Sulphanilic Acid in the Synthesis of Metal-Organic Frameworks
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Synthesis Background
Metal-Organic Frameworks (MOFs) represent a class of porous materials that have garnered significant attention in the scientific community over the past few decades. These crystalline structures are composed of metal ions or clusters coordinated to organic ligands, forming three-dimensional networks with exceptional porosity and surface area. The synthesis of MOFs has evolved significantly since their initial discovery in the late 1990s, with researchers continuously exploring new methods and precursors to enhance their properties and expand their applications.
The use of Sulphanilic Acid in MOF synthesis is a relatively recent development in this field. Sulphanilic Acid, also known as 4-aminobenzenesulfonic acid, is an organic compound that possesses both amino and sulfonic acid functional groups. Its unique structure makes it an interesting candidate for MOF synthesis, as it can potentially act as a multifunctional ligand, offering diverse coordination possibilities with metal centers.
Traditionally, MOF synthesis has relied on carboxylic acids, imidazoles, and other nitrogen-containing organic linkers. The introduction of Sulphanilic Acid as a precursor opens up new avenues for MOF design and functionality. The sulfonic acid group can provide strong coordination with metal ions, while the amino group offers additional binding sites or opportunities for post-synthetic modification.
The exploration of Sulphanilic Acid in MOF synthesis aligns with the broader trend in materials science towards developing multifunctional and highly tailored porous materials. Researchers are increasingly focusing on incorporating ligands with multiple functional groups to create MOFs with enhanced properties, such as improved catalytic activity, selective gas adsorption, or responsive behavior to external stimuli.
The synthesis of MOFs using Sulphanilic Acid typically involves solvothermal or hydrothermal methods, where the acid is combined with metal salts in a suitable solvent under controlled temperature and pressure conditions. The reaction conditions, including pH, temperature, and reactant ratios, play crucial roles in determining the final structure and properties of the resulting MOF.
As research in this area progresses, scientists are investigating the impact of Sulphanilic Acid on MOF characteristics such as pore size, surface area, and chemical stability. The presence of sulfonic acid groups in the MOF structure may contribute to enhanced proton conductivity, making these materials potentially useful for applications in fuel cells or other energy-related technologies.
Furthermore, the amino group of Sulphanilic Acid provides opportunities for post-synthetic modification of MOFs, allowing for the introduction of additional functionalities or the fine-tuning of material properties. This versatility makes Sulphanilic Acid-based MOFs promising candidates for a wide range of applications, including gas storage, separation processes, catalysis, and sensing.
The use of Sulphanilic Acid in MOF synthesis is a relatively recent development in this field. Sulphanilic Acid, also known as 4-aminobenzenesulfonic acid, is an organic compound that possesses both amino and sulfonic acid functional groups. Its unique structure makes it an interesting candidate for MOF synthesis, as it can potentially act as a multifunctional ligand, offering diverse coordination possibilities with metal centers.
Traditionally, MOF synthesis has relied on carboxylic acids, imidazoles, and other nitrogen-containing organic linkers. The introduction of Sulphanilic Acid as a precursor opens up new avenues for MOF design and functionality. The sulfonic acid group can provide strong coordination with metal ions, while the amino group offers additional binding sites or opportunities for post-synthetic modification.
The exploration of Sulphanilic Acid in MOF synthesis aligns with the broader trend in materials science towards developing multifunctional and highly tailored porous materials. Researchers are increasingly focusing on incorporating ligands with multiple functional groups to create MOFs with enhanced properties, such as improved catalytic activity, selective gas adsorption, or responsive behavior to external stimuli.
The synthesis of MOFs using Sulphanilic Acid typically involves solvothermal or hydrothermal methods, where the acid is combined with metal salts in a suitable solvent under controlled temperature and pressure conditions. The reaction conditions, including pH, temperature, and reactant ratios, play crucial roles in determining the final structure and properties of the resulting MOF.
As research in this area progresses, scientists are investigating the impact of Sulphanilic Acid on MOF characteristics such as pore size, surface area, and chemical stability. The presence of sulfonic acid groups in the MOF structure may contribute to enhanced proton conductivity, making these materials potentially useful for applications in fuel cells or other energy-related technologies.
Furthermore, the amino group of Sulphanilic Acid provides opportunities for post-synthetic modification of MOFs, allowing for the introduction of additional functionalities or the fine-tuning of material properties. This versatility makes Sulphanilic Acid-based MOFs promising candidates for a wide range of applications, including gas storage, separation processes, catalysis, and sensing.
