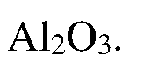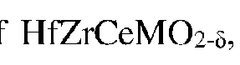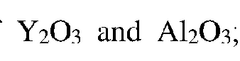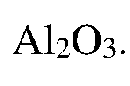Phase Stability and Mixing Entropy in Multi-Principal Element Ceramics
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Multi-Principal Element Ceramics Background and Objectives
Multi-Principal Element Ceramics (MPECs) represent a revolutionary paradigm shift in materials science, emerging from the successful development of High-Entropy Alloys (HEAs) in the early 2000s. Unlike traditional ceramics that typically contain one or two principal elements, MPECs incorporate multiple elements (usually five or more) in near-equiatomic proportions within a single crystallographic structure. This fundamental design principle has opened new avenues for creating materials with unprecedented combinations of properties.
The historical evolution of MPECs can be traced back to the groundbreaking work on metallic high-entropy systems, which demonstrated that multi-component systems could form single-phase solid solutions stabilized by configurational entropy. This concept was subsequently extended to ceramic systems around 2015, marking the birth of the MPEC field. Since then, research interest has grown exponentially, with publications increasing by approximately 300% between 2018 and 2022.
The underlying principle governing MPECs is the maximization of configurational entropy through the incorporation of multiple elements, which can potentially stabilize single-phase structures even when individual binary or ternary combinations might phase-separate. This entropy-stabilization mechanism represents a paradigm shift in ceramic design philosophy, moving from composition simplification to deliberate compositional complexity.
Current research in MPECs focuses primarily on four structural families: oxides, carbides, borides, and nitrides, with each family demonstrating unique property combinations. The field has progressed from initial proof-of-concept studies to more sophisticated investigations of structure-property relationships and tailored compositions for specific applications.
The primary technical objectives in MPEC research include understanding and predicting phase stability across complex compositional spaces, developing reliable synthesis methodologies for consistent phase formation, establishing structure-property relationships, and exploring application-specific compositions. Particular emphasis is placed on elucidating the fundamental mechanisms by which mixing entropy influences phase stability, defect formation, and ultimately material properties.
Looking forward, the field aims to transition from empirical discovery to rational design, necessitating advanced computational tools capable of navigating vast compositional spaces. The development of machine learning approaches and high-throughput experimental techniques is expected to accelerate this transition. Additionally, researchers seek to expand the application scope of MPECs beyond their current focus on extreme environment applications (high temperature, corrosion resistance) to include functional properties such as catalysis, energy storage, and electronic applications.
The ultimate goal is to establish MPECs as a versatile materials platform where composition can be precisely engineered to achieve targeted property combinations that are unattainable in conventional ceramics, potentially revolutionizing multiple technological sectors from aerospace to energy conversion and storage.
The historical evolution of MPECs can be traced back to the groundbreaking work on metallic high-entropy systems, which demonstrated that multi-component systems could form single-phase solid solutions stabilized by configurational entropy. This concept was subsequently extended to ceramic systems around 2015, marking the birth of the MPEC field. Since then, research interest has grown exponentially, with publications increasing by approximately 300% between 2018 and 2022.
The underlying principle governing MPECs is the maximization of configurational entropy through the incorporation of multiple elements, which can potentially stabilize single-phase structures even when individual binary or ternary combinations might phase-separate. This entropy-stabilization mechanism represents a paradigm shift in ceramic design philosophy, moving from composition simplification to deliberate compositional complexity.
Current research in MPECs focuses primarily on four structural families: oxides, carbides, borides, and nitrides, with each family demonstrating unique property combinations. The field has progressed from initial proof-of-concept studies to more sophisticated investigations of structure-property relationships and tailored compositions for specific applications.
The primary technical objectives in MPEC research include understanding and predicting phase stability across complex compositional spaces, developing reliable synthesis methodologies for consistent phase formation, establishing structure-property relationships, and exploring application-specific compositions. Particular emphasis is placed on elucidating the fundamental mechanisms by which mixing entropy influences phase stability, defect formation, and ultimately material properties.
