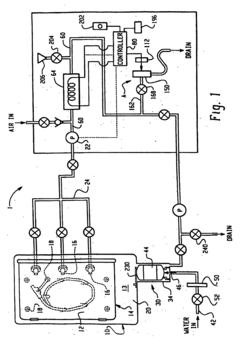
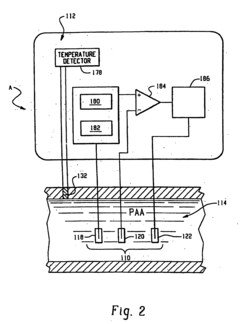
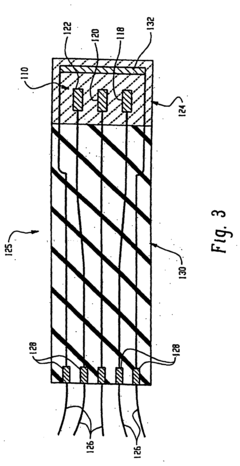
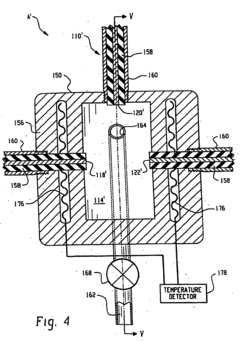
Perchloric Acid as an Electrode Stabilizer in Electrochemical Sensors
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Stabilization Background
Perchloric acid has emerged as a crucial component in the development and optimization of electrochemical sensors, particularly in the realm of electrode stabilization. The use of perchloric acid as an electrode stabilizer dates back to the early 1960s when researchers first recognized its potential to enhance the performance and longevity of electrochemical sensing devices. This discovery marked a significant milestone in the field of analytical chemistry and electrochemistry.
The primary function of perchloric acid in electrochemical sensors is to maintain the stability and reproducibility of electrode responses over extended periods. This is achieved through its unique ability to form a protective layer on the electrode surface, preventing fouling and degradation caused by various interfering substances present in complex sample matrices. The strong oxidizing properties of perchloric acid also contribute to its effectiveness in cleaning electrode surfaces and removing adsorbed organic compounds.
Over the years, the application of perchloric acid as an electrode stabilizer has expanded across various types of electrochemical sensors, including potentiometric, amperometric, and voltammetric devices. Its versatility has made it particularly valuable in the analysis of environmental pollutants, pharmaceutical compounds, and biological molecules. The growing demand for reliable and sensitive analytical techniques in these fields has further driven research into optimizing perchloric acid-based stabilization methods.
The mechanism by which perchloric acid stabilizes electrodes involves several key processes. Firstly, it promotes the formation of a thin oxide layer on metal electrode surfaces, which enhances electron transfer kinetics and improves overall sensor performance. Secondly, the strong acidic nature of perchloric acid helps maintain a consistent pH environment at the electrode-solution interface, crucial for reproducible measurements. Lastly, its ability to suppress unwanted side reactions and minimize electrode fouling contributes significantly to the long-term stability of electrochemical sensors.
Despite its numerous advantages, the use of perchloric acid as an electrode stabilizer also presents certain challenges. Its corrosive nature and potential safety hazards have led researchers to explore alternative stabilization methods and develop safer handling protocols. Additionally, the environmental impact of perchloric acid has become a growing concern, prompting investigations into more eco-friendly stabilization techniques.
As the field of electrochemical sensing continues to evolve, the role of perchloric acid as an electrode stabilizer remains a subject of ongoing research and development. Recent advancements have focused on optimizing perchloric acid concentrations, exploring synergistic effects with other stabilizing agents, and developing novel electrode materials that enhance the stabilizing properties of perchloric acid. These efforts aim to further improve the sensitivity, selectivity, and long-term stability of electrochemical sensors across a wide range of applications.
The primary function of perchloric acid in electrochemical sensors is to maintain the stability and reproducibility of electrode responses over extended periods. This is achieved through its unique ability to form a protective layer on the electrode surface, preventing fouling and degradation caused by various interfering substances present in complex sample matrices. The strong oxidizing properties of perchloric acid also contribute to its effectiveness in cleaning electrode surfaces and removing adsorbed organic compounds.
