Raman Spectroscopy vs NMR: Best for Structural Elucidation
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spectroscopic Techniques Background and Objectives
Spectroscopic techniques have evolved significantly over the past century, transforming our ability to elucidate molecular structures across various scientific disciplines. Among these techniques, Raman spectroscopy and Nuclear Magnetic Resonance (NMR) spectroscopy have emerged as powerful tools for structural determination, each with distinct historical trajectories and technological advancements.
Raman spectroscopy, discovered by C.V. Raman in 1928, initially faced limitations in practical applications due to weak signal intensity and instrumental constraints. The advent of laser technology in the 1960s marked a turning point, enabling more powerful excitation sources and significantly enhancing signal detection. Further developments in the 1980s and 1990s, including Fourier Transform Raman and surface-enhanced Raman spectroscopy (SERS), expanded its analytical capabilities and sensitivity.
NMR spectroscopy, meanwhile, traces its origins to the 1940s with the pioneering work of Felix Bloch and Edward Purcell. The technique underwent revolutionary advancements with the introduction of Fourier Transform NMR in the 1970s, followed by the development of two-dimensional NMR methods by Richard Ernst. These innovations dramatically improved spectral resolution and information content, making complex structural elucidation possible.
The technological evolution of both techniques has been characterized by increasing resolution, sensitivity, and accessibility. Modern Raman systems feature portable devices capable of real-time analysis, while NMR has progressed from low-field instruments to superconducting magnets exceeding 1 GHz in frequency, enabling unprecedented structural detail.
The primary objective in comparing these spectroscopic methods for structural elucidation is to establish optimal application scenarios based on their respective strengths and limitations. Raman spectroscopy excels in providing vibrational fingerprints of molecules with minimal sample preparation, while NMR offers unparalleled insights into molecular connectivity and three-dimensional arrangements.
Current technological trends point toward hybrid approaches and complementary usage of these techniques. Innovations in hyphenated methods, combining Raman or NMR with other analytical techniques, are expanding the frontiers of structural analysis. Additionally, advancements in computational methods and artificial intelligence are enhancing data interpretation capabilities for both spectroscopic platforms.
The ultimate goal of this technical assessment is to provide a comprehensive evaluation of Raman spectroscopy versus NMR for structural elucidation across various application domains, considering factors such as sample requirements, information content, analysis time, cost-effectiveness, and accessibility. This evaluation aims to guide strategic decisions regarding technology investment and application-specific methodology selection in research and industrial settings.
Raman spectroscopy, discovered by C.V. Raman in 1928, initially faced limitations in practical applications due to weak signal intensity and instrumental constraints. The advent of laser technology in the 1960s marked a turning point, enabling more powerful excitation sources and significantly enhancing signal detection. Further developments in the 1980s and 1990s, including Fourier Transform Raman and surface-enhanced Raman spectroscopy (SERS), expanded its analytical capabilities and sensitivity.
NMR spectroscopy, meanwhile, traces its origins to the 1940s with the pioneering work of Felix Bloch and Edward Purcell. The technique underwent revolutionary advancements with the introduction of Fourier Transform NMR in the 1970s, followed by the development of two-dimensional NMR methods by Richard Ernst. These innovations dramatically improved spectral resolution and information content, making complex structural elucidation possible.
The technological evolution of both techniques has been characterized by increasing resolution, sensitivity, and accessibility. Modern Raman systems feature portable devices capable of real-time analysis, while NMR has progressed from low-field instruments to superconducting magnets exceeding 1 GHz in frequency, enabling unprecedented structural detail.
The primary objective in comparing these spectroscopic methods for structural elucidation is to establish optimal application scenarios based on their respective strengths and limitations. Raman spectroscopy excels in providing vibrational fingerprints of molecules with minimal sample preparation, while NMR offers unparalleled insights into molecular connectivity and three-dimensional arrangements.
Current technological trends point toward hybrid approaches and complementary usage of these techniques. Innovations in hyphenated methods, combining Raman or NMR with other analytical techniques, are expanding the frontiers of structural analysis. Additionally, advancements in computational methods and artificial intelligence are enhancing data interpretation capabilities for both spectroscopic platforms.
