Revolutionizing Pharmaceutical Use with Isocyanates
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isocyanate Pharma Evolution
The evolution of isocyanates in the pharmaceutical industry represents a significant paradigm shift in drug development and manufacturing. Initially utilized primarily in industrial applications, isocyanates have gradually found their way into pharmaceutical research due to their unique chemical properties and versatility.
In the early stages, isocyanates were primarily used in the synthesis of polyurethanes for medical devices and drug delivery systems. However, as researchers delved deeper into their potential, a new frontier emerged in drug design. The ability of isocyanates to form covalent bonds with various functional groups opened up possibilities for creating novel drug molecules with enhanced stability and bioavailability.
The 1980s marked a turning point when scientists began exploring isocyanates as building blocks for active pharmaceutical ingredients (APIs). This period saw the development of isocyanate-based prodrugs, which could be metabolized in the body to release the active compound. These innovations paved the way for improved drug targeting and reduced side effects.
By the late 1990s and early 2000s, advances in synthetic chemistry and a better understanding of isocyanate reactivity led to the creation of more sophisticated drug candidates. Researchers began incorporating isocyanate moieties into existing drug scaffolds to modify their pharmacokinetic properties. This approach resulted in the development of several promising compounds with enhanced therapeutic profiles.
The past decade has witnessed a surge in isocyanate-based pharmaceuticals entering clinical trials. These compounds span a wide range of therapeutic areas, including oncology, neurology, and infectious diseases. The ability to fine-tune drug properties through isocyanate chemistry has become a valuable tool in addressing challenges such as poor solubility and rapid metabolism.
Recent breakthroughs in click chemistry and bioorthogonal reactions have further expanded the potential of isocyanates in drug discovery. Scientists are now exploring their use in targeted drug delivery systems and theranostic agents, combining diagnostic and therapeutic capabilities in a single molecule.
Looking ahead, the integration of artificial intelligence and machine learning in drug design is expected to accelerate the discovery of novel isocyanate-based pharmaceuticals. These technologies can predict optimal molecular structures and guide the synthesis of compounds with desired properties, potentially revolutionizing the drug development process.
In the early stages, isocyanates were primarily used in the synthesis of polyurethanes for medical devices and drug delivery systems. However, as researchers delved deeper into their potential, a new frontier emerged in drug design. The ability of isocyanates to form covalent bonds with various functional groups opened up possibilities for creating novel drug molecules with enhanced stability and bioavailability.
The 1980s marked a turning point when scientists began exploring isocyanates as building blocks for active pharmaceutical ingredients (APIs). This period saw the development of isocyanate-based prodrugs, which could be metabolized in the body to release the active compound. These innovations paved the way for improved drug targeting and reduced side effects.
By the late 1990s and early 2000s, advances in synthetic chemistry and a better understanding of isocyanate reactivity led to the creation of more sophisticated drug candidates. Researchers began incorporating isocyanate moieties into existing drug scaffolds to modify their pharmacokinetic properties. This approach resulted in the development of several promising compounds with enhanced therapeutic profiles.
The past decade has witnessed a surge in isocyanate-based pharmaceuticals entering clinical trials. These compounds span a wide range of therapeutic areas, including oncology, neurology, and infectious diseases. The ability to fine-tune drug properties through isocyanate chemistry has become a valuable tool in addressing challenges such as poor solubility and rapid metabolism.
Recent breakthroughs in click chemistry and bioorthogonal reactions have further expanded the potential of isocyanates in drug discovery. Scientists are now exploring their use in targeted drug delivery systems and theranostic agents, combining diagnostic and therapeutic capabilities in a single molecule.
Looking ahead, the integration of artificial intelligence and machine learning in drug design is expected to accelerate the discovery of novel isocyanate-based pharmaceuticals. These technologies can predict optimal molecular structures and guide the synthesis of compounds with desired properties, potentially revolutionizing the drug development process.
Market Demand Analysis
The market demand for isocyanates in the pharmaceutical industry has been steadily growing, driven by their versatile applications in drug delivery systems, medical devices, and pharmaceutical packaging. Isocyanates play a crucial role in the development of polyurethane-based materials, which are increasingly utilized in controlled-release formulations and biocompatible implants.
In the drug delivery sector, isocyanate-derived polymers are gaining traction due to their ability to enhance drug solubility, improve bioavailability, and provide targeted release mechanisms. This has led to a surge in research and development activities focused on novel drug delivery systems, particularly for challenging therapeutic compounds with poor solubility or stability profiles.
