Perchloric Acid in the Synthesis of Bioactive Glasses
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Bioactive Glass Synthesis: Background and Objectives
Bioactive glasses have emerged as a revolutionary material in the field of biomedicine, offering unique properties that promote bone regeneration and tissue engineering. The synthesis of these glasses has been a subject of extensive research, with various methods and precursors being explored to enhance their bioactivity and performance. Among these, the use of perchloric acid in the synthesis process has gained attention due to its potential to improve the structural and functional characteristics of bioactive glasses.
The development of bioactive glasses can be traced back to the 1960s when Larry Hench discovered the first bioactive glass composition, known as 45S5 Bioglass. Since then, researchers have been continuously working on improving the synthesis methods and exploring new compositions to enhance the material's bioactivity and expand its applications. The incorporation of perchloric acid in the synthesis process represents a significant step in this ongoing evolution.
Perchloric acid, a strong oxidizing agent, has been traditionally used in various chemical processes and analytical techniques. Its application in bioactive glass synthesis aims to address several key challenges in the field, including the need for improved control over the glass network formation, enhanced bioactivity, and tailored degradation rates. The use of perchloric acid is expected to influence the sol-gel process, which is one of the primary methods for synthesizing bioactive glasses.
The primary objective of researching perchloric acid in bioactive glass synthesis is to develop a more efficient and controlled synthesis process that can yield glasses with superior properties. This includes achieving a more homogeneous glass structure, optimizing the release of therapeutic ions, and enhancing the overall bioactivity of the material. Additionally, researchers aim to investigate how perchloric acid affects the incorporation of various elements into the glass network, potentially leading to new compositions with enhanced functionalities.
Another crucial goal is to understand the impact of perchloric acid on the surface properties of bioactive glasses. The surface characteristics play a vital role in the material's ability to bond with living tissues and promote cellular responses. By modifying the synthesis process with perchloric acid, researchers hope to create bioactive glasses with improved surface reactivity and controlled dissolution rates, which are essential for their performance in biological environments.
Furthermore, this research seeks to explore the potential of perchloric acid in developing bioactive glasses with tailored properties for specific medical applications. This includes creating glasses with controlled porosity for drug delivery systems, glasses with enhanced antibacterial properties, and compositions optimized for specific types of tissue regeneration, such as bone, cartilage, or soft tissue repair.
The development of bioactive glasses can be traced back to the 1960s when Larry Hench discovered the first bioactive glass composition, known as 45S5 Bioglass. Since then, researchers have been continuously working on improving the synthesis methods and exploring new compositions to enhance the material's bioactivity and expand its applications. The incorporation of perchloric acid in the synthesis process represents a significant step in this ongoing evolution.
Perchloric acid, a strong oxidizing agent, has been traditionally used in various chemical processes and analytical techniques. Its application in bioactive glass synthesis aims to address several key challenges in the field, including the need for improved control over the glass network formation, enhanced bioactivity, and tailored degradation rates. The use of perchloric acid is expected to influence the sol-gel process, which is one of the primary methods for synthesizing bioactive glasses.
The primary objective of researching perchloric acid in bioactive glass synthesis is to develop a more efficient and controlled synthesis process that can yield glasses with superior properties. This includes achieving a more homogeneous glass structure, optimizing the release of therapeutic ions, and enhancing the overall bioactivity of the material. Additionally, researchers aim to investigate how perchloric acid affects the incorporation of various elements into the glass network, potentially leading to new compositions with enhanced functionalities.
Another crucial goal is to understand the impact of perchloric acid on the surface properties of bioactive glasses. The surface characteristics play a vital role in the material's ability to bond with living tissues and promote cellular responses. By modifying the synthesis process with perchloric acid, researchers hope to create bioactive glasses with improved surface reactivity and controlled dissolution rates, which are essential for their performance in biological environments.
Furthermore, this research seeks to explore the potential of perchloric acid in developing bioactive glasses with tailored properties for specific medical applications. This includes creating glasses with controlled porosity for drug delivery systems, glasses with enhanced antibacterial properties, and compositions optimized for specific types of tissue regeneration, such as bone, cartilage, or soft tissue repair.
