchloric acid must be thoroughly decontaminated before disposal or reuse.
Regular safety audits and inspections are necessary to ensure compliance with regulations and identify potential hazards. Documentation of all safety procedures, incident reports, and training records should be maintained and regularly reviewed to improve safety protocols continuously.
Storage of perchloric acid must be conducted in dedicated, well-ventilated areas away from combustible materials and other chemicals. Containers should be made of compatible materials such as glass or high-density polyethylene, and secondary containment is mandatory to prevent spills. Temperature control is crucial, as perchloric acid becomes unstable at elevated temperatures.
Personal protective equipment (PPE) is paramount when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and acid-resistant aprons or suits. Proper training on the use of PPE and emergency procedures is essential for all personnel involved in handling perchloric acid.
Workplace design plays a critical role in safety. Fume hoods specifically designed for perchloric acid use, equipped with wash-down systems, are required to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these systems are vital to ensure their effectiveness.
Strict protocols must be in place for the dilution and mixing of perchloric acid. Adding water to concentrated perchloric acid can result in violent reactions, so the acid should always be added to water slowly and with constant stirring. Mixing with organic compounds or reducing agents must be avoided due to the risk of explosive reactions.
Emergency response plans specific to perchloric acid incidents must be developed and regularly practiced. This includes spill containment procedures, evacuation protocols, and first aid measures. Specialized fire suppression equipment suitable for oxidizer fires should be readily available.
Disposal of perchloric acid and its waste products requires careful consideration. Neutralization and dilution procedures must be followed, and disposal should be conducted through licensed hazardous waste management facilities. Any equipment or materials that have come into contact with perchloric acid must be thoroughly decontaminated before disposal or reuse.
Regular safety audits and inspections are necessary to ensure compliance with regulations and identify potential hazards. Documentation of all safety procedures, incident reports, and training records should be maintained and regularly reviewed to improve safety protocols continuously.
Environmental Impact Assessment
The use of perchloric acid in low-temperature electrosynthesis processes necessitates a thorough environmental impact assessment due to its potential hazards and ecological implications. Perchloric acid, being a strong oxidizing agent, poses significant risks to both aquatic and terrestrial ecosystems if not properly managed.
In aquatic environments, the release of perchloric acid can lead to severe pH imbalances, potentially causing harm to fish, aquatic plants, and microorganisms. Even small quantities can disrupt the delicate ecological balance of water bodies, affecting biodiversity and ecosystem functions. The persistence of perchlorate ions in water systems is a particular concern, as they can contaminate drinking water sources and bioaccumulate in aquatic food chains.
Soil contamination is another critical aspect to consider. Perchloric acid spills or improper disposal can result in soil acidification, negatively impacting soil fertility and microbial communities. This, in turn, can affect plant growth and agricultural productivity in affected areas. The mobility of perchlorate ions in soil further exacerbates the risk of groundwater contamination.
Air quality may also be compromised if perchloric acid vapors are released during the electrosynthesis process. These emissions can contribute to the formation of acid rain, potentially causing damage to vegetation, buildings, and aquatic ecosystems over a wider area.
The production and use of perchloric acid in industrial processes can lead to increased energy consumption and greenhouse gas emissions, contributing to climate change. This indirect environmental impact should be factored into the overall assessment of the technology's ecological footprint.
Waste management is a crucial consideration in the environmental impact assessment. The disposal of perchloric acid and its byproducts requires specialized handling and treatment facilities to prevent environmental contamination. Improper disposal can lead to long-term environmental degradation and pose risks to human health.
To mitigate these environmental risks, stringent safety protocols and containment measures must be implemented in facilities using perchloric acid for low-temperature electrosynthesis. This includes proper storage, handling, and disposal procedures, as well as the use of advanced air and water treatment systems to minimize emissions and effluents.
Continuous environmental monitoring is essential to detect any potential leaks or contamination early. This should include regular testing of soil, water, and air quality in and around the facilities using this technology. Additionally, the development of more environmentally friendly alternatives or process modifications that reduce the reliance on perchloric acid should be actively pursued as part of ongoing research and development efforts.
In aquatic environments, the release of perchloric acid can lead to severe pH imbalances, potentially causing harm to fish, aquatic plants, and microorganisms. Even small quantities can disrupt the delicate ecological balance of water bodies, affecting biodiversity and ecosystem functions. The persistence of perchlorate ions in water systems is a particular concern, as they can contaminate drinking water sources and bioaccumulate in aquatic food chains.
Soil contamination is another critical aspect to consider. Perchloric acid spills or improper disposal can result in soil acidification, negatively impacting soil fertility and microbial communities. This, in turn, can affect plant growth and agricultural productivity in affected areas. The mobility of perchlorate ions in soil further exacerbates the risk of groundwater contamination.
Air quality may also be compromised if perchloric acid vapors are released during the electrosynthesis process. These emissions can contribute to the formation of acid rain, potentially causing damage to vegetation, buildings, and aquatic ecosystems over a wider area.
The production and use of perchloric acid in industrial processes can lead to increased energy consumption and greenhouse gas emissions, contributing to climate change. This indirect environmental impact should be factored into the overall assessment of the technology's ecological footprint.
Waste management is a crucial consideration in the environmental impact assessment. The disposal of perchloric acid and its byproducts requires specialized handling and treatment facilities to prevent environmental contamination. Improper disposal can lead to long-term environmental degradation and pose risks to human health.
To mitigate these environmental risks, stringent safety protocols and containment measures must be implemented in facilities using perchloric acid for low-temperature electrosynthesis. This includes proper storage, handling, and disposal procedures, as well as the use of advanced air and water treatment systems to minimize emissions and effluents.
Continuous environmental monitoring is essential to detect any potential leaks or contamination early. This should include regular testing of soil, water, and air quality in and around the facilities using this technology. Additionally, the development of more environmentally friendly alternatives or process modifications that reduce the reliance on perchloric acid should be actively pursued as part of ongoing research and development efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!