The medical device industry has also witnessed a rising demand for isocyanate-based materials, especially in the production of catheters, stents, and wound dressings. These materials offer excellent mechanical properties, biocompatibility, and the ability to incorporate antimicrobial agents, addressing key challenges in infection control and patient comfort.
Pharmaceutical packaging represents another significant market segment for isocyanates. The industry's shift towards more sustainable and intelligent packaging solutions has spurred the development of isocyanate-based barrier coatings and smart packaging materials. These innovations aim to extend product shelf life, improve tamper-evidence, and enhance drug stability during storage and transportation.
The global trend towards personalized medicine and advanced therapies has further amplified the demand for specialized drug delivery systems and medical devices, many of which rely on isocyanate chemistry. This trend is expected to continue, driving innovation in materials science and opening new avenues for isocyanate applications in the pharmaceutical sector.
However, the market growth is not without challenges. Regulatory scrutiny regarding the safety of isocyanates and their potential environmental impact has intensified. This has led to increased investment in research aimed at developing safer, more environmentally friendly isocyanate alternatives and improved handling protocols.
Despite these challenges, the overall market outlook for isocyanates in pharmaceuticals remains positive. The industry's focus on innovation, coupled with the expanding applications of isocyanate-based materials in drug delivery, medical devices, and packaging, suggests a robust growth trajectory. As pharmaceutical companies continue to seek advanced solutions for complex therapeutic challenges, the demand for isocyanate-derived technologies is expected to expand, fostering collaborations between material scientists, pharmaceutical researchers, and medical device manufacturers.
In the drug delivery sector, isocyanate-derived polymers are gaining traction due to their ability to enhance drug solubility, improve bioavailability, and provide targeted release mechanisms. This has led to a surge in research and development activities focused on novel drug delivery systems, particularly for challenging therapeutic compounds with poor solubility or stability profiles.
The medical device industry has also witnessed a rising demand for isocyanate-based materials, especially in the production of catheters, stents, and wound dressings. These materials offer excellent mechanical properties, biocompatibility, and the ability to incorporate antimicrobial agents, addressing key challenges in infection control and patient comfort.
Pharmaceutical packaging represents another significant market segment for isocyanates. The industry's shift towards more sustainable and intelligent packaging solutions has spurred the development of isocyanate-based barrier coatings and smart packaging materials. These innovations aim to extend product shelf life, improve tamper-evidence, and enhance drug stability during storage and transportation.
The global trend towards personalized medicine and advanced therapies has further amplified the demand for specialized drug delivery systems and medical devices, many of which rely on isocyanate chemistry. This trend is expected to continue, driving innovation in materials science and opening new avenues for isocyanate applications in the pharmaceutical sector.
However, the market growth is not without challenges. Regulatory scrutiny regarding the safety of isocyanates and their potential environmental impact has intensified. This has led to increased investment in research aimed at developing safer, more environmentally friendly isocyanate alternatives and improved handling protocols.
Despite these challenges, the overall market outlook for isocyanates in pharmaceuticals remains positive. The industry's focus on innovation, coupled with the expanding applications of isocyanate-based materials in drug delivery, medical devices, and packaging, suggests a robust growth trajectory. As pharmaceutical companies continue to seek advanced solutions for complex therapeutic challenges, the demand for isocyanate-derived technologies is expected to expand, fostering collaborations between material scientists, pharmaceutical researchers, and medical device manufacturers.
Technical Challenges
The use of isocyanates in pharmaceutical applications presents several significant technical challenges that researchers and manufacturers must overcome. One of the primary obstacles is the high reactivity of isocyanates, which can lead to unintended side reactions and product instability. This reactivity, while beneficial for certain applications, requires careful control and handling to prevent degradation of the active pharmaceutical ingredients or unwanted modifications to the drug's structure.
Another major challenge lies in the toxicity of isocyanates, particularly their potential to cause respiratory sensitization and occupational asthma. This necessitates stringent safety measures and protective equipment during manufacturing processes, as well as the development of novel formulations that minimize exposure risks for both workers and end-users. The implementation of these safety protocols can significantly increase production costs and complexity.
The moisture sensitivity of isocyanates poses additional difficulties in pharmaceutical manufacturing. Exposure to ambient humidity can trigger unwanted reactions, leading to product degradation or loss of efficacy. This requires the development of specialized storage and handling techniques, as well as moisture-resistant packaging solutions to maintain product integrity throughout the supply chain.