Market Analysis for Bioactive Glass Applications
The bioactive glass market has experienced significant growth in recent years, driven by increasing applications in healthcare and dentistry. The global bioactive glass market size was valued at approximately $1.5 billion in 2020 and is projected to reach $2.7 billion by 2027, growing at a CAGR of around 7.8% during the forecast period. This growth is primarily attributed to the rising demand for advanced biomaterials in orthopedic and dental applications.
The healthcare sector, particularly orthopedics, remains the largest application segment for bioactive glasses. The increasing prevalence of bone-related disorders, coupled with an aging population, has fueled the demand for bioactive glass-based implants and bone grafts. In the dental industry, bioactive glasses are gaining traction in tooth remineralization products and as components in dental composites, contributing to market expansion.
Geographically, North America and Europe dominate the bioactive glass market, accounting for over 60% of the global market share. This dominance is due to advanced healthcare infrastructure, higher healthcare expenditure, and greater adoption of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare facilities, increasing disposable income, and growing awareness about advanced medical treatments.
The market for bioactive glasses is characterized by intense competition and continuous innovation. Key players are focusing on developing novel compositions and manufacturing techniques to enhance the performance and biocompatibility of bioactive glasses. The use of perchloric acid in the synthesis of bioactive glasses represents one such area of innovation, potentially offering improved control over glass composition and properties.
Despite the positive outlook, the bioactive glass market faces challenges such as high production costs and stringent regulatory requirements. These factors may hinder market growth, particularly in emerging economies. However, ongoing research and development efforts, including the exploration of new synthesis methods like those involving perchloric acid, are expected to address some of these challenges and open up new opportunities in the market.
In conclusion, the bioactive glass market shows promising growth potential, driven by expanding applications in healthcare and dentistry. The industry's focus on innovation, including research into novel synthesis methods, is likely to further propel market growth and diversify product offerings in the coming years.
The healthcare sector, particularly orthopedics, remains the largest application segment for bioactive glasses. The increasing prevalence of bone-related disorders, coupled with an aging population, has fueled the demand for bioactive glass-based implants and bone grafts. In the dental industry, bioactive glasses are gaining traction in tooth remineralization products and as components in dental composites, contributing to market expansion.
Geographically, North America and Europe dominate the bioactive glass market, accounting for over 60% of the global market share. This dominance is due to advanced healthcare infrastructure, higher healthcare expenditure, and greater adoption of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare facilities, increasing disposable income, and growing awareness about advanced medical treatments.
The market for bioactive glasses is characterized by intense competition and continuous innovation. Key players are focusing on developing novel compositions and manufacturing techniques to enhance the performance and biocompatibility of bioactive glasses. The use of perchloric acid in the synthesis of bioactive glasses represents one such area of innovation, potentially offering improved control over glass composition and properties.
Despite the positive outlook, the bioactive glass market faces challenges such as high production costs and stringent regulatory requirements. These factors may hinder market growth, particularly in emerging economies. However, ongoing research and development efforts, including the exploration of new synthesis methods like those involving perchloric acid, are expected to address some of these challenges and open up new opportunities in the market.
In conclusion, the bioactive glass market shows promising growth potential, driven by expanding applications in healthcare and dentistry. The industry's focus on innovation, including research into novel synthesis methods, is likely to further propel market growth and diversify product offerings in the coming years.
Current Challenges in Bioactive Glass Synthesis
The synthesis of bioactive glasses using perchloric acid presents several significant challenges that researchers and manufacturers must address. One of the primary obstacles is the inherent instability and high reactivity of perchloric acid, which necessitates stringent safety protocols and specialized handling equipment. This reactivity can lead to unpredictable reactions during the synthesis process, potentially affecting the composition and properties of the resulting bioactive glass.
Another major challenge lies in controlling the precise stoichiometry of the final product. The use of perchloric acid can introduce additional ions into the glass network, which may alter its bioactivity and degradation characteristics. Achieving the desired calcium-to-phosphorus ratio, crucial for optimal bioactivity, becomes more complex when perchloric acid is involved in the synthesis process.