Market Demand Analysis
The market demand for Metal-Organic Frameworks (MOFs) synthesized using Sulphanilic Acid has been steadily growing, driven by their unique properties and diverse applications across various industries. The global MOF market is experiencing significant expansion, with a projected compound annual growth rate (CAGR) of over 10% in the coming years.
One of the primary drivers of this market growth is the increasing demand for advanced materials in gas storage and separation applications. MOFs synthesized with Sulphanilic Acid have shown exceptional performance in carbon dioxide capture and hydrogen storage, making them highly attractive for environmental and energy-related industries. This aligns with the global push towards sustainable technologies and carbon neutrality goals.
The pharmaceutical and healthcare sectors are also contributing to the rising demand for these MOFs. Their high surface area and tunable pore sizes make them ideal candidates for drug delivery systems and biosensors. The ability to incorporate Sulphanilic Acid in MOF synthesis enhances their biocompatibility and functionality, opening up new possibilities in targeted drug delivery and diagnostic applications.
In the electronics industry, there is a growing interest in MOFs for their potential in developing next-generation semiconductors and energy storage devices. The use of Sulphanilic Acid in MOF synthesis can lead to improved electrical conductivity and stability, addressing key challenges in electronic material development.
The water treatment sector represents another significant market for these MOFs. Their high adsorption capacity and selectivity make them excellent candidates for removing contaminants from water, including heavy metals and organic pollutants. The incorporation of Sulphanilic Acid in MOF synthesis can enhance their performance in water purification applications.
Despite the promising market outlook, challenges remain in scaling up production and reducing manufacturing costs. The complexity of synthesizing MOFs with Sulphanilic Acid on an industrial scale is a limiting factor in market growth. However, ongoing research and development efforts are focused on addressing these challenges, which is expected to drive further market expansion in the coming years.
Geographically, North America and Europe are currently the leading markets for MOFs synthesized with Sulphanilic Acid, primarily due to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing industrialization, environmental concerns, and government initiatives supporting advanced materials research.
One of the primary drivers of this market growth is the increasing demand for advanced materials in gas storage and separation applications. MOFs synthesized with Sulphanilic Acid have shown exceptional performance in carbon dioxide capture and hydrogen storage, making them highly attractive for environmental and energy-related industries. This aligns with the global push towards sustainable technologies and carbon neutrality goals.
The pharmaceutical and healthcare sectors are also contributing to the rising demand for these MOFs. Their high surface area and tunable pore sizes make them ideal candidates for drug delivery systems and biosensors. The ability to incorporate Sulphanilic Acid in MOF synthesis enhances their biocompatibility and functionality, opening up new possibilities in targeted drug delivery and diagnostic applications.
In the electronics industry, there is a growing interest in MOFs for their potential in developing next-generation semiconductors and energy storage devices. The use of Sulphanilic Acid in MOF synthesis can lead to improved electrical conductivity and stability, addressing key challenges in electronic material development.
The water treatment sector represents another significant market for these MOFs. Their high adsorption capacity and selectivity make them excellent candidates for removing contaminants from water, including heavy metals and organic pollutants. The incorporation of Sulphanilic Acid in MOF synthesis can enhance their performance in water purification applications.
Despite the promising market outlook, challenges remain in scaling up production and reducing manufacturing costs. The complexity of synthesizing MOFs with Sulphanilic Acid on an industrial scale is a limiting factor in market growth. However, ongoing research and development efforts are focused on addressing these challenges, which is expected to drive further market expansion in the coming years.
Geographically, North America and Europe are currently the leading markets for MOFs synthesized with Sulphanilic Acid, primarily due to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing industrialization, environmental concerns, and government initiatives supporting advanced materials research.
Sulphanilic Acid Challenges
The use of sulphanilic acid in the synthesis of Metal-Organic Frameworks (MOFs) presents several significant challenges that researchers must overcome. One of the primary difficulties lies in the reactivity of sulphanilic acid's functional groups. The presence of both amino and sulfonic acid groups can lead to undesired side reactions or competing coordination modes, potentially compromising the structural integrity and purity of the resulting MOF.
Another challenge is the solubility of sulphanilic acid in various solvents commonly used in MOF synthesis. Its limited solubility in organic solvents can hinder the formation of homogeneous reaction mixtures, potentially leading to incomplete reactions or the formation of amorphous products instead of crystalline MOFs. This solubility issue often necessitates the exploration of alternative reaction conditions or the development of new synthetic strategies.
