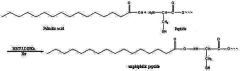
Sulphanilic Acid as a Moderator in Nanostructure Self-Assembly
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid in Nanostructure Self-Assembly: Background
Sulphanilic acid, a versatile organic compound, has emerged as a significant player in the field of nanostructure self-assembly. This aromatic molecule, characterized by its sulfonic acid and amino functional groups, has garnered increasing attention from researchers due to its unique properties and potential applications in nanotechnology.
The journey of sulphanilic acid in nanostructure self-assembly began in the early 2000s when scientists started exploring its potential as a molecular building block. Its ability to form hydrogen bonds and engage in π-π stacking interactions made it an attractive candidate for creating complex nanostructures. The compound's amphiphilic nature, stemming from its hydrophilic sulfonic acid group and relatively hydrophobic aromatic ring, further enhanced its self-assembly capabilities.
Over the past two decades, the role of sulphanilic acid in nanostructure formation has evolved significantly. Initially used primarily as a simple molecular component, it has now become recognized as a powerful moderator in self-assembly processes. This shift in perspective has opened up new avenues for controlling and fine-tuning the assembly of nanomaterials.
The growing interest in sulphanilic acid for nanostructure self-assembly is driven by several factors. Firstly, its biocompatibility and low toxicity make it an attractive option for biomedical applications. Secondly, its ability to form stable structures under various environmental conditions enhances its versatility. Lastly, the ease of functionalization of sulphanilic acid allows for the creation of a wide range of derivatives, each with unique self-assembly properties.
Recent advancements in analytical techniques, particularly in high-resolution microscopy and spectroscopy, have greatly contributed to our understanding of sulphanilic acid's behavior in nanostructure formation. These tools have enabled researchers to observe and manipulate self-assembly processes at the molecular level, leading to more precise control over the resulting nanostructures.
The current research landscape focuses on exploiting sulphanilic acid's moderating effects to create increasingly complex and functional nanostructures. This includes the development of smart materials, drug delivery systems, and novel catalysts. The compound's ability to respond to external stimuli such as pH, temperature, and light has also sparked interest in creating responsive and adaptive nanomaterials.
As we look towards the future, the potential applications of sulphanilic acid in nanostructure self-assembly continue to expand. From advanced electronics to environmental remediation, the versatility of this compound promises to play a crucial role in addressing some of the most pressing technological challenges of our time.
The journey of sulphanilic acid in nanostructure self-assembly began in the early 2000s when scientists started exploring its potential as a molecular building block. Its ability to form hydrogen bonds and engage in π-π stacking interactions made it an attractive candidate for creating complex nanostructures. The compound's amphiphilic nature, stemming from its hydrophilic sulfonic acid group and relatively hydrophobic aromatic ring, further enhanced its self-assembly capabilities.
Over the past two decades, the role of sulphanilic acid in nanostructure formation has evolved significantly. Initially used primarily as a simple molecular component, it has now become recognized as a powerful moderator in self-assembly processes. This shift in perspective has opened up new avenues for controlling and fine-tuning the assembly of nanomaterials.
The growing interest in sulphanilic acid for nanostructure self-assembly is driven by several factors. Firstly, its biocompatibility and low toxicity make it an attractive option for biomedical applications. Secondly, its ability to form stable structures under various environmental conditions enhances its versatility. Lastly, the ease of functionalization of sulphanilic acid allows for the creation of a wide range of derivatives, each with unique self-assembly properties.
Recent advancements in analytical techniques, particularly in high-resolution microscopy and spectroscopy, have greatly contributed to our understanding of sulphanilic acid's behavior in nanostructure formation. These tools have enabled researchers to observe and manipulate self-assembly processes at the molecular level, leading to more precise control over the resulting nanostructures.
The current research landscape focuses on exploiting sulphanilic acid's moderating effects to create increasingly complex and functional nanostructures. This includes the development of smart materials, drug delivery systems, and novel catalysts. The compound's ability to respond to external stimuli such as pH, temperature, and light has also sparked interest in creating responsive and adaptive nanomaterials.
As we look towards the future, the potential applications of sulphanilic acid in nanostructure self-assembly continue to expand. From advanced electronics to environmental remediation, the versatility of this compound promises to play a crucial role in addressing some of the most pressing technological challenges of our time.