The high acidity of perchloric acid also poses difficulties in maintaining the pH balance during synthesis. This can affect the formation of the glass network and the incorporation of therapeutic ions, potentially compromising the bioactive properties of the final material. Researchers must develop robust methods to neutralize or buffer the acid without introducing unwanted contaminants or altering the intended composition of the bioactive glass.
Furthermore, the use of perchloric acid in bioactive glass synthesis raises environmental concerns. The disposal of waste products and unreacted perchloric acid requires specialized treatment facilities, adding to the overall cost and complexity of the manufacturing process. This aspect also necessitates the development of more environmentally friendly synthesis routes that minimize the use of hazardous chemicals while maintaining the desired properties of the bioactive glass.
Scalability remains a significant challenge in the production of bioactive glasses using perchloric acid. The safety considerations and specialized equipment required for handling perchloric acid make it difficult to scale up laboratory processes to industrial production levels. This limitation hinders the widespread adoption of perchloric acid-based synthesis methods in commercial bioactive glass manufacturing.
Lastly, the long-term stability and shelf life of bioactive glasses synthesized using perchloric acid require thorough investigation. The potential presence of residual perchlorate ions in the final product may affect its long-term performance and biocompatibility. Researchers must develop comprehensive testing protocols to ensure the safety and efficacy of these materials over extended periods, particularly in medical applications where long-term stability is crucial.
Another major challenge lies in controlling the precise stoichiometry of the final product. The use of perchloric acid can introduce additional ions into the glass network, which may alter its bioactivity and degradation characteristics. Achieving the desired calcium-to-phosphorus ratio, crucial for optimal bioactivity, becomes more complex when perchloric acid is involved in the synthesis process.
The high acidity of perchloric acid also poses difficulties in maintaining the pH balance during synthesis. This can affect the formation of the glass network and the incorporation of therapeutic ions, potentially compromising the bioactive properties of the final material. Researchers must develop robust methods to neutralize or buffer the acid without introducing unwanted contaminants or altering the intended composition of the bioactive glass.
Furthermore, the use of perchloric acid in bioactive glass synthesis raises environmental concerns. The disposal of waste products and unreacted perchloric acid requires specialized treatment facilities, adding to the overall cost and complexity of the manufacturing process. This aspect also necessitates the development of more environmentally friendly synthesis routes that minimize the use of hazardous chemicals while maintaining the desired properties of the bioactive glass.
Scalability remains a significant challenge in the production of bioactive glasses using perchloric acid. The safety considerations and specialized equipment required for handling perchloric acid make it difficult to scale up laboratory processes to industrial production levels. This limitation hinders the widespread adoption of perchloric acid-based synthesis methods in commercial bioactive glass manufacturing.
Lastly, the long-term stability and shelf life of bioactive glasses synthesized using perchloric acid require thorough investigation. The potential presence of residual perchlorate ions in the final product may affect its long-term performance and biocompatibility. Researchers must develop comprehensive testing protocols to ensure the safety and efficacy of these materials over extended periods, particularly in medical applications where long-term stability is crucial.
Existing Perchloric Acid-Based Synthesis Protocols
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.- Synthesis and production of perchloric acid: Various methods and processes for synthesizing and producing perchloric acid are described. These may include electrochemical processes, chemical reactions, or specialized equipment designed for the production of high-purity perchloric acid.
- Applications of perchloric acid in chemical analysis: Perchloric acid is widely used in chemical analysis, particularly in sample preparation and digestion processes. It is effective in breaking down complex organic compounds and extracting metals from various matrices for subsequent analysis.
- Safety measures and handling of perchloric acid: Due to its strong oxidizing properties, special safety measures are required for handling and storing perchloric acid. This includes the use of specialized equipment, protective gear, and proper storage facilities to prevent accidents and ensure safe usage.
- Perchloric acid in battery technology: Perchloric acid and its derivatives are used in certain types of batteries and energy storage devices. This includes applications in electrolytes, electrode materials, or as components in advanced battery systems.