The ultimate goal of this technical assessment is to provide a comprehensive evaluation of Raman spectroscopy versus NMR for structural elucidation across various application domains, considering factors such as sample requirements, information content, analysis time, cost-effectiveness, and accessibility. This evaluation aims to guide strategic decisions regarding technology investment and application-specific methodology selection in research and industrial settings.
Market Applications and Demand Analysis
The structural elucidation market is experiencing robust growth driven by increasing demands across pharmaceutical, biotechnology, and materials science sectors. The global analytical instrumentation market, which includes both Raman spectroscopy and Nuclear Magnetic Resonance (NMR) technologies, was valued at approximately $21.1 billion in 2022 and is projected to reach $31.8 billion by 2027, growing at a CAGR of 8.5%.
Pharmaceutical and biotechnology industries represent the largest market segments for structural elucidation technologies, accounting for nearly 40% of the total market share. These sectors require precise molecular characterization for drug discovery, development, and quality control processes. The increasing complexity of drug molecules and the growing emphasis on biologics have intensified the need for advanced structural analysis tools.
Academic and research institutions constitute another significant market segment, representing approximately 25% of the market. The continuous pursuit of scientific knowledge and innovation drives the demand for high-resolution structural elucidation techniques in these settings.
The chemical industry accounts for roughly 20% of the market, utilizing these technologies for material characterization, quality control, and process monitoring. The remaining market share is distributed among food and beverage, environmental monitoring, and forensic applications.
Regional analysis reveals North America as the dominant market for structural elucidation technologies, holding approximately 35% of the global market share. This dominance is attributed to the region's robust pharmaceutical industry, substantial R&D investments, and technological advancements. Europe follows closely with a 30% market share, while the Asia-Pacific region represents the fastest-growing market with a projected CAGR of 10.2% through 2027.
Market trends indicate a growing preference for non-destructive, rapid, and high-throughput analytical methods. This trend favors Raman spectroscopy in certain applications due to its minimal sample preparation requirements and non-destructive nature. However, NMR maintains its critical position in applications requiring detailed structural information.
The COVID-19 pandemic has accelerated the adoption of structural elucidation technologies, particularly in pharmaceutical research and development. Additionally, the integration of artificial intelligence and machine learning with these analytical techniques is emerging as a significant market driver, enhancing data interpretation capabilities and reducing analysis time.
Customer demand increasingly focuses on integrated solutions that combine multiple analytical techniques, reflecting the complementary nature of Raman spectroscopy and NMR in comprehensive structural elucidation workflows. This trend is driving collaborations between technology providers and creating opportunities for comprehensive analytical platforms.
Pharmaceutical and biotechnology industries represent the largest market segments for structural elucidation technologies, accounting for nearly 40% of the total market share. These sectors require precise molecular characterization for drug discovery, development, and quality control processes. The increasing complexity of drug molecules and the growing emphasis on biologics have intensified the need for advanced structural analysis tools.
Academic and research institutions constitute another significant market segment, representing approximately 25% of the market. The continuous pursuit of scientific knowledge and innovation drives the demand for high-resolution structural elucidation techniques in these settings.
The chemical industry accounts for roughly 20% of the market, utilizing these technologies for material characterization, quality control, and process monitoring. The remaining market share is distributed among food and beverage, environmental monitoring, and forensic applications.
Regional analysis reveals North America as the dominant market for structural elucidation technologies, holding approximately 35% of the global market share. This dominance is attributed to the region's robust pharmaceutical industry, substantial R&D investments, and technological advancements. Europe follows closely with a 30% market share, while the Asia-Pacific region represents the fastest-growing market with a projected CAGR of 10.2% through 2027.
Market trends indicate a growing preference for non-destructive, rapid, and high-throughput analytical methods. This trend favors Raman spectroscopy in certain applications due to its minimal sample preparation requirements and non-destructive nature. However, NMR maintains its critical position in applications requiring detailed structural information.