Achieving precise control over the reaction kinetics of isocyanates in pharmaceutical formulations is another technical hurdle. The rate and extent of isocyanate reactions must be carefully managed to ensure consistent product quality and performance. This demands advanced process control systems and in-depth understanding of reaction mechanisms under various conditions.
The potential for isocyanate residues in final pharmaceutical products raises concerns about long-term stability and patient safety. Developing analytical methods for detecting and quantifying trace amounts of unreacted isocyanates or their byproducts is crucial for quality control and regulatory compliance. This challenge is compounded by the diverse range of isocyanate compounds and their potential degradation products.
Compatibility issues between isocyanates and other pharmaceutical excipients or active ingredients present yet another technical obstacle. Formulation scientists must carefully consider potential interactions and develop strategies to prevent adverse effects on drug stability, bioavailability, or therapeutic efficacy. This often requires extensive compatibility studies and innovative formulation approaches.
Lastly, the scalability of isocyanate-based pharmaceutical processes from laboratory to industrial production levels presents significant engineering challenges. Maintaining reaction control, product consistency, and safety standards at larger scales requires sophisticated equipment design and process optimization. Overcoming these scaling issues is essential for the commercial viability of isocyanate-based pharmaceutical technologies.
Another major challenge lies in the toxicity of isocyanates, particularly their potential to cause respiratory sensitization and occupational asthma. This necessitates stringent safety measures and protective equipment during manufacturing processes, as well as the development of novel formulations that minimize exposure risks for both workers and end-users. The implementation of these safety protocols can significantly increase production costs and complexity.
The moisture sensitivity of isocyanates poses additional difficulties in pharmaceutical manufacturing. Exposure to ambient humidity can trigger unwanted reactions, leading to product degradation or loss of efficacy. This requires the development of specialized storage and handling techniques, as well as moisture-resistant packaging solutions to maintain product integrity throughout the supply chain.
Achieving precise control over the reaction kinetics of isocyanates in pharmaceutical formulations is another technical hurdle. The rate and extent of isocyanate reactions must be carefully managed to ensure consistent product quality and performance. This demands advanced process control systems and in-depth understanding of reaction mechanisms under various conditions.
The potential for isocyanate residues in final pharmaceutical products raises concerns about long-term stability and patient safety. Developing analytical methods for detecting and quantifying trace amounts of unreacted isocyanates or their byproducts is crucial for quality control and regulatory compliance. This challenge is compounded by the diverse range of isocyanate compounds and their potential degradation products.
Compatibility issues between isocyanates and other pharmaceutical excipients or active ingredients present yet another technical obstacle. Formulation scientists must carefully consider potential interactions and develop strategies to prevent adverse effects on drug stability, bioavailability, or therapeutic efficacy. This often requires extensive compatibility studies and innovative formulation approaches.
Lastly, the scalability of isocyanate-based pharmaceutical processes from laboratory to industrial production levels presents significant engineering challenges. Maintaining reaction control, product consistency, and safety standards at larger scales requires sophisticated equipment design and process optimization. Overcoming these scaling issues is essential for the commercial viability of isocyanate-based pharmaceutical technologies.
Current Pharma Solutions
01 Synthesis and production of isocyanates
Various methods and processes for synthesizing and producing isocyanates are described. These include novel reaction pathways, catalysts, and production techniques to improve yield, purity, and efficiency in isocyanate manufacturing.- Synthesis and production of isocyanates: Various methods and processes for synthesizing and producing isocyanates are described. These include novel catalysts, reaction conditions, and precursor materials to improve yield, purity, and efficiency in isocyanate production.
- Applications of isocyanates in polymer chemistry: Isocyanates are widely used in polymer chemistry, particularly in the production of polyurethanes. The patents describe various applications, including coatings, adhesives, foams, and elastomers, as well as novel formulations and processing techniques.
- Isocyanate-based catalysts and additives: Several patents focus on the development of isocyanate-based catalysts and additives for various chemical processes. These include novel catalyst systems, stabilizers, and modifiers that enhance reaction rates, selectivity, or product properties.
- Safety and handling of isocyanates: Given the reactive nature of isocyanates, patents in this category address safety concerns and improved handling methods. This includes storage techniques, exposure prevention, and the development of less hazardous isocyanate derivatives or alternatives.
- Isocyanate-free alternatives and substitutes: Some patents focus on developing alternatives to traditional isocyanates, aiming to address environmental and health concerns. These include novel chemistries, bio-based materials, and modified production processes that reduce or eliminate the use of conventional isocyanates.