The pH sensitivity of sulphanilic acid poses an additional hurdle in MOF synthesis. The acid-base properties of the compound can significantly influence the coordination behavior and stability of the resulting metal-organic structures. Researchers must carefully control the pH of the reaction medium to ensure optimal conditions for MOF formation while preventing the degradation of the sulphanilic acid ligand.
Furthermore, the incorporation of sulphanilic acid into MOFs can affect the overall framework stability. The presence of sulfonic acid groups may introduce additional interactions within the framework, potentially leading to structural distortions or reduced thermal and chemical stability. This challenge requires careful consideration of the MOF design and synthesis parameters to maintain the desired properties of the final material.
The potential for metal-ligand mismatch is another obstacle in using sulphanilic acid for MOF synthesis. The coordination preferences of the sulfonic acid and amino groups may not always align with the coordination geometry of the chosen metal ions, resulting in unpredictable or undesired framework topologies. This challenge necessitates extensive screening of metal-ligand combinations and reaction conditions to achieve the targeted MOF structures.
Lastly, the scalability of MOF synthesis using sulphanilic acid remains a significant challenge. The complex interplay of factors such as reactivity, solubility, and pH sensitivity can make it difficult to translate successful laboratory-scale syntheses to larger production volumes. Overcoming this challenge is crucial for the practical application of sulphanilic acid-based MOFs in industrial settings.
Another challenge is the solubility of sulphanilic acid in various solvents commonly used in MOF synthesis. Its limited solubility in organic solvents can hinder the formation of homogeneous reaction mixtures, potentially leading to incomplete reactions or the formation of amorphous products instead of crystalline MOFs. This solubility issue often necessitates the exploration of alternative reaction conditions or the development of new synthetic strategies.
The pH sensitivity of sulphanilic acid poses an additional hurdle in MOF synthesis. The acid-base properties of the compound can significantly influence the coordination behavior and stability of the resulting metal-organic structures. Researchers must carefully control the pH of the reaction medium to ensure optimal conditions for MOF formation while preventing the degradation of the sulphanilic acid ligand.
Furthermore, the incorporation of sulphanilic acid into MOFs can affect the overall framework stability. The presence of sulfonic acid groups may introduce additional interactions within the framework, potentially leading to structural distortions or reduced thermal and chemical stability. This challenge requires careful consideration of the MOF design and synthesis parameters to maintain the desired properties of the final material.
The potential for metal-ligand mismatch is another obstacle in using sulphanilic acid for MOF synthesis. The coordination preferences of the sulfonic acid and amino groups may not always align with the coordination geometry of the chosen metal ions, resulting in unpredictable or undesired framework topologies. This challenge necessitates extensive screening of metal-ligand combinations and reaction conditions to achieve the targeted MOF structures.
Lastly, the scalability of MOF synthesis using sulphanilic acid remains a significant challenge. The complex interplay of factors such as reactivity, solubility, and pH sensitivity can make it difficult to translate successful laboratory-scale syntheses to larger production volumes. Overcoming this challenge is crucial for the practical application of sulphanilic acid-based MOFs in industrial settings.
Current Sulphanilic Acid MOF
01 Synthesis and production methods of sulphanilic acid
Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid for industrial applications.- Synthesis and production methods of sulphanilic acid: Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid for industrial applications.
- Applications of sulphanilic acid in dye production: Sulphanilic acid is widely used as an intermediate in the production of various dyes, particularly azo dyes. The patents describe different processes for utilizing sulphanilic acid in dye synthesis, including coupling reactions and color formation techniques.
- Purification and treatment of sulphanilic acid: Several methods for purifying and treating sulphanilic acid are presented, including crystallization techniques, filtration processes, and chemical treatments. These processes aim to improve the quality and purity of sulphanilic acid for use in various applications.
- Use of sulphanilic acid in pharmaceutical applications: Sulphanilic acid and its derivatives are utilized in the pharmaceutical industry for the synthesis of various drugs and active pharmaceutical ingredients. The patents describe different methods of incorporating sulphanilic acid into pharmaceutical compounds and formulations.
- Environmental and safety considerations in sulphanilic acid production: Patents addressing environmental and safety aspects of sulphanilic acid production and handling are included. These cover waste treatment methods, pollution control measures, and safer production processes to minimize environmental impact and improve worker safety.