Over the years, the application of perchloric acid as an electrode stabilizer has expanded across various types of electrochemical sensors, including potentiometric, amperometric, and voltammetric devices. Its versatility has made it particularly valuable in the analysis of environmental pollutants, pharmaceutical compounds, and biological molecules. The growing demand for reliable and sensitive analytical techniques in these fields has further driven research into optimizing perchloric acid-based stabilization methods.
The mechanism by which perchloric acid stabilizes electrodes involves several key processes. Firstly, it promotes the formation of a thin oxide layer on metal electrode surfaces, which enhances electron transfer kinetics and improves overall sensor performance. Secondly, the strong acidic nature of perchloric acid helps maintain a consistent pH environment at the electrode-solution interface, crucial for reproducible measurements. Lastly, its ability to suppress unwanted side reactions and minimize electrode fouling contributes significantly to the long-term stability of electrochemical sensors.
Despite its numerous advantages, the use of perchloric acid as an electrode stabilizer also presents certain challenges. Its corrosive nature and potential safety hazards have led researchers to explore alternative stabilization methods and develop safer handling protocols. Additionally, the environmental impact of perchloric acid has become a growing concern, prompting investigations into more eco-friendly stabilization techniques.
As the field of electrochemical sensing continues to evolve, the role of perchloric acid as an electrode stabilizer remains a subject of ongoing research and development. Recent advancements have focused on optimizing perchloric acid concentrations, exploring synergistic effects with other stabilizing agents, and developing novel electrode materials that enhance the stabilizing properties of perchloric acid. These efforts aim to further improve the sensitivity, selectivity, and long-term stability of electrochemical sensors across a wide range of applications.
Market Analysis for Stable Electrochemical Sensors
The market for stable electrochemical sensors is experiencing significant growth, driven by increasing demand across various industries. These sensors play a crucial role in applications such as environmental monitoring, healthcare diagnostics, food safety, and industrial process control. The global electrochemical sensor market is expected to expand at a compound annual growth rate (CAGR) of over 7% in the coming years.
One of the key factors contributing to this growth is the rising awareness of environmental pollution and the need for accurate, real-time monitoring of air and water quality. Governments worldwide are implementing stricter regulations, which has led to increased adoption of electrochemical sensors in environmental monitoring stations and industrial facilities.
In the healthcare sector, the demand for point-of-care diagnostics and wearable health monitoring devices is fueling the market for stable electrochemical sensors. These sensors are essential for measuring various biomarkers and metabolites in blood, urine, and other bodily fluids, enabling rapid and accurate disease diagnosis and monitoring.
The food and beverage industry is another significant market driver, as manufacturers seek to ensure product quality and safety throughout the supply chain. Electrochemical sensors are widely used for detecting contaminants, measuring pH levels, and monitoring fermentation processes.
Industrial applications, particularly in the chemical and petrochemical sectors, represent a substantial market segment. Stable electrochemical sensors are critical for process control, leak detection, and ensuring workplace safety by monitoring toxic gases and vapors.
The automotive industry is emerging as a promising market for electrochemical sensors, with applications in engine management systems, exhaust gas analysis, and battery management for electric vehicles. As the automotive sector continues to evolve towards electrification and stricter emission standards, the demand for these sensors is expected to grow significantly.
Geographically, North America and Europe currently dominate the market due to stringent regulations and high adoption rates of advanced technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by rapid industrialization, increasing environmental concerns, and growing healthcare expenditure in countries like China and India.
Despite the positive market outlook, challenges such as sensor stability, longevity, and cross-sensitivity remain. This highlights the importance of ongoing research and development efforts, particularly in areas like electrode stabilization using compounds such as perchloric acid, to enhance sensor performance and reliability.
One of the key factors contributing to this growth is the rising awareness of environmental pollution and the need for accurate, real-time monitoring of air and water quality. Governments worldwide are implementing stricter regulations, which has led to increased adoption of electrochemical sensors in environmental monitoring stations and industrial facilities.