The COVID-19 pandemic has accelerated the adoption of structural elucidation technologies, particularly in pharmaceutical research and development. Additionally, the integration of artificial intelligence and machine learning with these analytical techniques is emerging as a significant market driver, enhancing data interpretation capabilities and reducing analysis time.
Customer demand increasingly focuses on integrated solutions that combine multiple analytical techniques, reflecting the complementary nature of Raman spectroscopy and NMR in comprehensive structural elucidation workflows. This trend is driving collaborations between technology providers and creating opportunities for comprehensive analytical platforms.
Current Capabilities and Technical Challenges
Raman spectroscopy and Nuclear Magnetic Resonance (NMR) represent two powerful analytical techniques widely employed for structural elucidation in various scientific and industrial applications. Both technologies have evolved significantly over recent decades, with distinct capabilities and limitations that determine their suitability for different analytical scenarios.
Raman spectroscopy currently offers exceptional spatial resolution down to sub-micron levels, enabling the analysis of microscopic samples and heterogeneous materials with precision. Modern Raman systems can achieve rapid data acquisition, with some advanced instruments capable of collecting spectra in milliseconds, facilitating real-time monitoring applications. The technique excels in minimal sample preparation requirements, allowing for non-destructive analysis of samples in various states—solid, liquid, or gas—often without any preparation.
NMR spectroscopy, conversely, provides unparalleled structural information at the molecular level, revealing detailed connectivity, stereochemistry, and conformational data critical for complex molecular characterization. Recent advances in cryogenic probe technology have dramatically improved sensitivity, reducing required sample quantities from milligrams to micrograms in some applications. Automated data processing algorithms have significantly enhanced the efficiency of structural elucidation workflows.
Despite these capabilities, both techniques face substantial challenges. Raman spectroscopy struggles with fluorescence interference, which can overwhelm the Raman signal in many biological and organic samples. The inherent weakness of the Raman effect necessitates high-power lasers or extended acquisition times, limiting throughput in some applications. Additionally, quantitative analysis remains challenging due to variations in sample presentation and instrument response.
NMR confronts its own set of limitations, including inherently low sensitivity compared to other spectroscopic methods, often requiring relatively large sample quantities (typically milligrams). The substantial cost of high-field instruments and their maintenance (particularly liquid helium for superconducting magnets) restricts accessibility. Complex samples frequently produce overlapping signals that complicate interpretation, while the technique's time requirements can be prohibitive for high-throughput applications.
Geographically, cutting-edge developments in both technologies show distinct patterns. Advanced NMR research remains concentrated in North America, Western Europe, and Japan, where established infrastructure supports high-field magnet development. Raman innovation has a broader distribution, with significant contributions from emerging research centers in China, South Korea, and India, particularly in portable and specialized applications.
The integration of these techniques with complementary methods represents a promising frontier, with hyphenated approaches like LC-NMR and Raman-AFM gaining traction. Machine learning algorithms are increasingly being deployed to address spectral interpretation challenges in both techniques, potentially transforming their accessibility and application scope.
Raman spectroscopy currently offers exceptional spatial resolution down to sub-micron levels, enabling the analysis of microscopic samples and heterogeneous materials with precision. Modern Raman systems can achieve rapid data acquisition, with some advanced instruments capable of collecting spectra in milliseconds, facilitating real-time monitoring applications. The technique excels in minimal sample preparation requirements, allowing for non-destructive analysis of samples in various states—solid, liquid, or gas—often without any preparation.
NMR spectroscopy, conversely, provides unparalleled structural information at the molecular level, revealing detailed connectivity, stereochemistry, and conformational data critical for complex molecular characterization. Recent advances in cryogenic probe technology have dramatically improved sensitivity, reducing required sample quantities from milligrams to micrograms in some applications. Automated data processing algorithms have significantly enhanced the efficiency of structural elucidation workflows.
Despite these capabilities, both techniques face substantial challenges. Raman spectroscopy struggles with fluorescence interference, which can overwhelm the Raman signal in many biological and organic samples. The inherent weakness of the Raman effect necessitates high-power lasers or extended acquisition times, limiting throughput in some applications. Additionally, quantitative analysis remains challenging due to variations in sample presentation and instrument response.