Market Analysis for Nanostructure Applications
The market for nanostructure applications is experiencing rapid growth and diversification across multiple industries. The use of sulphanilic acid as a moderator in nanostructure self-assembly has opened up new possibilities for creating advanced materials with tailored properties. This innovation has significant implications for sectors such as electronics, healthcare, energy, and environmental remediation.
In the electronics industry, nanostructures facilitated by sulphanilic acid moderation are showing promise in the development of more efficient and compact devices. The ability to control the self-assembly process at the nanoscale allows for the creation of materials with enhanced electrical and optical properties. This has potential applications in the production of next-generation semiconductors, displays, and sensors.
The healthcare sector is another area where nanostructure applications are gaining traction. The precise control over nanostructure formation enabled by sulphanilic acid moderation is particularly valuable in drug delivery systems and diagnostic tools. These nanostructures can be designed to target specific cells or tissues, improving the efficacy of treatments while reducing side effects.
In the energy sector, nanostructures are being explored for their potential to enhance the performance of solar cells, batteries, and fuel cells. The use of sulphanilic acid in the self-assembly process allows for the creation of materials with increased surface area and improved catalytic properties, which can lead to more efficient energy conversion and storage devices.
Environmental applications of nanostructures are also gaining attention. The ability to create highly specific and reactive surfaces through controlled self-assembly has implications for water purification, air filtration, and pollutant remediation. Nanostructures moderated by sulphanilic acid could potentially offer more effective and sustainable solutions to environmental challenges.
The market demand for these nanostructure applications is driven by several factors. Firstly, there is a growing need for miniaturization and increased functionality in consumer electronics. Secondly, the healthcare industry is constantly seeking more targeted and effective treatments. Thirdly, the global push for renewable energy and energy efficiency is creating opportunities for advanced materials. Lastly, environmental concerns are spurring the development of more effective remediation technologies.
As research on sulphanilic acid as a moderator in nanostructure self-assembly progresses, it is expected to unlock new market opportunities. The ability to fine-tune material properties at the nanoscale could lead to the development of novel products and solutions across various industries. This technology has the potential to disrupt existing markets and create entirely new ones, particularly in areas where precise control over material structure and properties is crucial.
In the electronics industry, nanostructures facilitated by sulphanilic acid moderation are showing promise in the development of more efficient and compact devices. The ability to control the self-assembly process at the nanoscale allows for the creation of materials with enhanced electrical and optical properties. This has potential applications in the production of next-generation semiconductors, displays, and sensors.
The healthcare sector is another area where nanostructure applications are gaining traction. The precise control over nanostructure formation enabled by sulphanilic acid moderation is particularly valuable in drug delivery systems and diagnostic tools. These nanostructures can be designed to target specific cells or tissues, improving the efficacy of treatments while reducing side effects.
In the energy sector, nanostructures are being explored for their potential to enhance the performance of solar cells, batteries, and fuel cells. The use of sulphanilic acid in the self-assembly process allows for the creation of materials with increased surface area and improved catalytic properties, which can lead to more efficient energy conversion and storage devices.
Environmental applications of nanostructures are also gaining attention. The ability to create highly specific and reactive surfaces through controlled self-assembly has implications for water purification, air filtration, and pollutant remediation. Nanostructures moderated by sulphanilic acid could potentially offer more effective and sustainable solutions to environmental challenges.
The market demand for these nanostructure applications is driven by several factors. Firstly, there is a growing need for miniaturization and increased functionality in consumer electronics. Secondly, the healthcare industry is constantly seeking more targeted and effective treatments. Thirdly, the global push for renewable energy and energy efficiency is creating opportunities for advanced materials. Lastly, environmental concerns are spurring the development of more effective remediation technologies.
As research on sulphanilic acid as a moderator in nanostructure self-assembly progresses, it is expected to unlock new market opportunities. The ability to fine-tune material properties at the nanoscale could lead to the development of novel products and solutions across various industries. This technology has the potential to disrupt existing markets and create entirely new ones, particularly in areas where precise control over material structure and properties is crucial.
Current Challenges in Nanostructure Self-Assembly
Nanostructure self-assembly has emerged as a powerful approach for creating complex and functional materials at the nanoscale. However, several challenges persist in this field, hindering its widespread application and commercialization. One of the primary obstacles is the lack of precise control over the assembly process, which often results in defects and inconsistencies in the final structures.