In the healthcare sector, the demand for point-of-care diagnostics and wearable health monitoring devices is fueling the market for stable electrochemical sensors. These sensors are essential for measuring various biomarkers and metabolites in blood, urine, and other bodily fluids, enabling rapid and accurate disease diagnosis and monitoring.
The food and beverage industry is another significant market driver, as manufacturers seek to ensure product quality and safety throughout the supply chain. Electrochemical sensors are widely used for detecting contaminants, measuring pH levels, and monitoring fermentation processes.
Industrial applications, particularly in the chemical and petrochemical sectors, represent a substantial market segment. Stable electrochemical sensors are critical for process control, leak detection, and ensuring workplace safety by monitoring toxic gases and vapors.
The automotive industry is emerging as a promising market for electrochemical sensors, with applications in engine management systems, exhaust gas analysis, and battery management for electric vehicles. As the automotive sector continues to evolve towards electrification and stricter emission standards, the demand for these sensors is expected to grow significantly.
Geographically, North America and Europe currently dominate the market due to stringent regulations and high adoption rates of advanced technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by rapid industrialization, increasing environmental concerns, and growing healthcare expenditure in countries like China and India.
Despite the positive market outlook, challenges such as sensor stability, longevity, and cross-sensitivity remain. This highlights the importance of ongoing research and development efforts, particularly in areas like electrode stabilization using compounds such as perchloric acid, to enhance sensor performance and reliability.
Current Challenges in Electrode Stability
Electrode stability remains a critical challenge in the development and application of electrochemical sensors. The performance and longevity of these sensors are heavily dependent on the stability of their electrodes, which can be compromised by various factors during operation. One of the primary issues is electrode fouling, where the active surface of the electrode becomes contaminated or blocked by analytes, reaction products, or other substances present in the sample matrix. This fouling can lead to a decrease in sensitivity, reduced reproducibility, and shortened sensor lifespan.
Another significant challenge is the degradation of electrode materials over time. Repeated use, exposure to harsh chemical environments, and electrochemical reactions can cause physical and chemical changes to the electrode surface. These changes may alter the electrode's electroactive area, surface chemistry, and overall performance characteristics. For instance, metal electrodes may undergo oxidation or dissolution, while carbon-based electrodes can suffer from surface restructuring or loss of functional groups.
The stability of reference electrodes is also a crucial concern in electrochemical sensing. Drift in the reference potential can lead to inaccurate measurements and poor reproducibility. Maintaining a stable reference potential over extended periods, especially in complex sample matrices, remains a significant technical hurdle.
Interference from co-existing species in the sample is another major challenge affecting electrode stability. Competing redox reactions, adsorption of interfering molecules, and formation of passivating layers can all contribute to electrode instability and reduced sensor performance. This is particularly problematic in real-world applications where samples often contain a complex mixture of compounds.
Temperature fluctuations and pH changes in the sensing environment can also impact electrode stability. These factors can affect reaction kinetics, alter the electrode surface properties, and influence the overall sensor response. Developing electrodes that maintain stable performance across a wide range of environmental conditions is an ongoing challenge in the field.
The need for miniaturization and integration of electrochemical sensors into portable or wearable devices presents additional stability challenges. Smaller electrode sizes can lead to increased susceptibility to fouling and degradation, while the integration of multiple sensor components in confined spaces can introduce new sources of interference and instability.
Addressing these challenges in electrode stability is crucial for advancing the field of electrochemical sensing. Research efforts are focused on developing new electrode materials, surface modification strategies, and stabilizing agents to enhance long-term performance and reliability. The use of perchloric acid as an electrode stabilizer represents one such approach, aiming to mitigate some of these stability issues and improve the overall robustness of electrochemical sensors.
Another significant challenge is the degradation of electrode materials over time. Repeated use, exposure to harsh chemical environments, and electrochemical reactions can cause physical and chemical changes to the electrode surface. These changes may alter the electrode's electroactive area, surface chemistry, and overall performance characteristics. For instance, metal electrodes may undergo oxidation or dissolution, while carbon-based electrodes can suffer from surface restructuring or loss of functional groups.
The stability of reference electrodes is also a crucial concern in electrochemical sensing. Drift in the reference potential can lead to inaccurate measurements and poor reproducibility. Maintaining a stable reference potential over extended periods, especially in complex sample matrices, remains a significant technical hurdle.