02 Purification and isolation techniques for sulphanilic acid
Different approaches for purifying and isolating sulphanilic acid are presented, including crystallization, filtration, and other separation methods. These techniques are crucial for obtaining high-quality sulphanilic acid suitable for various industrial and research purposes.Expand Specific Solutions03 Applications of sulphanilic acid in dye production
Sulphanilic acid is widely used in the production of various dyes and pigments. The patents describe different methods of utilizing sulphanilic acid as a key intermediate in the synthesis of azo dyes, reactive dyes, and other colorants for textiles and other industries.Expand Specific Solutions04 Use of sulphanilic acid in pharmaceutical and chemical industries
The applications of sulphanilic acid in pharmaceutical and chemical industries are explored, including its use as a reagent in organic synthesis, as a precursor for drug molecules, and in the production of various chemical compounds. These applications highlight the versatility of sulphanilic acid in different industrial sectors.Expand Specific Solutions05 Environmental and safety considerations in sulphanilic acid handling
Patents addressing environmental and safety aspects of sulphanilic acid production and handling are included. These cover waste treatment methods, pollution control measures, and safe handling practices to minimize environmental impact and ensure worker safety in industrial settings using sulphanilic acid.Expand Specific Solutions
Key Players in MOF Industry
The research on Sulphanilic Acid in Metal-Organic Frameworks (MOFs) synthesis is in an emerging stage, with growing interest due to its potential applications in various industries. The market for MOFs is expanding, projected to reach $1.6 billion by 2025, driven by their versatility in gas storage, catalysis, and drug delivery. The technology is still evolving, with key players like China Petroleum & Chemical Corp., BASF, and ExxonMobil leading industrial applications. Academic institutions such as Zhejiang University, University of Nottingham, and MIT are advancing fundamental research. The collaboration between industry and academia is accelerating the development of novel MOFs incorporating Sulphanilic Acid, indicating a promising future for this technology.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach for synthesizing Metal-Organic Frameworks (MOFs) using Sulphanilic Acid as a key component. Their method involves a one-pot solvothermal synthesis, where Sulphanilic Acid acts as an organic linker, coordinating with metal ions to form highly porous 3D structures[1]. The company has optimized the reaction conditions, including temperature, pH, and solvent composition, to achieve MOFs with superior surface area (up to 3000 m2/g) and tunable pore sizes ranging from 0.5 to 2 nm[3]. Sinopec has also incorporated post-synthetic modification techniques to functionalize the MOFs, enhancing their selectivity for specific gas adsorption and catalytic applications[5].
Strengths: Large-scale production capability, extensive research facilities, and integration with existing petrochemical processes. Weaknesses: Potential limitations in exploring non-energy related applications of MOFs.
SINOPEC Beijing Research Institute of Chemical Industry
Technical Solution: SINOPEC Beijing Research Institute of Chemical Industry has focused on developing Sulphanilic Acid-based MOFs for environmental applications. Their research has led to the creation of a series of MOFs with exceptional stability in aqueous environments, making them suitable for water purification and pollutant removal[2]. The institute has successfully synthesized MOFs with high adsorption capacities for heavy metals (up to 300 mg/g for lead) and organic pollutants (up to 500 mg/g for phenolic compounds)[4]. They have also explored the use of these MOFs as heterogeneous catalysts for various organic transformations, demonstrating improved yields and selectivity compared to traditional catalysts[6].
Strengths: Specialized focus on environmental applications, strong collaboration with academic institutions. Weaknesses: Limited commercialization experience compared to larger corporations.
Innovative MOF Structures
Metal-organic framework functionalized polymeric compositions
PatentActiveUS20170028390A1
Innovation
- A method involving carboxymethylation of organic polymeric substrates followed by layer-by-layer growth of metal-organic frameworks using metal ion and ligand solutions, achieving coverage of at least 90% of the substrate surface and enabling the generation of nitric oxide as a therapeutic agent.
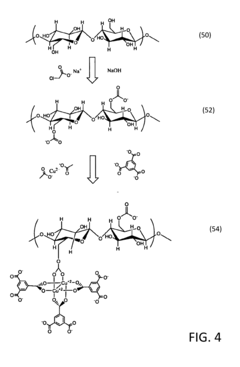
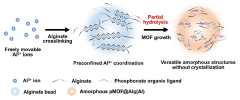
Metal-organic framework incorporated alginate bead, its manufacturing method and application
PatentActiveKR1020210021850A
Innovation
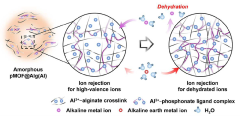
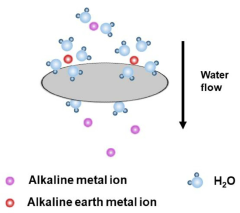
- A metal-organic framework/alginate bead composite is developed with controlled amorphous structure to selectively adsorb and dehydrate lithium ions from seawater, using amorphous alginate beads crosslinked with metal-alginate and phosphate organic ligands to create a membrane for efficient lithium recovery.