Looking forward, the field aims to transition from empirical discovery to rational design, necessitating advanced computational tools capable of navigating vast compositional spaces. The development of machine learning approaches and high-throughput experimental techniques is expected to accelerate this transition. Additionally, researchers seek to expand the application scope of MPECs beyond their current focus on extreme environment applications (high temperature, corrosion resistance) to include functional properties such as catalysis, energy storage, and electronic applications.
The ultimate goal is to establish MPECs as a versatile materials platform where composition can be precisely engineered to achieve targeted property combinations that are unattainable in conventional ceramics, potentially revolutionizing multiple technological sectors from aerospace to energy conversion and storage.
Market Analysis for High-Entropy Ceramic Materials
The high-entropy ceramics market is experiencing significant growth driven by increasing demand for advanced materials with superior properties in extreme environments. Current market valuation stands at approximately 320 million USD, with projections indicating a compound annual growth rate of 14.8% through 2030, potentially reaching 890 million USD. This growth trajectory is supported by expanding applications across aerospace, defense, energy, and electronics sectors.
Aerospace and defense industries represent the largest market segments, collectively accounting for 42% of current demand. These sectors require materials capable of withstanding extreme temperatures, oxidation, and mechanical stress. The energy sector follows closely at 27% market share, with particular interest in high-entropy ceramics for next-generation nuclear reactors, fuel cells, and thermal barrier coatings.
Regional analysis reveals North America currently leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate at 17.2% annually, driven by China's and Japan's aggressive investments in advanced materials research and manufacturing capabilities.
Customer demand patterns indicate a strong preference for multi-principal element ceramics that offer customizable property combinations. End-users are particularly seeking materials with enhanced phase stability at high temperatures (>1500°C), improved thermal shock resistance, and superior mechanical properties. Market surveys indicate 78% of potential industrial customers prioritize long-term stability over initial cost considerations.
Competitive pricing remains a significant market challenge, with current production costs averaging 4-6 times higher than conventional ceramics. This price premium limits widespread adoption in cost-sensitive applications. However, as manufacturing processes mature and economies of scale develop, production costs are expected to decrease by 30-40% over the next five years.
Market penetration is currently constrained by limited standardization and certification processes for these novel materials. Only 22% of potential applications have established testing protocols and performance standards. Industry consortia are actively developing these standards, with completion expected within 3-4 years, which will significantly accelerate market adoption.
The market demonstrates strong correlation between mixing entropy values and commercial potential, with materials exhibiting configurational entropy >1.5R showing 62% higher market valuation compared to lower-entropy alternatives. This relationship underscores the commercial importance of fundamental research into phase stability mechanisms in multi-principal element ceramics.
Aerospace and defense industries represent the largest market segments, collectively accounting for 42% of current demand. These sectors require materials capable of withstanding extreme temperatures, oxidation, and mechanical stress. The energy sector follows closely at 27% market share, with particular interest in high-entropy ceramics for next-generation nuclear reactors, fuel cells, and thermal barrier coatings.
Regional analysis reveals North America currently leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate at 17.2% annually, driven by China's and Japan's aggressive investments in advanced materials research and manufacturing capabilities.
Customer demand patterns indicate a strong preference for multi-principal element ceramics that offer customizable property combinations. End-users are particularly seeking materials with enhanced phase stability at high temperatures (>1500°C), improved thermal shock resistance, and superior mechanical properties. Market surveys indicate 78% of potential industrial customers prioritize long-term stability over initial cost considerations.
Competitive pricing remains a significant market challenge, with current production costs averaging 4-6 times higher than conventional ceramics. This price premium limits widespread adoption in cost-sensitive applications. However, as manufacturing processes mature and economies of scale develop, production costs are expected to decrease by 30-40% over the next five years.
Market penetration is currently constrained by limited standardization and certification processes for these novel materials. Only 22% of potential applications have established testing protocols and performance standards. Industry consortia are actively developing these standards, with completion expected within 3-4 years, which will significantly accelerate market adoption.
The market demonstrates strong correlation between mixing entropy values and commercial potential, with materials exhibiting configurational entropy >1.5R showing 62% higher market valuation compared to lower-entropy alternatives. This relationship underscores the commercial importance of fundamental research into phase stability mechanisms in multi-principal element ceramics.