The inherent complexity of molecular interactions and the sensitivity of self-assembly processes to environmental conditions pose significant challenges. Slight variations in temperature, pH, or concentration can lead to dramatic changes in the resulting nanostructures. This sensitivity makes it difficult to achieve reproducible results and scale up production for industrial applications.
Another major challenge is the limited understanding of the fundamental mechanisms governing self-assembly at the molecular level. While researchers have made significant progress in recent years, many aspects of the process remain poorly understood. This knowledge gap hampers the ability to design and predict the formation of desired nanostructures with specific properties and functionalities.
The integration of multiple components with different chemical and physical properties into a single self-assembled system presents additional challenges. Achieving precise spatial organization and maintaining the stability of these complex structures often requires intricate balancing of various intermolecular forces.
Furthermore, the development of robust and versatile characterization techniques for analyzing self-assembled nanostructures in real-time and under various conditions remains a significant challenge. Current methods often provide limited information or require sample preparation that may alter the original structure.
The use of sulphanilic acid as a moderator in nanostructure self-assembly introduces its own set of challenges. While sulphanilic acid shows promise in influencing the assembly process, controlling its interactions with other components and understanding its exact role in the formation of nanostructures requires further investigation.
Achieving long-range order and minimizing defects in self-assembled nanostructures continues to be a significant hurdle. The presence of defects can dramatically affect the properties and performance of the resulting materials, limiting their potential applications in areas such as electronics, photonics, and energy storage.
Lastly, bridging the gap between laboratory-scale synthesis and industrial-scale production of self-assembled nanostructures remains a formidable challenge. Developing scalable and cost-effective manufacturing processes while maintaining the quality and uniformity of the nanostructures is crucial for the commercial viability of this technology.
The inherent complexity of molecular interactions and the sensitivity of self-assembly processes to environmental conditions pose significant challenges. Slight variations in temperature, pH, or concentration can lead to dramatic changes in the resulting nanostructures. This sensitivity makes it difficult to achieve reproducible results and scale up production for industrial applications.
Another major challenge is the limited understanding of the fundamental mechanisms governing self-assembly at the molecular level. While researchers have made significant progress in recent years, many aspects of the process remain poorly understood. This knowledge gap hampers the ability to design and predict the formation of desired nanostructures with specific properties and functionalities.
The integration of multiple components with different chemical and physical properties into a single self-assembled system presents additional challenges. Achieving precise spatial organization and maintaining the stability of these complex structures often requires intricate balancing of various intermolecular forces.
Furthermore, the development of robust and versatile characterization techniques for analyzing self-assembled nanostructures in real-time and under various conditions remains a significant challenge. Current methods often provide limited information or require sample preparation that may alter the original structure.
The use of sulphanilic acid as a moderator in nanostructure self-assembly introduces its own set of challenges. While sulphanilic acid shows promise in influencing the assembly process, controlling its interactions with other components and understanding its exact role in the formation of nanostructures requires further investigation.
Achieving long-range order and minimizing defects in self-assembled nanostructures continues to be a significant hurdle. The presence of defects can dramatically affect the properties and performance of the resulting materials, limiting their potential applications in areas such as electronics, photonics, and energy storage.
Lastly, bridging the gap between laboratory-scale synthesis and industrial-scale production of self-assembled nanostructures remains a formidable challenge. Developing scalable and cost-effective manufacturing processes while maintaining the quality and uniformity of the nanostructures is crucial for the commercial viability of this technology.
Existing Sulphanilic Acid Moderation Methods
01 Synthesis and purification of sulphanilic acid
Various methods for synthesizing and purifying sulphanilic acid are described. These processes involve different reaction conditions, catalysts, and purification techniques to obtain high-quality sulphanilic acid suitable for self-assembly applications.- Synthesis and purification of sulphanilic acid: Various methods for synthesizing and purifying sulphanilic acid are described. These processes involve different reaction conditions, catalysts, and purification techniques to obtain high-quality sulphanilic acid suitable for self-assembly applications.
- Self-assembly of sulphanilic acid derivatives: Research on the self-assembly behavior of sulphanilic acid derivatives, including their ability to form supramolecular structures. This involves studying the intermolecular interactions, assembly conditions, and resulting nanostructures formed by these compounds.