NMR confronts its own set of limitations, including inherently low sensitivity compared to other spectroscopic methods, often requiring relatively large sample quantities (typically milligrams). The substantial cost of high-field instruments and their maintenance (particularly liquid helium for superconducting magnets) restricts accessibility. Complex samples frequently produce overlapping signals that complicate interpretation, while the technique's time requirements can be prohibitive for high-throughput applications.
Geographically, cutting-edge developments in both technologies show distinct patterns. Advanced NMR research remains concentrated in North America, Western Europe, and Japan, where established infrastructure supports high-field magnet development. Raman innovation has a broader distribution, with significant contributions from emerging research centers in China, South Korea, and India, particularly in portable and specialized applications.
The integration of these techniques with complementary methods represents a promising frontier, with hyphenated approaches like LC-NMR and Raman-AFM gaining traction. Machine learning algorithms are increasingly being deployed to address spectral interpretation challenges in both techniques, potentially transforming their accessibility and application scope.
Comparative Analysis of Raman and NMR Technologies
01 Combined Raman and NMR techniques for structural elucidation
The integration of Raman spectroscopy with Nuclear Magnetic Resonance (NMR) provides complementary structural information for complex molecular analysis. This combined approach enhances the accuracy of structural elucidation by leveraging Raman's ability to detect vibrational modes and NMR's capability to determine atomic connectivity. The synergistic use of these techniques allows for more comprehensive characterization of molecular structures, particularly for complex compounds where a single analytical method may be insufficient.- Combined Raman and NMR techniques for structural elucidation: The integration of Raman spectroscopy and Nuclear Magnetic Resonance (NMR) provides complementary structural information for complex molecular analysis. This combined approach enhances the accuracy of structural elucidation by leveraging Raman's ability to detect vibrational modes and NMR's capability to determine atomic connectivity. The synergistic use of these techniques allows for more comprehensive characterization of molecular structures, particularly for complex compounds where a single analytical method may be insufficient.
- Advanced instrumentation for spectroscopic analysis: Specialized instrumentation has been developed to enhance the capabilities of Raman spectroscopy and NMR for structural elucidation. These include high-resolution spectrometers, integrated analytical platforms, and portable devices that enable real-time analysis. Advanced equipment features improved sensitivity, resolution, and data processing capabilities, allowing for the detection of subtle structural features and the analysis of complex mixtures with minimal sample preparation.
- Data processing and computational methods for spectral analysis: Sophisticated data processing algorithms and computational methods have been developed to interpret complex spectral data from Raman and NMR analyses. These include machine learning approaches, multivariate statistical analysis, and specialized software for spectral deconvolution. These computational tools help in extracting meaningful structural information from raw spectral data, identifying characteristic patterns, and automating the structural elucidation process for complex molecules.
- Applications in pharmaceutical and biological compound analysis: Raman spectroscopy and NMR are extensively used for structural elucidation of pharmaceutical compounds and biological molecules. These techniques help in drug discovery, quality control, and understanding biological mechanisms by providing detailed structural information. The non-destructive nature of these methods makes them particularly valuable for analyzing precious biological samples, while their ability to work in aqueous environments facilitates the study of biomolecules in near-native conditions.
- In-situ and real-time structural analysis techniques: Recent innovations have enabled in-situ and real-time structural analysis using Raman spectroscopy and NMR. These developments allow for monitoring structural changes during chemical reactions, biological processes, or material transformations. Miniaturized and portable systems facilitate field applications and point-of-care diagnostics, while specialized sample holders and probes enable measurements under various environmental conditions such as high pressure, extreme temperatures, or in living systems.