02 Applications of isocyanates in polymer chemistry
Isocyanates are widely used in polymer chemistry, particularly in the production of polyurethanes. The patents discuss various applications, including coatings, adhesives, foams, and elastomers, as well as novel formulations and processing techniques.Expand Specific Solutions03 Isocyanate-based catalysts and reaction modifiers
Several patents focus on the development of isocyanate-based catalysts and reaction modifiers. These compounds are used to enhance chemical reactions, improve product properties, or facilitate specific industrial processes.Expand Specific Solutions04 Safety and handling of isocyanates
Given the reactive nature of isocyanates, patents address safety concerns and handling procedures. This includes methods for reducing toxicity, improving storage stability, and developing safer alternatives or modified isocyanates with reduced health risks.Expand Specific Solutions05 Isocyanate-free alternatives and substitutes
Some patents explore alternatives to traditional isocyanates, aiming to address environmental and health concerns. These include bio-based substitutes, non-isocyanate polyurethanes, and alternative chemistries that can replace isocyanates in certain applications.Expand Specific Solutions
Key Industry Players
The pharmaceutical industry's use of isocyanates is in a mature stage, with established players and well-developed applications. The market size is substantial, driven by the growing demand for polyurethane-based products in various sectors. Technologically, isocyanates are well-understood, but innovation continues in areas like safety and sustainability. Key players such as Wanhua Chemical Group, BASF, and Covestro dominate the market, leveraging their extensive R&D capabilities and global presence. Emerging companies like Wanhua Chemical (Ningbo) and JSR Corp are also making significant contributions, particularly in specialized applications and regional markets. The industry faces challenges in developing greener alternatives and addressing health concerns, spurring ongoing research and development efforts.
Wanhua Chemical Group Co., Ltd.
Technical Solution: Wanhua Chemical Group has developed innovative isocyanate-based pharmaceutical solutions, focusing on controlled release drug delivery systems. Their technology utilizes the reactivity of isocyanates to create polyurethane-based matrices that encapsulate active pharmaceutical ingredients (APIs). This approach allows for precise control over drug release rates, improving therapeutic efficacy and reducing side effects[1]. The company has also pioneered the use of bio-based isocyanates derived from renewable resources, addressing sustainability concerns in pharmaceutical manufacturing[2]. Their research extends to the development of isocyanate-functionalized polymers for targeted drug delivery, enhancing the specificity of treatments for various diseases[3].
Strengths: Advanced controlled release technology, sustainable bio-based materials, and targeted delivery systems. Weaknesses: Potential toxicity concerns with some isocyanates, regulatory challenges for novel drug delivery systems.
BASF Corp.
Technical Solution: BASF Corp. has made significant strides in revolutionizing pharmaceutical use with isocyanates through their development of novel polyurethane-based drug delivery systems. Their technology utilizes the versatile chemistry of isocyanates to create tailored polymer matrices that can encapsulate a wide range of APIs. BASF's approach focuses on enhancing drug bioavailability and controlling release kinetics, resulting in improved therapeutic outcomes[1]. The company has also developed isocyanate-based crosslinking agents for the production of hydrogels used in advanced wound care and tissue engineering applications[2]. Additionally, BASF has pioneered the use of water-based polyurethane dispersions for pharmaceutical coatings, offering improved stability and reduced environmental impact compared to traditional solvent-based systems[3].
Strengths: Diverse applications in drug delivery and biomaterials, strong focus on sustainability, extensive R&D capabilities. Weaknesses: Complexity in scaling up novel formulations, potential regulatory hurdles for innovative delivery systems.
Isocyanate Innovations
Novel isocyanate and isothiocyanate compounds for cancer treatment
PatentInactiveEP2758367A1
Innovation
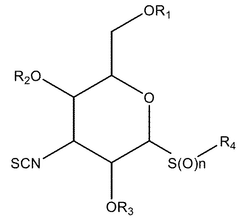
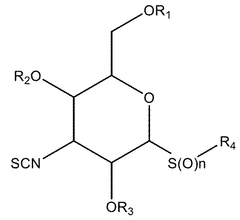
- Development of novel isocyanate and isothiocyanate compounds with specific structural modifications, such as those in formula (1), which reduce toxicity and enhance cytostatic activity, allowing for targeted therapy with fewer side effects.