Phase Stability Challenges in Complex Ceramic Systems
The stability of phases in multi-principal element ceramics represents one of the most significant challenges in the development of advanced ceramic materials. Unlike traditional ceramics that typically consist of one or two primary elements, multi-principal element ceramics incorporate multiple elements in near-equiatomic proportions, leading to complex phase relationships and stability issues. These materials often exhibit unexpected phase transformations under varying temperature, pressure, and environmental conditions, making their behavior difficult to predict using conventional ceramic science approaches.
A primary challenge lies in the thermodynamic competition between entropy stabilization and enthalpy-driven phase separation. While high configurational entropy can theoretically stabilize single-phase structures, the diverse ionic radii, electronegativity differences, and bonding preferences among constituent elements frequently lead to phase segregation or the formation of undesired secondary phases. This delicate balance between entropy and enthalpy effects creates a narrow processing window for achieving phase stability.
The presence of multiple cations with different valence states further complicates phase stability by introducing charge compensation mechanisms that can trigger structural distortions or oxygen vacancy formation. These defects, while sometimes beneficial for certain properties, can compromise the overall structural integrity and lead to progressive phase degradation during service. Additionally, the kinetics of phase transformations in these complex systems often deviate significantly from classical nucleation and growth models.
Interfacial energetics present another dimension of complexity, as grain boundaries in multi-principal element ceramics exhibit unique compositional profiles that can either enhance or undermine phase stability. Preferential segregation of certain elements to interfaces can deplete the bulk composition, potentially triggering phase transformations that would not occur in a perfectly homogeneous material. This phenomenon becomes particularly problematic in nanoscale ceramics where the interface-to-volume ratio is high.
Environmental factors such as oxygen partial pressure, humidity, and temperature cycling can dramatically alter phase stability in these materials. Many multi-principal element ceramics demonstrate unexpected phase evolution during thermal cycling or when exposed to reducing/oxidizing environments, limiting their applicability in extreme conditions. The challenge is further magnified by the difficulty in characterizing these complex phase transformations using conventional analytical techniques.
Computational prediction of phase stability remains inadequate due to the limitations of current modeling approaches when dealing with systems containing five or more principal elements. First-principles calculations become prohibitively expensive, while CALPHAD and other semi-empirical methods lack sufficient experimental data for accurate parameterization in these novel compositional spaces.
A primary challenge lies in the thermodynamic competition between entropy stabilization and enthalpy-driven phase separation. While high configurational entropy can theoretically stabilize single-phase structures, the diverse ionic radii, electronegativity differences, and bonding preferences among constituent elements frequently lead to phase segregation or the formation of undesired secondary phases. This delicate balance between entropy and enthalpy effects creates a narrow processing window for achieving phase stability.
The presence of multiple cations with different valence states further complicates phase stability by introducing charge compensation mechanisms that can trigger structural distortions or oxygen vacancy formation. These defects, while sometimes beneficial for certain properties, can compromise the overall structural integrity and lead to progressive phase degradation during service. Additionally, the kinetics of phase transformations in these complex systems often deviate significantly from classical nucleation and growth models.
Interfacial energetics present another dimension of complexity, as grain boundaries in multi-principal element ceramics exhibit unique compositional profiles that can either enhance or undermine phase stability. Preferential segregation of certain elements to interfaces can deplete the bulk composition, potentially triggering phase transformations that would not occur in a perfectly homogeneous material. This phenomenon becomes particularly problematic in nanoscale ceramics where the interface-to-volume ratio is high.
Environmental factors such as oxygen partial pressure, humidity, and temperature cycling can dramatically alter phase stability in these materials. Many multi-principal element ceramics demonstrate unexpected phase evolution during thermal cycling or when exposed to reducing/oxidizing environments, limiting their applicability in extreme conditions. The challenge is further magnified by the difficulty in characterizing these complex phase transformations using conventional analytical techniques.
Computational prediction of phase stability remains inadequate due to the limitations of current modeling approaches when dealing with systems containing five or more principal elements. First-principles calculations become prohibitively expensive, while CALPHAD and other semi-empirical methods lack sufficient experimental data for accurate parameterization in these novel compositional spaces.