- Applications of sulphanilic acid self-assemblies: Exploration of potential applications for sulphanilic acid self-assemblies in various fields such as materials science, nanotechnology, and biomedicine. This includes their use in drug delivery systems, sensors, and functional materials.
- Characterization techniques for sulphanilic acid self-assemblies: Methods and instruments used to analyze and characterize the structure, properties, and behavior of sulphanilic acid self-assemblies. This may include spectroscopic techniques, microscopy, and other analytical methods to study their morphology and interactions.
- Factors influencing sulphanilic acid self-assembly: Investigation of various factors that affect the self-assembly process of sulphanilic acid, such as pH, temperature, concentration, and the presence of additives or co-solvents. Understanding these factors helps in controlling and optimizing the self-assembly process.
02 Self-assembly of sulphanilic acid derivatives
Research on the self-assembly behavior of sulphanilic acid derivatives, including their ability to form supramolecular structures. This involves studying the intermolecular interactions, assembly conditions, and resulting nanostructures formed by these compounds.Expand Specific Solutions03 Applications of sulphanilic acid self-assemblies
Exploration of potential applications for sulphanilic acid self-assemblies in various fields such as materials science, nanotechnology, and biomedicine. This includes their use in drug delivery systems, sensors, and functional materials.Expand Specific Solutions04 Characterization techniques for sulphanilic acid self-assemblies
Methods and instruments used to analyze and characterize the structure, properties, and behavior of sulphanilic acid self-assemblies. This may include spectroscopic techniques, microscopy, and computational modeling.Expand Specific Solutions05 Factors influencing sulphanilic acid self-assembly
Investigation of various factors that affect the self-assembly process of sulphanilic acid, such as pH, temperature, concentration, and the presence of additives or co-solvents. Understanding these factors is crucial for controlling and optimizing the self-assembly process.Expand Specific Solutions
Key Players in Nanostructure Research
The research on sulphanilic acid as a moderator in nanostructure self-assembly is in an early developmental stage, with significant potential for growth. The market size is currently limited but expected to expand as applications in nanotechnology and materials science evolve. Leading academic institutions such as Harvard College, Northwestern University, and MIT are at the forefront of this research, indicating its cutting-edge nature. Companies like Xerox Holdings Corp. and Micron Technology, Inc. are also involved, suggesting growing industrial interest. The technology's maturity is still low, with most work concentrated in research laboratories, but collaborative efforts between academia and industry are likely to accelerate its development and potential commercialization in the coming years.
President & Fellows of Harvard College
Technical Solution: Harvard College has made significant contributions to the research on sulphanilic acid as a moderator in nanostructure self-assembly. Their approach involves using sulphanilic acid as a pH-responsive molecular switch to control the self-assembly process of nanostructures[1]. By exploiting the acid-base properties of sulphanilic acid, researchers have developed a method to precisely tune the formation and disassembly of nanostructures in response to pH changes[2]. This technique allows for the creation of smart materials with reversible properties, which can be applied in drug delivery systems, sensors, and adaptive materials[3]. Harvard's research also explores the use of sulphanilic acid in conjunction with other organic molecules to create complex, hierarchical nanostructures with tailored functionalities[4].
Strengths: Precise control over nanostructure formation, reversible assembly process, and potential for smart material applications. Weaknesses: May be limited to specific pH ranges and require careful environmental control for practical applications.
Massachusetts Institute of Technology
Technical Solution: MIT's research on sulphanilic acid as a moderator in nanostructure self-assembly focuses on leveraging its unique molecular structure to create novel nanomaterials. Their approach utilizes the amphiphilic nature of sulphanilic acid to direct the self-assembly of nanoparticles into ordered structures[5]. By carefully controlling the concentration and environmental conditions, MIT researchers have developed methods to create a wide range of nanostructures, including nanosheets, nanotubes, and 3D networks[6]. The team has also investigated the use of sulphanilic acid in combination with metal ions to form coordination complexes that can act as building blocks for more complex nanostructures[7]. This research has led to the development of new materials with applications in catalysis, energy storage, and environmental remediation[8].
Strengths: Versatility in creating diverse nanostructures, potential for multifunctional materials, and applications in various fields. Weaknesses: May require precise control of reaction conditions and could be sensitive to impurities or competing interactions.