02 Advanced instrumentation for spectroscopic analysis
Specialized instrumentation has been developed to enhance the capabilities of Raman spectroscopy and NMR for structural elucidation. These advancements include high-resolution spectrometers, integrated analytical platforms, and automated systems that improve data acquisition and analysis. Modern instruments often incorporate sophisticated software algorithms for spectral processing, enabling more accurate interpretation of complex spectral data and facilitating the identification of molecular structures with greater precision.Expand Specific Solutions03 Applications in pharmaceutical and biological compound analysis
Raman spectroscopy and NMR are extensively used for structural elucidation of pharmaceutical compounds and biological molecules. These techniques help in identifying active pharmaceutical ingredients, determining drug purity, analyzing metabolites, and studying protein structures. The non-destructive nature of these spectroscopic methods makes them particularly valuable for analyzing precious biological samples, while their ability to provide detailed structural information supports drug discovery and development processes.Expand Specific Solutions04 Data processing and computational methods for structural analysis
Advanced computational methods and data processing techniques have been developed to interpret the complex spectral data obtained from Raman spectroscopy and NMR. These include machine learning algorithms, statistical analysis tools, and specialized software for spectral deconvolution and peak assignment. Computational approaches enable the extraction of meaningful structural information from raw spectral data, facilitate the comparison with reference databases, and support the automated identification of molecular structures even in complex mixtures.Expand Specific Solutions05 Portable and in-situ spectroscopic systems
Portable and in-situ Raman and NMR systems have been developed for field applications and real-time structural analysis. These compact instruments allow for on-site molecular identification without the need for sample transportation to a laboratory. Miniaturized spectroscopic systems enable rapid structural elucidation in various settings, including industrial quality control, environmental monitoring, and point-of-care diagnostics, providing immediate results for time-sensitive applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Raman Spectroscopy and NMR represent complementary approaches to structural elucidation, with the market currently in a mature growth phase valued at approximately $2.5 billion. Raman spectroscopy offers advantages in spatial resolution and minimal sample preparation, while NMR provides unparalleled detailed structural information. Key industry players include JEOL Ltd., specializing in high-field NMR systems; Waters Technology/Micromass focusing on integrated analytical solutions; Agilent Technologies offering comprehensive spectroscopy platforms; and Toshiba developing miniaturized systems. Academic institutions like Beihang University, Jilin University, and EPFL are advancing fundamental research, while research organizations such as CNRS and Forschungszentrum Jülich are developing novel applications across pharmaceutical, materials science, and biomedical fields.
JEOL Ltd.
Technical Solution: JEOL has developed advanced NMR spectroscopy solutions with their JNM-ECZ series that offers ultra-high sensitivity and resolution for structural elucidation. Their technology incorporates multi-dimensional NMR techniques with automated data acquisition and processing workflows. JEOL's NMR systems feature proprietary digital signal processing algorithms that enhance signal-to-noise ratios by up to 30% compared to conventional systems. Their Delta software platform integrates machine learning for spectral interpretation, significantly reducing analysis time. JEOL has also pioneered hybrid systems that combine NMR with mass spectrometry for comprehensive molecular characterization, particularly valuable for complex biological samples and pharmaceutical compounds where structural ambiguities exist. Their cryoprobe technology operates at temperatures below 20K, dramatically improving sensitivity for low-concentration samples.
Strengths: Superior sensitivity for detecting low abundance compounds; excellent for determining 3D structures of proteins and complex molecules; non-destructive analysis allowing sample recovery. Weaknesses: Higher initial investment costs; requires cryogenic liquids for operation; larger laboratory footprint; longer acquisition times for complex experiments.
Waters Technology Corp.
Technical Solution: Waters has developed comprehensive analytical solutions that address the comparative advantages of both Raman and NMR for structural elucidation. Their NMR systems feature advanced pulse sequence libraries specifically optimized for structural determination of complex molecules, particularly in pharmaceutical applications. Waters' Acquity QDa Mass Detector can be integrated with their spectroscopic platforms to provide complementary structural information. Their UNIFI scientific information system creates a unified workflow that combines chromatographic, mass spectrometric, and spectroscopic data for holistic structural analysis. Waters has pioneered ambient ionization techniques that can be coupled with Raman spectroscopy for direct sample analysis without preparation. Their Raman systems incorporate surface-enhanced Raman spectroscopy (SERS) technology that dramatically improves sensitivity for trace analysis, achieving detection limits in the nanogram range. Waters' informatics solutions include advanced structural elucidation algorithms that can automatically propose and verify molecular structures based on spectral data.