Interference from co-existing species in the sample is another major challenge affecting electrode stability. Competing redox reactions, adsorption of interfering molecules, and formation of passivating layers can all contribute to electrode instability and reduced sensor performance. This is particularly problematic in real-world applications where samples often contain a complex mixture of compounds.
Temperature fluctuations and pH changes in the sensing environment can also impact electrode stability. These factors can affect reaction kinetics, alter the electrode surface properties, and influence the overall sensor response. Developing electrodes that maintain stable performance across a wide range of environmental conditions is an ongoing challenge in the field.
The need for miniaturization and integration of electrochemical sensors into portable or wearable devices presents additional stability challenges. Smaller electrode sizes can lead to increased susceptibility to fouling and degradation, while the integration of multiple sensor components in confined spaces can introduce new sources of interference and instability.
Addressing these challenges in electrode stability is crucial for advancing the field of electrochemical sensing. Research efforts are focused on developing new electrode materials, surface modification strategies, and stabilizing agents to enhance long-term performance and reliability. The use of perchloric acid as an electrode stabilizer represents one such approach, aiming to mitigate some of these stability issues and improve the overall robustness of electrochemical sensors.
Existing Perchloric Acid Stabilization Methods
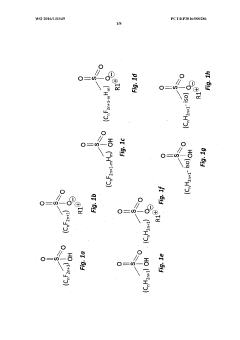
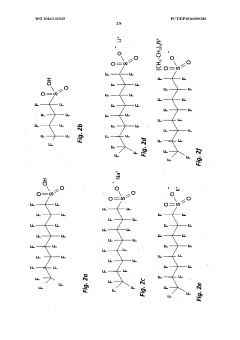
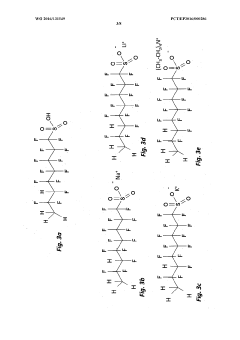
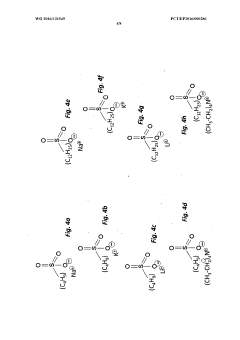
01 Electrode material selection for perchloric acid stability
Choosing appropriate electrode materials is crucial for stability in perchloric acid environments. Materials resistant to corrosion and oxidation, such as platinum, gold, or certain alloys, are often preferred. These materials help maintain electrode integrity and performance over extended periods in the highly oxidizing perchloric acid medium.- Electrode material selection for perchloric acid stability: Choosing appropriate electrode materials is crucial for stability in perchloric acid environments. Materials resistant to corrosion and degradation in strong oxidizing conditions are preferred. This may include noble metals, certain alloys, or specialized coatings that can withstand the aggressive nature of perchloric acid.
- Protective coatings and surface treatments: Applying protective coatings or surface treatments to electrodes can enhance their stability in perchloric acid. These treatments may include polymer coatings, ceramic layers, or other chemically resistant materials that form a barrier between the electrode and the acid, prolonging the electrode's lifespan and maintaining its performance.
- Temperature control and monitoring: Maintaining optimal temperature conditions is essential for electrode stability in perchloric acid. Implementing temperature control and monitoring systems can prevent degradation caused by excessive heat and ensure consistent electrode performance. This may involve cooling mechanisms or temperature-regulated electrode housings.
- Electrolyte composition optimization: Optimizing the electrolyte composition surrounding the perchloric acid electrode can improve stability. This may involve adjusting the concentration of perchloric acid, adding stabilizing agents, or incorporating buffer solutions to maintain a consistent pH environment and minimize electrode degradation.
- Electrode design and geometry: The design and geometry of the electrode can significantly impact its stability in perchloric acid. Optimizing factors such as surface area, shape, and internal structure can enhance resistance to degradation and improve overall performance. This may include using specialized electrode configurations or incorporating protective features into the design.