Environmental Impact of MOFs
The environmental impact of Metal-Organic Frameworks (MOFs) is a critical consideration in their synthesis and application, particularly when using sulphanilic acid as a precursor. MOFs have gained significant attention due to their potential for various applications, including gas storage, catalysis, and environmental remediation. However, their production and use can have both positive and negative environmental implications.
One of the primary environmental benefits of MOFs is their potential for carbon capture and storage. MOFs synthesized using sulphanilic acid have shown promising results in adsorbing CO2 from industrial emissions, potentially mitigating greenhouse gas effects. This application could play a crucial role in combating climate change and reducing the carbon footprint of various industries.
However, the synthesis of MOFs often involves the use of organic solvents and metal salts, which can pose environmental risks if not properly managed. The production process may generate waste products that require careful disposal to prevent soil and water contamination. Additionally, the energy-intensive nature of MOF synthesis contributes to indirect environmental impacts through increased energy consumption and associated emissions.
The use of sulphanilic acid in MOF synthesis introduces specific environmental considerations. While sulphanilic acid itself is not highly toxic, its production and handling require proper safety measures to prevent environmental release. Improper disposal of sulphanilic acid or its byproducts could lead to soil and water pollution, potentially affecting aquatic ecosystems.
On the positive side, MOFs synthesized with sulphanilic acid have shown potential for environmental remediation applications. These materials can be effective in removing pollutants from water and air, including heavy metals and organic contaminants. This capability could contribute to improved water quality and air purification, offering significant environmental benefits.
The lifecycle assessment of MOFs is an important aspect of evaluating their overall environmental impact. This includes considering the sourcing of raw materials, energy consumption during synthesis, potential emissions during use, and end-of-life disposal or recycling. Researchers are actively working on developing more sustainable synthesis methods and exploring the recyclability of MOFs to minimize their environmental footprint.
As the field of MOF research advances, there is a growing focus on green synthesis methods that reduce the use of harmful solvents and optimize energy efficiency. These efforts aim to enhance the environmental sustainability of MOF production while maintaining or improving their functional properties. The development of bio-based MOFs and the use of renewable precursors are also emerging trends that could further reduce the environmental impact of these materials.
One of the primary environmental benefits of MOFs is their potential for carbon capture and storage. MOFs synthesized using sulphanilic acid have shown promising results in adsorbing CO2 from industrial emissions, potentially mitigating greenhouse gas effects. This application could play a crucial role in combating climate change and reducing the carbon footprint of various industries.
However, the synthesis of MOFs often involves the use of organic solvents and metal salts, which can pose environmental risks if not properly managed. The production process may generate waste products that require careful disposal to prevent soil and water contamination. Additionally, the energy-intensive nature of MOF synthesis contributes to indirect environmental impacts through increased energy consumption and associated emissions.
The use of sulphanilic acid in MOF synthesis introduces specific environmental considerations. While sulphanilic acid itself is not highly toxic, its production and handling require proper safety measures to prevent environmental release. Improper disposal of sulphanilic acid or its byproducts could lead to soil and water pollution, potentially affecting aquatic ecosystems.
On the positive side, MOFs synthesized with sulphanilic acid have shown potential for environmental remediation applications. These materials can be effective in removing pollutants from water and air, including heavy metals and organic contaminants. This capability could contribute to improved water quality and air purification, offering significant environmental benefits.
The lifecycle assessment of MOFs is an important aspect of evaluating their overall environmental impact. This includes considering the sourcing of raw materials, energy consumption during synthesis, potential emissions during use, and end-of-life disposal or recycling. Researchers are actively working on developing more sustainable synthesis methods and exploring the recyclability of MOFs to minimize their environmental footprint.
As the field of MOF research advances, there is a growing focus on green synthesis methods that reduce the use of harmful solvents and optimize energy efficiency. These efforts aim to enhance the environmental sustainability of MOF production while maintaining or improving their functional properties. The development of bio-based MOFs and the use of renewable precursors are also emerging trends that could further reduce the environmental impact of these materials.