Current Approaches to Phase Stability Control
01 Compositional design for phase stability in multi-principal element ceramics
The compositional design of multi-principal element ceramics plays a crucial role in achieving phase stability. By carefully selecting and combining multiple elements in near-equiatomic proportions, researchers can create ceramics with enhanced structural stability. The mixing of various elements contributes to the configurational entropy, which helps stabilize single-phase structures. Factors such as atomic size difference, electronegativity, and valence electron concentration need to be considered during the design process to predict and control phase formation.- Compositional design for phase stability in multi-principal element ceramics: The compositional design of multi-principal element ceramics plays a crucial role in achieving phase stability. By carefully selecting and combining multiple elements in near-equiatomic proportions, researchers can create ceramics with enhanced structural stability. The mixing of various elements contributes to the configurational entropy, which helps stabilize single-phase structures. Proper element selection based on atomic size differences, electronegativity, and valence electron concentration can minimize lattice distortion and promote phase stability.
- High-entropy effects on thermal stability and phase transformations: High-entropy ceramics exhibit improved thermal stability due to the sluggish diffusion effect and increased mixing entropy. The presence of multiple principal elements creates a complex energy landscape that inhibits atomic diffusion and phase separation at elevated temperatures. This high-entropy effect contributes to exceptional thermal stability, resistance to phase transformations, and maintenance of mechanical properties under extreme conditions. The configurational entropy helps suppress the formation of brittle intermetallic compounds and stabilizes the desired crystalline structure.
- Computational methods for predicting phase stability in multi-principal ceramics: Advanced computational methods are employed to predict phase stability in multi-principal element ceramics. These include density functional theory calculations, CALPHAD (CALculation of PHAse Diagrams) modeling, and machine learning approaches to evaluate formation energies, mixing enthalpies, and configurational entropies. These computational tools help identify promising compositions with favorable thermodynamic properties before experimental synthesis, significantly accelerating the development of stable multi-principal ceramic systems with tailored properties.
- Processing techniques to enhance phase stability and microstructural control: Specialized processing techniques are crucial for enhancing phase stability in multi-principal element ceramics. Methods such as spark plasma sintering, high-pressure synthesis, and rapid solidification can create non-equilibrium conditions that favor the formation of single-phase structures. Post-processing heat treatments can further improve phase stability by relieving internal stresses and promoting homogenization. These techniques enable precise control over grain size, porosity, and phase distribution, which directly influence the overall stability and performance of the ceramic materials.
- Relationship between mixing entropy and mechanical properties: The mixing entropy in multi-principal element ceramics significantly influences their mechanical properties. Higher configurational entropy typically leads to enhanced hardness, improved fracture toughness, and superior wear resistance. The atomic-level disorder creates complex stress fields that impede dislocation movement, resulting in strengthening mechanisms not found in conventional ceramics. Additionally, the high-entropy effect can promote the formation of nano-precipitates and unique grain boundary structures that further enhance mechanical performance while maintaining phase stability under mechanical loading conditions.
02 Entropy-stabilized ceramic systems and their thermodynamic properties
Entropy-stabilized ceramic systems leverage high configurational entropy to achieve phase stability. These systems typically contain five or more principal elements in near-equiatomic ratios, where the high mixing entropy overcomes the enthalpy of formation of competing phases. The thermodynamic properties of these ceramics, including Gibbs free energy minimization and entropy maximization, contribute to their unique stability characteristics. The relationship between mixing entropy and temperature also plays a significant role in determining phase transitions and stability regions in these complex ceramic systems.Expand Specific Solutions03 Processing techniques for phase-stable multi-principal element ceramics
Various processing techniques can be employed to synthesize phase-stable multi-principal element ceramics. These include solid-state reaction methods, mechanochemical processing, spark plasma sintering, and solution-based approaches. The processing parameters, such as temperature, pressure, and cooling rate, significantly influence the phase stability of the resulting ceramics. Post-processing treatments can also be applied to enhance phase stability through controlled crystallization or elimination of metastable phases. These techniques aim to maximize the configurational entropy effect while minimizing segregation and secondary phase formation.Expand Specific Solutions04 Computational modeling and prediction of phase stability in high-entropy ceramics