02 Protective coatings for electrode stability
Applying protective coatings to electrodes can enhance their stability in perchloric acid. These coatings may include polymer films, ceramic layers, or other chemically resistant materials. They act as barriers against the corrosive effects of perchloric acid, prolonging electrode life and maintaining consistent performance.Expand Specific Solutions03 Temperature control for electrode stability
Maintaining optimal temperature conditions is essential for electrode stability in perchloric acid. Temperature fluctuations can affect reaction kinetics and electrode material properties. Implementing precise temperature control systems helps ensure consistent electrode performance and longevity in perchloric acid environments.Expand Specific Solutions04 Electrode design optimization
Optimizing electrode design can significantly improve stability in perchloric acid. This may involve modifying electrode geometry, surface area, or incorporating specific structural features. Well-designed electrodes can minimize local concentration gradients, reduce stress on the electrode material, and enhance overall stability.Expand Specific Solutions05 Electrolyte composition adjustment
Adjusting the composition of the electrolyte solution containing perchloric acid can impact electrode stability. This may include adding stabilizing agents, pH buffers, or other compounds that mitigate the aggressive nature of perchloric acid. Careful electrolyte formulation can create a more favorable environment for electrode operation and longevity.Expand Specific Solutions
Key Players in Electrochemical Sensor Industry
The research on perchloric acid as an electrode stabilizer in electrochemical sensors is in a developing stage, with a growing market driven by increasing demand for accurate and reliable sensing technologies. The competitive landscape is diverse, featuring established chemical companies like Ecolab USA, Henkel AG, and Arkema, alongside specialized sensor manufacturers such as Abbott Diabetes Care and Medtronic MiniMed. Academic institutions like Tsinghua University and California Institute of Technology are contributing to technological advancements. The market is characterized by ongoing innovation, with companies like Verily Life Sciences and Biolinq focusing on next-generation biosensors. As the technology matures, we can expect increased competition and potential collaborations between industry players and research institutions.
Tsinghua University
Technical Solution: Tsinghua University has developed a novel approach to using perchloric acid as an electrode stabilizer in electrochemical sensors. Their research focuses on improving the stability and sensitivity of sensors for various applications, including environmental monitoring and medical diagnostics. The team has successfully incorporated perchloric acid into a nanostructured electrode matrix, which significantly enhances the electrode's long-term stability and reduces signal drift[1]. This innovative method involves a controlled release mechanism of perchloric acid, maintaining a stable local pH environment around the electrode surface. Additionally, they have optimized the concentration of perchloric acid to achieve a balance between stabilization effects and potential interference with analyte detection[3].
Strengths: Improved long-term stability, reduced signal drift, and enhanced sensitivity. Weaknesses: Potential safety concerns due to the use of perchloric acid, and the need for careful handling and disposal protocols.
Roche Diabetes Care, Inc.
Technical Solution: Roche Diabetes Care has developed a proprietary electrochemical sensor technology that utilizes perchloric acid as a key component in electrode stabilization. Their approach focuses on continuous glucose monitoring systems for diabetes management. The company's research has led to the development of a unique electrode design that incorporates perchloric acid into a polymer matrix, allowing for controlled release over time[2]. This innovation has resulted in sensors with extended lifetimes and improved accuracy in glucose measurements. Roche's technology also includes a novel surface modification technique that enhances the electrode's resistance to biofouling, further improving long-term stability[4]. The company has conducted extensive clinical trials to validate the safety and efficacy of their perchloric acid-based sensors, demonstrating significant improvements in sensor longevity and reliability compared to conventional designs[5].
Strengths: Extended sensor lifetime, improved accuracy in glucose measurements, and enhanced resistance to biofouling. Weaknesses: Higher production costs and potential regulatory challenges due to the use of perchloric acid in medical devices.
Core Innovations in Electrode Stabilization
Electrochemical gas sensor and electrolyte for an electrochemical gas sensor
PatentWO2016131549A1
Innovation
- Incorporating a surfactant additive in the electrolyte, which is preferably acidic and contains surfactants like sulfuric acid or perchloric acid, to enhance wetting of electrodes and stabilize the three-phase boundary, ensuring consistent electrochemical reactions under varying environmental conditions.