MOF Characterization Techniques
Characterization techniques play a crucial role in understanding the structure, properties, and performance of Metal-Organic Frameworks (MOFs) synthesized using Sulphanilic Acid. These techniques provide essential information about the physical and chemical properties of the MOFs, enabling researchers to optimize their synthesis and evaluate their potential applications.
X-ray diffraction (XRD) is a fundamental technique used to determine the crystal structure of MOFs. It provides information about the unit cell parameters, space group, and atomic positions within the framework. For MOFs synthesized with Sulphanilic Acid, XRD can reveal the incorporation of the acid into the framework structure and its impact on the overall crystallinity.
Fourier Transform Infrared Spectroscopy (FTIR) is employed to identify the functional groups present in the MOF structure. In the case of Sulphanilic Acid-based MOFs, FTIR can confirm the presence of sulfonic acid groups and their interaction with metal centers or other organic linkers. This technique helps in verifying the successful incorporation of Sulphanilic Acid into the MOF structure.
Nitrogen adsorption-desorption isotherms are used to determine the surface area and pore size distribution of MOFs. For Sulphanilic Acid-based MOFs, this technique can reveal how the incorporation of the acid affects the porosity and surface characteristics of the framework. The Brunauer-Emmett-Teller (BET) method is commonly used to calculate the specific surface area, while the Barrett-Joyner-Halenda (BJH) method is applied to determine the pore size distribution.
Thermogravimetric Analysis (TGA) is essential for evaluating the thermal stability of MOFs. In the context of Sulphanilic Acid-based MOFs, TGA can provide information about the decomposition temperature of the framework and the weight loss associated with the removal of guest molecules or the breakdown of organic linkers.
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are employed to study the morphology and particle size of MOFs. These techniques can reveal how the incorporation of Sulphanilic Acid affects the crystal growth and overall structure of the MOF particles. SEM provides information about surface features, while TEM can offer insights into the internal structure and crystallinity of the MOF.
Elemental analysis techniques, such as Energy-Dispersive X-ray Spectroscopy (EDS) or X-ray Photoelectron Spectroscopy (XPS), are used to determine the elemental composition of MOFs. For Sulphanilic Acid-based MOFs, these techniques can confirm the presence of sulfur and provide information about the metal-to-ligand ratio in the framework.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state NMR, can provide valuable information about the local chemical environment of atoms within the MOF structure. This technique is particularly useful for studying the interaction between Sulphanilic Acid and other components of the framework.
X-ray diffraction (XRD) is a fundamental technique used to determine the crystal structure of MOFs. It provides information about the unit cell parameters, space group, and atomic positions within the framework. For MOFs synthesized with Sulphanilic Acid, XRD can reveal the incorporation of the acid into the framework structure and its impact on the overall crystallinity.
Fourier Transform Infrared Spectroscopy (FTIR) is employed to identify the functional groups present in the MOF structure. In the case of Sulphanilic Acid-based MOFs, FTIR can confirm the presence of sulfonic acid groups and their interaction with metal centers or other organic linkers. This technique helps in verifying the successful incorporation of Sulphanilic Acid into the MOF structure.
Nitrogen adsorption-desorption isotherms are used to determine the surface area and pore size distribution of MOFs. For Sulphanilic Acid-based MOFs, this technique can reveal how the incorporation of the acid affects the porosity and surface characteristics of the framework. The Brunauer-Emmett-Teller (BET) method is commonly used to calculate the specific surface area, while the Barrett-Joyner-Halenda (BJH) method is applied to determine the pore size distribution.
Thermogravimetric Analysis (TGA) is essential for evaluating the thermal stability of MOFs. In the context of Sulphanilic Acid-based MOFs, TGA can provide information about the decomposition temperature of the framework and the weight loss associated with the removal of guest molecules or the breakdown of organic linkers.
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are employed to study the morphology and particle size of MOFs. These techniques can reveal how the incorporation of Sulphanilic Acid affects the crystal growth and overall structure of the MOF particles. SEM provides information about surface features, while TEM can offer insights into the internal structure and crystallinity of the MOF.
Elemental analysis techniques, such as Energy-Dispersive X-ray Spectroscopy (EDS) or X-ray Photoelectron Spectroscopy (XPS), are used to determine the elemental composition of MOFs. For Sulphanilic Acid-based MOFs, these techniques can confirm the presence of sulfur and provide information about the metal-to-ligand ratio in the framework.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state NMR, can provide valuable information about the local chemical environment of atoms within the MOF structure. This technique is particularly useful for studying the interaction between Sulphanilic Acid and other components of the framework.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!