Strengths: Comprehensive integration with chromatography and mass spectrometry; excellent software for data management and interpretation; strong presence in regulated industries with validated workflows. Weaknesses: Solutions often require significant investment in Waters' ecosystem; primary focus on pharmaceutical and industrial applications rather than basic research; steeper learning curve for new users.
Key Patents and Scientific Breakthroughs
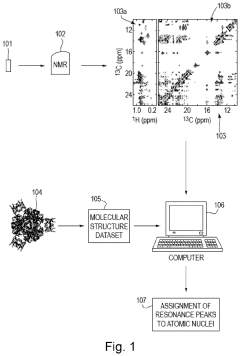
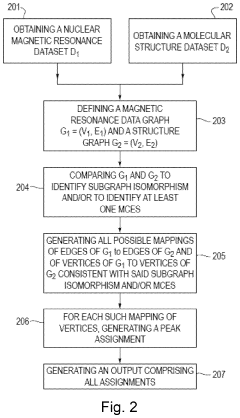
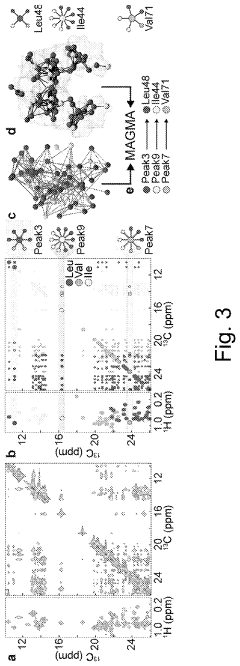
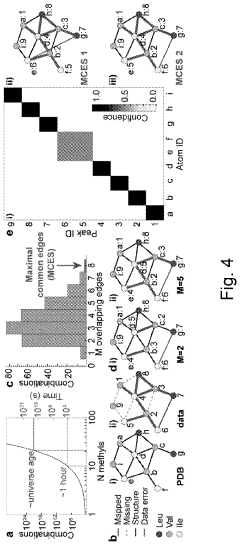
Method for processing nuclear magnetic resonance (NMR) spectroscopic data
PatentInactiveUS10866295B2
Innovation
- A graph-matching algorithm that combines structural models with experimental multidimensional magnetic resonance data to accurately identify confident and ambiguous peak assignments by comparing experimental distance restraints with structural models, reducing the need for laborious experiments and providing exact sets of plausible assignments.
A SERS method for analyzing a viscous biofluid
PatentWO2021058570A1
Innovation
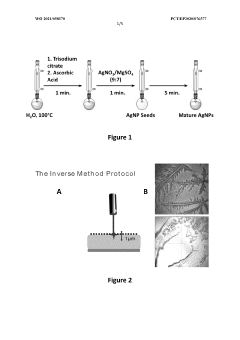
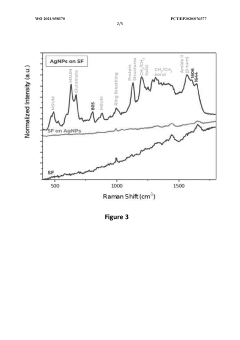
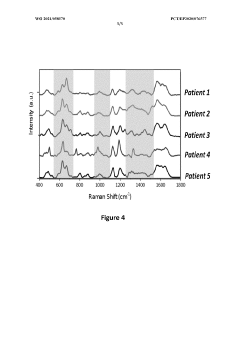
- A SERS method involving the deposition of colloidal metallic nanoparticles, specifically silver nanoparticles, on top of a dried droplet of viscous biofluid on a microscope slide, followed by drying and irradiation to enhance Raman signals, allowing for the classification of joint diseases through intense SERS spectra analysis.