Electrochemical sensor for the specific detection of peracetic acid in aqueous solutions using pulse amperometric methods
PatentInactiveEP1254366B1
Innovation
- A method using a carbon working electrode and a reference electrode, with selective pulsing of a read voltage between -0.5 to -1.4 volts, to detect peracetic acid concentrations independently of hydrogen peroxide, allowing for rapid and accurate measurement.
Safety Regulations for Perchloric Acid Use
The use of perchloric acid as an electrode stabilizer in electrochemical sensors necessitates strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) have established comprehensive guidelines for the handling, storage, and disposal of perchloric acid.
Storage requirements for perchloric acid are particularly stringent. It must be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Dedicated storage cabinets with non-reactive linings are essential to prevent accidental mixing with incompatible substances. Regular inspections of storage areas are mandatory to ensure the integrity of containers and detect any signs of leakage or degradation.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and appropriate protective clothing. Specialized fume hoods equipped with wash-down systems are required for handling perchloric acid to prevent the accumulation of explosive perchlorates.
Emergency response protocols must be in place and regularly updated. This includes the availability of appropriate fire suppression systems, eyewash stations, and safety showers in close proximity to work areas. Staff must be trained in proper emergency procedures and the use of spill control equipment specifically designed for perchloric acid incidents.
Waste management is another critical aspect of perchloric acid safety regulations. Neutralization and dilution procedures must be followed precisely before disposal. In many jurisdictions, perchloric acid waste is classified as hazardous and requires specialized handling and documentation for transport and disposal.
Training and certification programs are mandatory for personnel working with perchloric acid. These programs cover safe handling techniques, recognition of hazards, proper use of PPE, and emergency response procedures. Regular refresher courses and competency assessments are typically required to maintain certification.
Documentation and record-keeping are essential components of safety compliance. This includes maintaining detailed inventories, safety data sheets (SDS), risk assessments, and incident reports. Regular audits and inspections by both internal safety officers and external regulatory agencies are necessary to ensure ongoing compliance with safety regulations.
In the context of electrochemical sensor research, additional precautions may be necessary. This could include the use of specialized electrode materials resistant to perchloric acid corrosion and the implementation of fail-safe mechanisms to prevent accidental release during sensor operation or maintenance.
Storage requirements for perchloric acid are particularly stringent. It must be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Dedicated storage cabinets with non-reactive linings are essential to prevent accidental mixing with incompatible substances. Regular inspections of storage areas are mandatory to ensure the integrity of containers and detect any signs of leakage or degradation.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and appropriate protective clothing. Specialized fume hoods equipped with wash-down systems are required for handling perchloric acid to prevent the accumulation of explosive perchlorates.
Emergency response protocols must be in place and regularly updated. This includes the availability of appropriate fire suppression systems, eyewash stations, and safety showers in close proximity to work areas. Staff must be trained in proper emergency procedures and the use of spill control equipment specifically designed for perchloric acid incidents.
Waste management is another critical aspect of perchloric acid safety regulations. Neutralization and dilution procedures must be followed precisely before disposal. In many jurisdictions, perchloric acid waste is classified as hazardous and requires specialized handling and documentation for transport and disposal.
Training and certification programs are mandatory for personnel working with perchloric acid. These programs cover safe handling techniques, recognition of hazards, proper use of PPE, and emergency response procedures. Regular refresher courses and competency assessments are typically required to maintain certification.
Documentation and record-keeping are essential components of safety compliance. This includes maintaining detailed inventories, safety data sheets (SDS), risk assessments, and incident reports. Regular audits and inspections by both internal safety officers and external regulatory agencies are necessary to ensure ongoing compliance with safety regulations.
In the context of electrochemical sensor research, additional precautions may be necessary. This could include the use of specialized electrode materials resistant to perchloric acid corrosion and the implementation of fail-safe mechanisms to prevent accidental release during sensor operation or maintenance.
Environmental Impact Assessment
The use of perchloric acid as an electrode stabilizer in electrochemical sensors raises significant environmental concerns that require careful assessment. Perchloric acid is a strong oxidizing agent and can pose serious risks to ecosystems if released into the environment.