Core Innovations in Self-Assembly Moderation
Self-assembly NANO-composites comprising hydrophilic bioactive peptides and hydrophobic materials
PatentWO2007094570A1
Innovation
- Self-assembled nanostructures formed by conjugates of hydrophilic bioactive peptides and hydrophobic materials, linked by amide bonds, which are applied at concentrations above the critical micelle concentration to stabilize attachment to biomaterials and enhance tissue regeneration.
Self-assembly of nucleic acid nanostructures
PatentActiveHK1210628A
Innovation
- Controlled synthesis of nucleic acid structures with predefined size and shape using self-assembly of single-stranded oligonucleotides.
- Precise control over the location of each oligonucleotide in the resultant structure, allowing for specific modifications.
- Creation of diverse synthetic nucleic acid nanostructures including lattices, ribbons, tubes, and 2D/3D objects with defined shapes.
Environmental Impact of Sulphanilic Acid Use
The use of sulphanilic acid as a moderator in nanostructure self-assembly processes raises important environmental considerations. While this compound offers significant benefits in controlling and directing the formation of nanoscale structures, its potential impact on ecosystems and human health must be carefully evaluated.
Sulphanilic acid, being an organic compound, can potentially interact with various environmental components. When released into aquatic environments, it may affect water quality and aquatic life. Studies have shown that sulphanilic acid can be biodegraded by certain microorganisms, which is a positive aspect for its environmental fate. However, the rate and extent of biodegradation can vary depending on environmental conditions, potentially leading to accumulation in some ecosystems.
The persistence of sulphanilic acid in soil environments is another area of concern. Its mobility in soil can lead to groundwater contamination if not properly managed. Research has indicated that sulphanilic acid can adsorb to soil particles, which may limit its spread but also makes it challenging to remove once contamination occurs.
From an atmospheric perspective, sulphanilic acid has low volatility, reducing the risk of air pollution. However, the production and handling of this compound may release particulates or vapors that could contribute to local air quality issues if not properly controlled.
The potential for bioaccumulation in food chains is relatively low for sulphanilic acid, which is a positive aspect of its environmental profile. However, its presence in water bodies could still impact sensitive aquatic species, particularly during critical life stages.
In terms of human health, exposure to sulphanilic acid through environmental contamination is a concern. While acute toxicity is generally low, chronic exposure effects are less well understood and require further investigation. Occupational exposure during the production and use of sulphanilic acid in nanostructure assembly processes necessitates strict safety protocols.
Waste management is a critical aspect of mitigating the environmental impact of sulphanilic acid use. Proper disposal methods, including chemical treatment or incineration, must be employed to prevent environmental release. Additionally, recycling and recovery techniques for sulphanilic acid from industrial processes could significantly reduce its environmental footprint.
As research in nanostructure self-assembly advances, there is a growing need for green chemistry approaches to minimize the environmental impact of sulphanilic acid. This includes exploring bio-based alternatives, optimizing reaction conditions to reduce the amount of sulphanilic acid required, and developing closed-loop systems that minimize waste generation and maximize resource efficiency.
Sulphanilic acid, being an organic compound, can potentially interact with various environmental components. When released into aquatic environments, it may affect water quality and aquatic life. Studies have shown that sulphanilic acid can be biodegraded by certain microorganisms, which is a positive aspect for its environmental fate. However, the rate and extent of biodegradation can vary depending on environmental conditions, potentially leading to accumulation in some ecosystems.
The persistence of sulphanilic acid in soil environments is another area of concern. Its mobility in soil can lead to groundwater contamination if not properly managed. Research has indicated that sulphanilic acid can adsorb to soil particles, which may limit its spread but also makes it challenging to remove once contamination occurs.
From an atmospheric perspective, sulphanilic acid has low volatility, reducing the risk of air pollution. However, the production and handling of this compound may release particulates or vapors that could contribute to local air quality issues if not properly controlled.
The potential for bioaccumulation in food chains is relatively low for sulphanilic acid, which is a positive aspect of its environmental profile. However, its presence in water bodies could still impact sensitive aquatic species, particularly during critical life stages.
In terms of human health, exposure to sulphanilic acid through environmental contamination is a concern. While acute toxicity is generally low, chronic exposure effects are less well understood and require further investigation. Occupational exposure during the production and use of sulphanilic acid in nanostructure assembly processes necessitates strict safety protocols.