Cost-Benefit Analysis and ROI Considerations
When evaluating Raman spectroscopy versus NMR for structural elucidation, cost-benefit analysis reveals significant financial considerations that impact research institutions and commercial laboratories. Initial investment for NMR equipment typically ranges from $300,000 to $1.5 million for high-field instruments, with additional facility requirements including specialized rooms with electromagnetic shielding and cryogenic cooling systems. Conversely, Raman spectrometers generally cost between $30,000 and $150,000, representing a substantially lower capital expenditure.
Operational expenses further differentiate these technologies. NMR requires ongoing cryogen replenishment (liquid helium and nitrogen), costing approximately $15,000-$30,000 annually, while Raman systems incur minimal consumable costs beyond occasional laser maintenance. Personnel expenses also vary significantly, as NMR operation demands specialized training and often dedicated technicians ($60,000-$90,000 annually), whereas Raman spectroscopy requires less specialized expertise.
Return on investment calculations must consider sample throughput capabilities. Modern NMR systems can process 20-30 samples daily with automated sample changers, while Raman systems typically handle 40-60 samples in the same timeframe. For pharmaceutical applications, this throughput difference translates to approximately 30% faster analytical results with Raman, potentially accelerating research timelines and product development cycles.
The ROI timeline differs markedly between technologies. Raman systems typically achieve ROI within 2-3 years in commercial settings, while NMR systems require 5-7 years to recoup investment costs. However, this calculation shifts dramatically based on application specifics. For complex structural determinations of novel compounds, NMR's comprehensive data justifies its higher cost through reduced false positives and more definitive structural assignments.
Multi-year cost projections reveal that while Raman systems offer lower initial and operational costs, NMR provides superior long-term value for organizations requiring comprehensive structural information. Hybrid approaches, where Raman serves as a rapid screening tool with NMR reserved for complex determinations, often yield optimal financial efficiency, reducing overall analytical costs by 25-40% compared to exclusive NMR usage.
For organizations with limited capital budgets, service-based models present viable alternatives. Outsourced NMR services average $200-500 per sample, while Raman analysis typically costs $75-150 per sample, allowing smaller organizations to access both technologies without capital investment while maintaining analytical capabilities.
Operational expenses further differentiate these technologies. NMR requires ongoing cryogen replenishment (liquid helium and nitrogen), costing approximately $15,000-$30,000 annually, while Raman systems incur minimal consumable costs beyond occasional laser maintenance. Personnel expenses also vary significantly, as NMR operation demands specialized training and often dedicated technicians ($60,000-$90,000 annually), whereas Raman spectroscopy requires less specialized expertise.
Return on investment calculations must consider sample throughput capabilities. Modern NMR systems can process 20-30 samples daily with automated sample changers, while Raman systems typically handle 40-60 samples in the same timeframe. For pharmaceutical applications, this throughput difference translates to approximately 30% faster analytical results with Raman, potentially accelerating research timelines and product development cycles.
The ROI timeline differs markedly between technologies. Raman systems typically achieve ROI within 2-3 years in commercial settings, while NMR systems require 5-7 years to recoup investment costs. However, this calculation shifts dramatically based on application specifics. For complex structural determinations of novel compounds, NMR's comprehensive data justifies its higher cost through reduced false positives and more definitive structural assignments.
Multi-year cost projections reveal that while Raman systems offer lower initial and operational costs, NMR provides superior long-term value for organizations requiring comprehensive structural information. Hybrid approaches, where Raman serves as a rapid screening tool with NMR reserved for complex determinations, often yield optimal financial efficiency, reducing overall analytical costs by 25-40% compared to exclusive NMR usage.
For organizations with limited capital budgets, service-based models present viable alternatives. Outsourced NMR services average $200-500 per sample, while Raman analysis typically costs $75-150 per sample, allowing smaller organizations to access both technologies without capital investment while maintaining analytical capabilities.
Sample Preparation Requirements and Limitations
Sample preparation represents a critical factor when comparing Raman spectroscopy and Nuclear Magnetic Resonance (NMR) for structural elucidation applications. These techniques differ significantly in their requirements, which directly impacts their accessibility, cost-effectiveness, and suitability for various research contexts.