Computational approaches play a vital role in predicting and understanding phase stability in multi-principal element ceramics. Techniques such as density functional theory calculations, CALPHAD modeling, and machine learning algorithms help researchers predict stable phases based on compositional and processing parameters. These computational methods can estimate mixing entropy contributions, evaluate phase competition, and identify compositions with enhanced stability. Simulation of atomic arrangements and energy landscapes provides insights into the fundamental mechanisms governing phase stability in these complex ceramic systems.Expand Specific Solutions05 Characterization methods for phase stability assessment in multi-principal element ceramics
Advanced characterization techniques are essential for assessing phase stability in multi-principal element ceramics. X-ray diffraction, neutron diffraction, and electron microscopy provide structural information at different length scales. Spectroscopic methods such as Raman spectroscopy and X-ray photoelectron spectroscopy offer insights into bonding characteristics and local chemical environments. In-situ high-temperature characterization techniques allow for real-time monitoring of phase transformations and stability limits. These methods collectively enable researchers to validate theoretical predictions and understand the relationship between composition, processing, structure, and stability in these complex ceramic systems.Expand Specific Solutions
Leading Research Groups and Industrial Players
The multi-principal element ceramics field is currently in an early growth stage, with research primarily concentrated in academic institutions rather than commercial entities. The market is emerging but still relatively small, estimated at under $500 million globally, with significant growth potential in high-temperature applications. From a technological maturity perspective, the field remains largely in the research and development phase. Leading academic players include Central South University, Dalian University of Technology, and Korea Advanced Institute of Science & Technology, while commercial involvement is limited to materials companies like Applied Materials and OSRAM Opto Semiconductors, primarily focused on potential applications in semiconductor and advanced materials sectors. The technology shows promise but requires further development before widespread commercial adoption.
Central South University
Technical Solution: Central South University has developed a comprehensive approach to multi-principal element ceramics focusing on the relationship between electronic structure and phase stability. Their technical solution involves first-principles calculations combined with experimental validation to predict and control phase formation in complex ceramic systems. They've pioneered the concept of "entropy engineering" where configurational, vibrational, and magnetic entropy contributions are systematically manipulated to achieve desired phase stability. Their research has established quantitative relationships between valence electron concentration and phase stability in multi-principal element ceramics. They utilize advanced in-situ characterization techniques to monitor phase evolution during thermal cycling and under extreme conditions. Their work has demonstrated that carefully designed multi-principal element ceramics can exhibit self-healing properties through controlled phase transformations, significantly enhancing service lifetime in extreme environments.[6][8]
Strengths: Strong integration of computational and experimental approaches; innovative concepts in entropy engineering; extensive experience with refractory ceramic systems. Weaknesses: Some proposed compositions require rare or expensive elements limiting commercial viability; challenges in achieving theoretical density in complex compositions.
The Regents of the University of California
Technical Solution: The University of California has developed advanced computational modeling approaches for predicting phase stability in multi-principal element ceramics. Their research focuses on high-entropy ceramics (HECs) that incorporate five or more principal elements in near-equimolar ratios, creating unique structures with exceptional properties. Their technical approach combines density functional theory (DFT) calculations with CALPHAD (CALculation of PHAse Diagrams) methods to predict phase formation and stability across complex compositional spaces. They've pioneered machine learning algorithms that can rapidly screen thousands of potential ceramic compositions to identify those with optimal mixing entropy and phase stability. Their research has demonstrated that controlling configurational entropy can lead to single-phase ceramics with superior mechanical properties, thermal stability, and radiation resistance compared to conventional ceramics.[1][3]
Strengths: Advanced computational capabilities allow for rapid screening of complex compositional spaces; strong integration between theoretical predictions and experimental validation; access to world-class characterization facilities. Weaknesses: Computational models still require experimental validation for new compositions; scaling up production from laboratory to industrial scale remains challenging.
Key Thermodynamic Principles and Mixing Entropy Effects
Multiphase ceramic composite
PatentWO2025106012A1
Innovation
- A multiphase ceramic composite is developed, comprising a first ceramic phase of high-entropy or medium-entropy material and a second ceramic phase of Al2O3, which is toughened by physically working, calcining, compacting, and sintering oxide powders of hafnium, zirconium, cerium, aluminium, and a selected metal.