Waste management is a critical aspect of mitigating the environmental impact of sulphanilic acid use. Proper disposal methods, including chemical treatment or incineration, must be employed to prevent environmental release. Additionally, recycling and recovery techniques for sulphanilic acid from industrial processes could significantly reduce its environmental footprint.
As research in nanostructure self-assembly advances, there is a growing need for green chemistry approaches to minimize the environmental impact of sulphanilic acid. This includes exploring bio-based alternatives, optimizing reaction conditions to reduce the amount of sulphanilic acid required, and developing closed-loop systems that minimize waste generation and maximize resource efficiency.
Scalability of Sulphanilic Acid Moderation
The scalability of sulphanilic acid moderation in nanostructure self-assembly is a critical factor in determining its potential for large-scale applications. As research progresses, understanding the limitations and opportunities for scaling up this process becomes increasingly important.
One of the primary advantages of using sulphanilic acid as a moderator is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale applications, as the raw material supply chain is well-established. However, the scalability of the moderation process itself requires careful consideration.
In laboratory settings, sulphanilic acid has demonstrated effective moderation of nanostructure self-assembly at small scales. The challenge lies in maintaining this effectiveness as the production volume increases. Factors such as reaction kinetics, heat transfer, and mixing efficiency can all be affected by scale-up, potentially altering the final nanostructure properties.
To address these challenges, researchers are exploring various approaches to scale up sulphanilic acid moderation. One promising method involves the use of continuous flow reactors, which allow for better control of reaction conditions and can be more easily scaled than batch processes. These reactors can maintain consistent temperature and concentration profiles, crucial for uniform nanostructure formation.
Another area of focus is the development of advanced mixing technologies. As the volume of reactants increases, ensuring homogeneous distribution of sulphanilic acid becomes more challenging. Innovative mixing techniques, such as microfluidic devices or ultrasonic mixers, are being investigated to overcome this hurdle.
The environmental impact of scaling up sulphanilic acid moderation must also be considered. While the compound itself is not highly toxic, large-scale use may require additional waste treatment processes. Researchers are exploring recycling methods to minimize environmental impact and improve cost-effectiveness.
Computational modeling and simulation play a crucial role in predicting the behavior of sulphanilic acid-moderated systems at larger scales. These tools help identify potential issues before costly physical scale-up attempts and guide the optimization of process parameters.
As the field progresses, interdisciplinary collaboration between chemists, chemical engineers, and materials scientists will be essential to overcome the challenges of scaling up sulphanilic acid moderation. The successful translation of laboratory-scale results to industrial production will unlock the full potential of this promising approach to nanostructure self-assembly.
One of the primary advantages of using sulphanilic acid as a moderator is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale applications, as the raw material supply chain is well-established. However, the scalability of the moderation process itself requires careful consideration.
In laboratory settings, sulphanilic acid has demonstrated effective moderation of nanostructure self-assembly at small scales. The challenge lies in maintaining this effectiveness as the production volume increases. Factors such as reaction kinetics, heat transfer, and mixing efficiency can all be affected by scale-up, potentially altering the final nanostructure properties.
To address these challenges, researchers are exploring various approaches to scale up sulphanilic acid moderation. One promising method involves the use of continuous flow reactors, which allow for better control of reaction conditions and can be more easily scaled than batch processes. These reactors can maintain consistent temperature and concentration profiles, crucial for uniform nanostructure formation.
Another area of focus is the development of advanced mixing technologies. As the volume of reactants increases, ensuring homogeneous distribution of sulphanilic acid becomes more challenging. Innovative mixing techniques, such as microfluidic devices or ultrasonic mixers, are being investigated to overcome this hurdle.
The environmental impact of scaling up sulphanilic acid moderation must also be considered. While the compound itself is not highly toxic, large-scale use may require additional waste treatment processes. Researchers are exploring recycling methods to minimize environmental impact and improve cost-effectiveness.
Computational modeling and simulation play a crucial role in predicting the behavior of sulphanilic acid-moderated systems at larger scales. These tools help identify potential issues before costly physical scale-up attempts and guide the optimization of process parameters.
As the field progresses, interdisciplinary collaboration between chemists, chemical engineers, and materials scientists will be essential to overcome the challenges of scaling up sulphanilic acid moderation. The successful translation of laboratory-scale results to industrial production will unlock the full potential of this promising approach to nanostructure self-assembly.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!