Raman spectroscopy generally offers more straightforward sample preparation protocols. Samples can be analyzed in solid, liquid, or gas phases with minimal processing. For solid samples, materials can often be placed directly under the microscope without extensive preparation. Liquid samples typically require only a small volume (microliters) in appropriate containers like cuvettes or on slides. Additionally, Raman allows for non-destructive in situ measurements, enabling analysis through transparent packaging or containers, which proves advantageous for sensitive or valuable samples.
However, Raman spectroscopy does present certain limitations. Fluorescence interference remains a significant challenge, particularly with biological samples or those containing fluorophores. This often necessitates additional preparation steps such as photobleaching or using different excitation wavelengths. Sample homogeneity is also crucial, as Raman typically analyzes microscopic areas, potentially leading to sampling errors with heterogeneous materials.
NMR spectroscopy imposes more demanding sample preparation requirements. Samples generally must be in solution form, requiring appropriate deuterated solvents that can significantly increase costs. Sample quantities needed for conventional NMR are substantially higher than for Raman, typically requiring milligrams to grams of material, though advances in microcoil and cryoprobe technologies have reduced this requirement somewhat. Sample purity is particularly critical for NMR, as impurities can generate confounding signals that complicate interpretation.
The time factor also differentiates these techniques. NMR sample preparation often involves dissolution, filtration, and transfer to specialized tubes, followed by instrument setup procedures including shimming and tuning. Conversely, Raman sample preparation can be completed in minutes, allowing for higher throughput analysis.
Environmental sensitivity presents another consideration. NMR samples must be maintained at specific temperatures during analysis, while Raman spectroscopy offers greater flexibility regarding environmental conditions. Furthermore, NMR sample recovery can be challenging due to solvent interactions, whereas Raman's non-destructive nature typically allows complete sample recovery.
These preparation differences significantly influence technique selection based on sample availability, time constraints, and required information depth. The minimal preparation requirements of Raman make it advantageous for rapid screening or precious samples, while NMR's more involved preparation is justified by its unparalleled structural information yield.
Raman spectroscopy generally offers more straightforward sample preparation protocols. Samples can be analyzed in solid, liquid, or gas phases with minimal processing. For solid samples, materials can often be placed directly under the microscope without extensive preparation. Liquid samples typically require only a small volume (microliters) in appropriate containers like cuvettes or on slides. Additionally, Raman allows for non-destructive in situ measurements, enabling analysis through transparent packaging or containers, which proves advantageous for sensitive or valuable samples.
However, Raman spectroscopy does present certain limitations. Fluorescence interference remains a significant challenge, particularly with biological samples or those containing fluorophores. This often necessitates additional preparation steps such as photobleaching or using different excitation wavelengths. Sample homogeneity is also crucial, as Raman typically analyzes microscopic areas, potentially leading to sampling errors with heterogeneous materials.
NMR spectroscopy imposes more demanding sample preparation requirements. Samples generally must be in solution form, requiring appropriate deuterated solvents that can significantly increase costs. Sample quantities needed for conventional NMR are substantially higher than for Raman, typically requiring milligrams to grams of material, though advances in microcoil and cryoprobe technologies have reduced this requirement somewhat. Sample purity is particularly critical for NMR, as impurities can generate confounding signals that complicate interpretation.
The time factor also differentiates these techniques. NMR sample preparation often involves dissolution, filtration, and transfer to specialized tubes, followed by instrument setup procedures including shimming and tuning. Conversely, Raman sample preparation can be completed in minutes, allowing for higher throughput analysis.
Environmental sensitivity presents another consideration. NMR samples must be maintained at specific temperatures during analysis, while Raman spectroscopy offers greater flexibility regarding environmental conditions. Furthermore, NMR sample recovery can be challenging due to solvent interactions, whereas Raman's non-destructive nature typically allows complete sample recovery.
These preparation differences significantly influence technique selection based on sample availability, time constraints, and required information depth. The minimal preparation requirements of Raman make it advantageous for rapid screening or precious samples, while NMR's more involved preparation is justified by its unparalleled structural information yield.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!