- Purification and concentration of perchloric acid: Methods and apparatus for purifying and concentrating perchloric acid are described. These processes aim to produce high-purity perchloric acid for specialized applications in research and industry.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in chemical reactions, as well as its role in sample preparation and digestion for analytical chemistry applications.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.Expand Specific Solutions04 Perchloric acid in battery technology
Use of perchloric acid in the development and manufacturing of batteries, particularly in electrolyte formulations. This may involve its role in improving battery performance, longevity, or specific electrochemical properties in various types of batteries.Expand Specific Solutions05 Purification and concentration of perchloric acid
Techniques and processes for purifying and concentrating perchloric acid to meet specific purity requirements for various applications. This may include distillation methods, membrane separation, or other advanced purification technologies to remove impurities and achieve desired concentrations.Expand Specific Solutions
Key Players in Bioactive Glass Research and Production
The research on perchloric acid in bioactive glass synthesis is in a nascent stage, with the market still developing. The technology's maturity is relatively low, indicating significant potential for growth and innovation. Key players like Corning, Inc. and SCHOTT AG, with their extensive experience in glass technologies, are well-positioned to drive advancements. Academic institutions such as Queen Mary University of London and Xi'an Jiaotong University are contributing valuable research. The involvement of diverse entities, from established corporations to specialized biotech firms like NovaBone Products LLC, suggests a competitive landscape with opportunities for both breakthrough innovations and incremental improvements in this emerging field.
Corning, Inc.
Technical Solution: Corning has developed a novel approach for synthesizing bioactive glasses using perchloric acid as a catalyst. Their method involves a sol-gel process where perchloric acid is used to control the hydrolysis and condensation reactions of silica precursors. This results in bioactive glasses with enhanced porosity and surface area, leading to improved bioactivity[1]. The company has also incorporated rare earth elements into these glasses, which has shown to enhance their antibacterial properties and osteogenic potential[2]. Corning's research has demonstrated that perchloric acid-assisted synthesis can produce bioactive glasses with controlled release of therapeutic ions, making them suitable for various biomedical applications[3].
Strengths: Enhanced porosity and surface area, improved bioactivity, controlled ion release. Weaknesses: Potential safety concerns due to perchloric acid use, may require specialized handling and disposal procedures.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has made significant advancements in the use of perchloric acid for bioactive glass synthesis. Their approach focuses on using perchloric acid as a catalyst in a low-temperature sol-gel process, which allows for the incorporation of temperature-sensitive biomolecules into the glass network[13]. This method has enabled the development of bioactive glasses with controlled release of growth factors and antibiotics. The institute has also pioneered a perchloric acid-assisted microemulsion technique for synthesizing hollow bioactive glass microspheres with tailored size distributions and shell thicknesses[14]. These microspheres have shown promising results in drug delivery applications and as fillers in injectable bone cements. Furthermore, their research has demonstrated that perchloric acid can be used to create bioactive glasses with unique compositional gradients, allowing for the design of materials with spatially varied biological responses[15].
Strengths: Low-temperature synthesis, incorporation of biomolecules, tailored microsphere production, compositional gradients. Weaknesses: Potential environmental concerns related to perchloric acid use, complexity of scaling up the microemulsion technique.
Innovations in Perchloric Acid Use for Bioactive Glasses
Compositions comprising bioactive glass and amino acid
PatentPendingUS20240325264A1
Innovation
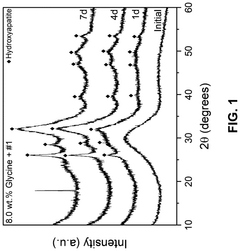
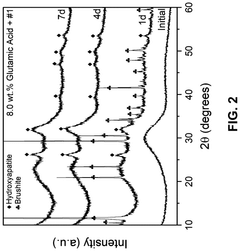
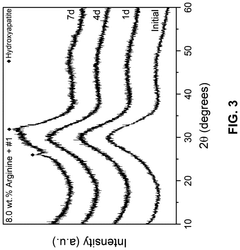
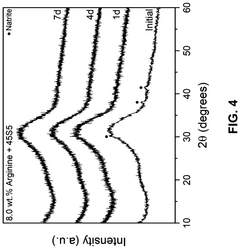
- A composition comprising 15-55 wt.% SiO2, 0.1-15 wt.% ZrO2, 20-60 wt.% CaO, and 5-30 wt.% P2O5 bioactive glass combined with an amino acid, which forms a bioactive crystalline phase within 7 days when subjected to artificial saliva at 37°C, enhancing mineralization rates and durability.