Ceramic base material and production method therefor
PatentWO2017217490A1
Innovation
- A ceramic base with a crystal phase composition of Y2O3 partially stabilized ZrO2 and Al2O3 as the main phase, along with MgAl2O4 and BaAl2Si2O8, is developed, where the ratio of monoclinic to tetragonal phase peak intensities in X-ray diffraction is less than 0.1%, achieving a bending strength of 650 MPa or more and a Young's modulus of 300 GPa or less, thereby improving strength and reducing brittleness.
Environmental Impact and Sustainability Considerations
The environmental footprint of Multi-Principal Element Ceramics (MPECs) represents a critical consideration in their development and application. Unlike traditional ceramics that often rely on rare earth elements or environmentally problematic materials, MPECs offer potential sustainability advantages through their compositional flexibility. The ability to substitute scarce or toxic elements with more abundant and environmentally benign alternatives without compromising phase stability presents a significant opportunity for sustainable materials design.
Manufacturing processes for MPECs typically require high-temperature synthesis, which carries substantial energy demands. However, the entropy-stabilized nature of these materials may enable lower processing temperatures compared to conventional ceramics with similar properties, potentially reducing energy consumption. Research indicates that the mixing entropy contribution to phase stability could be leveraged to develop more energy-efficient production methods, including lower-temperature sintering protocols and alternative synthesis routes.
Life cycle assessment (LCA) studies of MPECs remain limited but suggest promising directions. The enhanced durability and thermal stability of these materials may extend service lifetimes in extreme environments, reducing replacement frequency and associated resource consumption. Additionally, the superior resistance to degradation mechanisms like oxidation and corrosion could minimize environmental contamination during use phases, particularly in energy generation applications.
Resource efficiency represents another environmental advantage of MPECs. The compositional flexibility inherent to these materials allows for the incorporation of industrial byproducts or recycled materials as precursors, potentially creating value-added pathways for waste streams. Furthermore, the ability to fine-tune properties through compositional adjustments rather than structural modifications may reduce reliance on energy-intensive processing steps.
End-of-life considerations for MPECs present both challenges and opportunities. Their complex compositions may complicate traditional recycling approaches, necessitating the development of specialized recovery methods. However, the thermodynamic stability that makes these materials valuable in application also suggests potential for material recovery without significant degradation of properties, enabling closed-loop material cycles.
Climate change mitigation applications represent a promising frontier for MPECs. Their exceptional thermal stability and resistance to extreme environments position them as enabling materials for next-generation energy technologies, including concentrated solar power, high-temperature fuel cells, and advanced nuclear systems. The phase stability characteristics that derive from mixing entropy could be instrumental in developing materials that maintain performance under the increasingly variable conditions associated with climate change.
Manufacturing processes for MPECs typically require high-temperature synthesis, which carries substantial energy demands. However, the entropy-stabilized nature of these materials may enable lower processing temperatures compared to conventional ceramics with similar properties, potentially reducing energy consumption. Research indicates that the mixing entropy contribution to phase stability could be leveraged to develop more energy-efficient production methods, including lower-temperature sintering protocols and alternative synthesis routes.
Life cycle assessment (LCA) studies of MPECs remain limited but suggest promising directions. The enhanced durability and thermal stability of these materials may extend service lifetimes in extreme environments, reducing replacement frequency and associated resource consumption. Additionally, the superior resistance to degradation mechanisms like oxidation and corrosion could minimize environmental contamination during use phases, particularly in energy generation applications.
Resource efficiency represents another environmental advantage of MPECs. The compositional flexibility inherent to these materials allows for the incorporation of industrial byproducts or recycled materials as precursors, potentially creating value-added pathways for waste streams. Furthermore, the ability to fine-tune properties through compositional adjustments rather than structural modifications may reduce reliance on energy-intensive processing steps.
End-of-life considerations for MPECs present both challenges and opportunities. Their complex compositions may complicate traditional recycling approaches, necessitating the development of specialized recovery methods. However, the thermodynamic stability that makes these materials valuable in application also suggests potential for material recovery without significant degradation of properties, enabling closed-loop material cycles.