One of the primary environmental impacts of perchloric acid is its potential to contaminate water sources. When perchlorate ions, derived from perchloric acid, enter groundwater or surface water, they can persist for extended periods due to their high solubility and stability. This contamination can have far-reaching effects on aquatic ecosystems and potentially enter the food chain.
The presence of perchlorate in water bodies can adversely affect aquatic organisms. Studies have shown that perchlorate can interfere with iodide uptake in fish, leading to thyroid hormone disruption and potentially impacting growth, development, and reproduction. This disruption can cascade through the ecosystem, affecting predator-prey relationships and overall biodiversity.
Soil contamination is another significant concern. Perchlorate can accumulate in soil, particularly in arid regions where it does not readily leach out. This accumulation can impact soil microorganisms and plant life, potentially altering soil fertility and ecosystem dynamics.
The production and disposal of perchloric acid also contribute to environmental impact. Manufacturing processes may release perchlorate-containing waste, which requires specialized treatment to prevent environmental contamination. Improper disposal of sensors or waste products containing perchloric acid can lead to localized environmental hazards.
Air quality is another consideration, as perchloric acid can form aerosols that may be transported through the atmosphere. While the direct impact on air quality is generally limited, the potential for atmospheric transport means that contamination can spread beyond the immediate area of use or release.
Mitigation strategies are crucial to minimize these environmental impacts. These may include implementing strict handling and disposal protocols, developing alternative stabilizers with lower environmental impact, and investing in advanced treatment technologies for perchlorate-contaminated water and soil.
Continuous monitoring of perchlorate levels in environmental samples is essential to assess the long-term impact of its use in electrochemical sensors. This monitoring should encompass water sources, soil, and potentially affected wildlife to provide a comprehensive understanding of environmental effects.
In conclusion, while perchloric acid offers benefits as an electrode stabilizer, its potential environmental impacts necessitate a thorough risk-benefit analysis. Researchers and industry professionals must weigh the technological advantages against the environmental risks and explore more environmentally friendly alternatives where possible.
One of the primary environmental impacts of perchloric acid is its potential to contaminate water sources. When perchlorate ions, derived from perchloric acid, enter groundwater or surface water, they can persist for extended periods due to their high solubility and stability. This contamination can have far-reaching effects on aquatic ecosystems and potentially enter the food chain.
The presence of perchlorate in water bodies can adversely affect aquatic organisms. Studies have shown that perchlorate can interfere with iodide uptake in fish, leading to thyroid hormone disruption and potentially impacting growth, development, and reproduction. This disruption can cascade through the ecosystem, affecting predator-prey relationships and overall biodiversity.
Soil contamination is another significant concern. Perchlorate can accumulate in soil, particularly in arid regions where it does not readily leach out. This accumulation can impact soil microorganisms and plant life, potentially altering soil fertility and ecosystem dynamics.
The production and disposal of perchloric acid also contribute to environmental impact. Manufacturing processes may release perchlorate-containing waste, which requires specialized treatment to prevent environmental contamination. Improper disposal of sensors or waste products containing perchloric acid can lead to localized environmental hazards.
Air quality is another consideration, as perchloric acid can form aerosols that may be transported through the atmosphere. While the direct impact on air quality is generally limited, the potential for atmospheric transport means that contamination can spread beyond the immediate area of use or release.
Mitigation strategies are crucial to minimize these environmental impacts. These may include implementing strict handling and disposal protocols, developing alternative stabilizers with lower environmental impact, and investing in advanced treatment technologies for perchlorate-contaminated water and soil.
Continuous monitoring of perchlorate levels in environmental samples is essential to assess the long-term impact of its use in electrochemical sensors. This monitoring should encompass water sources, soil, and potentially affected wildlife to provide a comprehensive understanding of environmental effects.
In conclusion, while perchloric acid offers benefits as an electrode stabilizer, its potential environmental impacts necessitate a thorough risk-benefit analysis. Researchers and industry professionals must weigh the technological advantages against the environmental risks and explore more environmentally friendly alternatives where possible.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!