Method for treating a lead-containing glass that makes it possible to limit the migration in solution of the lead contained in this glass
PatentActiveUS11851365B2
Innovation
- A method involving a step of placing lead-containing glass in contact with a perchloric acid solution followed by a heat treatment below the glass transition temperature, specifically using an aqueous solution of perchloric acid at concentrations ranging from 10−3M to 10−1M for 12-36 hours at ambient temperature, and then heating to temperatures up to 150°C below the glass transition temperature for 12-36 hours, effectively reducing lead migration.
Safety Considerations for Perchloric Acid Handling
Perchloric acid is a powerful oxidizing agent widely used in various chemical processes, including the synthesis of bioactive glasses. However, its high reactivity and potential for explosive reactions necessitate stringent safety measures during handling and storage. When working with perchloric acid in bioactive glass synthesis, researchers must prioritize safety to prevent accidents and ensure a secure laboratory environment.
Proper personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. Additionally, a fume hood with a wash-down system specifically designed for perchloric acid use is crucial to contain and neutralize any vapors or spills. Regular maintenance and cleaning of these fume hoods are necessary to prevent the accumulation of potentially explosive perchlorate salts.
Storage of perchloric acid requires special considerations. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are preferred, as perchloric acid can react with some metals. Proper labeling and segregation from incompatible substances are vital to prevent accidental mixing and potential explosions.
Dilution of perchloric acid is a critical step that demands caution. Always add acid to water, never the reverse, to avoid violent reactions. This process should be performed in a fume hood with adequate ventilation. When preparing solutions for bioactive glass synthesis, it is advisable to use the lowest concentration of perchloric acid that will effectively serve the intended purpose.
Training and education are paramount for all personnel working with perchloric acid. This includes understanding the chemical properties, potential hazards, proper handling techniques, and emergency procedures. Regular safety briefings and updates on best practices should be conducted to maintain a high level of awareness among researchers and laboratory staff.
Emergency response planning is crucial when working with perchloric acid. This includes having readily available spill kits, neutralizing agents, and clear protocols for addressing accidents. Eye wash stations and safety showers should be easily accessible and regularly tested. Additionally, fire suppression systems suitable for chemical fires should be in place, as water alone may not be effective in combating perchloric acid-related fires.
Waste management is another critical aspect of perchloric acid safety. Proper disposal methods must be followed to prevent environmental contamination and potential hazards. Neutralization of waste solutions and appropriate containment for disposal are essential steps in the waste management process.
By adhering to these safety considerations, researchers can minimize risks associated with perchloric acid use in bioactive glass synthesis. Implementing and maintaining a culture of safety not only protects personnel but also ensures the integrity of research outcomes and the longevity of laboratory operations.
Proper personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. Additionally, a fume hood with a wash-down system specifically designed for perchloric acid use is crucial to contain and neutralize any vapors or spills. Regular maintenance and cleaning of these fume hoods are necessary to prevent the accumulation of potentially explosive perchlorate salts.
Storage of perchloric acid requires special considerations. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are preferred, as perchloric acid can react with some metals. Proper labeling and segregation from incompatible substances are vital to prevent accidental mixing and potential explosions.
Dilution of perchloric acid is a critical step that demands caution. Always add acid to water, never the reverse, to avoid violent reactions. This process should be performed in a fume hood with adequate ventilation. When preparing solutions for bioactive glass synthesis, it is advisable to use the lowest concentration of perchloric acid that will effectively serve the intended purpose.
Training and education are paramount for all personnel working with perchloric acid. This includes understanding the chemical properties, potential hazards, proper handling techniques, and emergency procedures. Regular safety briefings and updates on best practices should be conducted to maintain a high level of awareness among researchers and laboratory staff.
Emergency response planning is crucial when working with perchloric acid. This includes having readily available spill kits, neutralizing agents, and clear protocols for addressing accidents. Eye wash stations and safety showers should be easily accessible and regularly tested. Additionally, fire suppression systems suitable for chemical fires should be in place, as water alone may not be effective in combating perchloric acid-related fires.