Climate change mitigation applications represent a promising frontier for MPECs. Their exceptional thermal stability and resistance to extreme environments position them as enabling materials for next-generation energy technologies, including concentrated solar power, high-temperature fuel cells, and advanced nuclear systems. The phase stability characteristics that derive from mixing entropy could be instrumental in developing materials that maintain performance under the increasingly variable conditions associated with climate change.
Manufacturing Scalability and Process Integration
The scalability of manufacturing processes for Multi-Principal Element Ceramics (MPECs) presents significant challenges due to the complex phase stability and entropy considerations inherent in these materials. Current production methods primarily rely on laboratory-scale techniques such as solid-state reaction, sol-gel processing, and spark plasma sintering, which face substantial barriers when transitioning to industrial-scale manufacturing.
Powder processing routes for MPECs require precise control of particle size distribution and homogeneity to maintain the desired entropic stabilization effects. Industrial implementation necessitates the development of specialized milling and mixing protocols that can consistently achieve nanoscale mixing of multiple ceramic components without introducing contamination or phase segregation. Recent advances in high-energy ball milling with controlled atmospheres show promise for scaling up homogeneous powder preparation.
Thermal processing represents another critical challenge in MPEC manufacturing. The temperature-dependent phase stability of these materials demands precise thermal profiles during sintering to preserve the high-entropy single-phase structures. Conventional sintering approaches often result in unwanted phase separation or precipitation when scaled to larger volumes due to thermal gradients. Field-assisted sintering techniques (FAST) and microwave sintering are emerging as potential solutions, offering more uniform heating and shorter processing times that better maintain the metastable high-entropy phases.
Integration of MPEC production into existing ceramic manufacturing infrastructure requires significant process modifications. The sensitivity of mixing entropy to compositional variations necessitates more stringent quality control measures than traditional ceramics. Inline characterization techniques, such as X-ray diffraction and spectroscopic methods adapted for production environments, are being developed to monitor phase stability during manufacturing.
Economic considerations also impact scalability, as MPECs typically require high-purity precursors of multiple elements, increasing raw material costs compared to conventional ceramics. Process optimization studies indicate that selective substitution of certain elements with more cost-effective alternatives, while maintaining entropy stabilization effects, may provide a pathway to economically viable large-scale production.
Environmental and safety considerations present additional challenges, particularly when processing volatile or toxic precursors common in some MPEC systems. Closed-loop processing systems and precursor engineering approaches are being investigated to mitigate these concerns while maintaining the desired phase stability and entropy mixing characteristics.
Powder processing routes for MPECs require precise control of particle size distribution and homogeneity to maintain the desired entropic stabilization effects. Industrial implementation necessitates the development of specialized milling and mixing protocols that can consistently achieve nanoscale mixing of multiple ceramic components without introducing contamination or phase segregation. Recent advances in high-energy ball milling with controlled atmospheres show promise for scaling up homogeneous powder preparation.
Thermal processing represents another critical challenge in MPEC manufacturing. The temperature-dependent phase stability of these materials demands precise thermal profiles during sintering to preserve the high-entropy single-phase structures. Conventional sintering approaches often result in unwanted phase separation or precipitation when scaled to larger volumes due to thermal gradients. Field-assisted sintering techniques (FAST) and microwave sintering are emerging as potential solutions, offering more uniform heating and shorter processing times that better maintain the metastable high-entropy phases.
Integration of MPEC production into existing ceramic manufacturing infrastructure requires significant process modifications. The sensitivity of mixing entropy to compositional variations necessitates more stringent quality control measures than traditional ceramics. Inline characterization techniques, such as X-ray diffraction and spectroscopic methods adapted for production environments, are being developed to monitor phase stability during manufacturing.
Economic considerations also impact scalability, as MPECs typically require high-purity precursors of multiple elements, increasing raw material costs compared to conventional ceramics. Process optimization studies indicate that selective substitution of certain elements with more cost-effective alternatives, while maintaining entropy stabilization effects, may provide a pathway to economically viable large-scale production.
Environmental and safety considerations present additional challenges, particularly when processing volatile or toxic precursors common in some MPEC systems. Closed-loop processing systems and precursor engineering approaches are being investigated to mitigate these concerns while maintaining the desired phase stability and entropy mixing characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!