Waste management is another critical aspect of perchloric acid safety. Proper disposal methods must be followed to prevent environmental contamination and potential hazards. Neutralization of waste solutions and appropriate containment for disposal are essential steps in the waste management process.
By adhering to these safety considerations, researchers can minimize risks associated with perchloric acid use in bioactive glass synthesis. Implementing and maintaining a culture of safety not only protects personnel but also ensures the integrity of research outcomes and the longevity of laboratory operations.
Environmental Impact of Perchloric Acid in Synthesis
The use of perchloric acid in the synthesis of bioactive glasses raises significant environmental concerns due to its highly reactive and potentially hazardous nature. When released into the environment, perchloric acid can have severe impacts on ecosystems and human health. The primary environmental risks associated with perchloric acid stem from its strong oxidizing properties and its ability to form persistent perchlorate salts.
In aquatic environments, perchloric acid can disrupt the natural balance of ecosystems by altering pH levels and oxidizing organic matter. This can lead to the death of aquatic organisms and the degradation of water quality. Furthermore, perchlorate contamination in water sources poses a threat to both wildlife and human populations. Perchlorate ions can interfere with iodine uptake in the thyroid gland, potentially causing hormonal imbalances and developmental issues in exposed organisms.
Soil contamination is another significant concern when perchloric acid is improperly handled or disposed of during the synthesis process. Perchlorate salts formed in soil can persist for extended periods, leading to long-term environmental damage. These salts can be taken up by plants, entering the food chain and potentially accumulating in higher trophic levels.
The atmospheric release of perchloric acid vapors during synthesis can contribute to air pollution and pose respiratory risks to both workers and nearby communities. Additionally, the reaction of perchloric acid with organic materials in the air can lead to the formation of explosive compounds, presenting a safety hazard.
To mitigate these environmental impacts, strict protocols for the handling, use, and disposal of perchloric acid in bioactive glass synthesis must be implemented. This includes the use of closed systems to prevent releases, proper neutralization techniques for waste streams, and the development of alternative synthesis methods that reduce or eliminate the need for perchloric acid.
Research into green chemistry approaches for bioactive glass synthesis is ongoing, with a focus on finding less hazardous alternatives to perchloric acid. These efforts aim to develop environmentally friendly processes that maintain the desired properties of bioactive glasses while minimizing ecological risks.
Regulatory bodies have implemented stringent guidelines for the use and disposal of perchloric acid in industrial and research settings. Compliance with these regulations is crucial for minimizing environmental impact and ensuring the sustainable development of bioactive glass technologies.
In aquatic environments, perchloric acid can disrupt the natural balance of ecosystems by altering pH levels and oxidizing organic matter. This can lead to the death of aquatic organisms and the degradation of water quality. Furthermore, perchlorate contamination in water sources poses a threat to both wildlife and human populations. Perchlorate ions can interfere with iodine uptake in the thyroid gland, potentially causing hormonal imbalances and developmental issues in exposed organisms.
Soil contamination is another significant concern when perchloric acid is improperly handled or disposed of during the synthesis process. Perchlorate salts formed in soil can persist for extended periods, leading to long-term environmental damage. These salts can be taken up by plants, entering the food chain and potentially accumulating in higher trophic levels.
The atmospheric release of perchloric acid vapors during synthesis can contribute to air pollution and pose respiratory risks to both workers and nearby communities. Additionally, the reaction of perchloric acid with organic materials in the air can lead to the formation of explosive compounds, presenting a safety hazard.
To mitigate these environmental impacts, strict protocols for the handling, use, and disposal of perchloric acid in bioactive glass synthesis must be implemented. This includes the use of closed systems to prevent releases, proper neutralization techniques for waste streams, and the development of alternative synthesis methods that reduce or eliminate the need for perchloric acid.
Research into green chemistry approaches for bioactive glass synthesis is ongoing, with a focus on finding less hazardous alternatives to perchloric acid. These efforts aim to develop environmentally friendly processes that maintain the desired properties of bioactive glasses while minimizing ecological risks.
Regulatory bodies have implemented stringent guidelines for the use and disposal of perchloric acid in industrial and research settings. Compliance with these regulations is crucial for minimizing environmental impact and ensuring the sustainable development of bioactive glass technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!