Thiosugar based isothiocyanates and uses thereof
PatentWO2025073693A1
Innovation
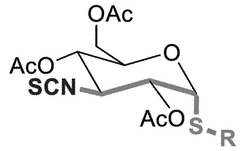
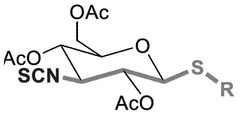
- Development of novel isothiocyanate derivatives, specifically thiosugar-based isothiocyanates, which exhibit activation capacity of the Nrf2 factor, cytotoxic activity against solid tumors, and antileukemic activity, making them potential lead compounds for cancer therapy.
Regulatory Framework
The regulatory framework surrounding the use of isocyanates in pharmaceutical applications is complex and multifaceted, reflecting the potential hazards associated with these compounds as well as their significant therapeutic potential. Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and various national health authorities, have established stringent guidelines for the use of isocyanates in drug development and manufacturing processes.
One of the primary concerns addressed by regulatory frameworks is the potential toxicity of isocyanates. These compounds are known respiratory sensitizers and can cause occupational asthma. As a result, regulatory agencies have implemented strict exposure limits and safety protocols for workers in pharmaceutical manufacturing facilities. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, has set permissible exposure limits (PELs) for various isocyanates, requiring employers to implement engineering controls and personal protective equipment to minimize worker exposure.
In the context of drug development, regulatory bodies require extensive safety and efficacy data before approving isocyanate-containing pharmaceuticals. This includes comprehensive toxicology studies, long-term stability assessments, and detailed information on the manufacturing process. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has established guidelines for impurities in drug substances and products, which are particularly relevant for isocyanate-based pharmaceuticals due to the potential for residual unreacted isocyanates.
Environmental regulations also play a crucial role in the use of isocyanates in the pharmaceutical industry. Many countries have implemented strict controls on the release of isocyanates into the environment, requiring pharmaceutical companies to develop robust waste management and disposal protocols. The Environmental Protection Agency (EPA) in the United States, for example, regulates isocyanates under the Toxic Substances Control Act (TSCA) and requires reporting of significant new uses of these compounds.
As the pharmaceutical industry continues to explore novel applications of isocyanates, regulatory frameworks are evolving to keep pace with technological advancements. There is an increasing focus on developing guidelines for the use of isocyanates in targeted drug delivery systems and nanotechnology-based pharmaceuticals. Regulatory agencies are also working to harmonize international standards to facilitate global drug development and marketing while maintaining rigorous safety standards.
The regulatory landscape for isocyanates in pharmaceuticals is characterized by a balance between promoting innovation and ensuring public safety. As research continues to uncover new potential applications for these compounds in drug development, it is likely that regulatory frameworks will continue to adapt, potentially leading to more nuanced and application-specific guidelines in the future.
One of the primary concerns addressed by regulatory frameworks is the potential toxicity of isocyanates. These compounds are known respiratory sensitizers and can cause occupational asthma. As a result, regulatory agencies have implemented strict exposure limits and safety protocols for workers in pharmaceutical manufacturing facilities. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, has set permissible exposure limits (PELs) for various isocyanates, requiring employers to implement engineering controls and personal protective equipment to minimize worker exposure.
In the context of drug development, regulatory bodies require extensive safety and efficacy data before approving isocyanate-containing pharmaceuticals. This includes comprehensive toxicology studies, long-term stability assessments, and detailed information on the manufacturing process. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has established guidelines for impurities in drug substances and products, which are particularly relevant for isocyanate-based pharmaceuticals due to the potential for residual unreacted isocyanates.
Environmental regulations also play a crucial role in the use of isocyanates in the pharmaceutical industry. Many countries have implemented strict controls on the release of isocyanates into the environment, requiring pharmaceutical companies to develop robust waste management and disposal protocols. The Environmental Protection Agency (EPA) in the United States, for example, regulates isocyanates under the Toxic Substances Control Act (TSCA) and requires reporting of significant new uses of these compounds.
As the pharmaceutical industry continues to explore novel applications of isocyanates, regulatory frameworks are evolving to keep pace with technological advancements. There is an increasing focus on developing guidelines for the use of isocyanates in targeted drug delivery systems and nanotechnology-based pharmaceuticals. Regulatory agencies are also working to harmonize international standards to facilitate global drug development and marketing while maintaining rigorous safety standards.
The regulatory landscape for isocyanates in pharmaceuticals is characterized by a balance between promoting innovation and ensuring public safety. As research continues to uncover new potential applications for these compounds in drug development, it is likely that regulatory frameworks will continue to adapt, potentially leading to more nuanced and application-specific guidelines in the future.
Environmental Impact
The use of isocyanates in pharmaceutical applications has raised significant environmental concerns due to their potential impact on ecosystems and human health. These compounds, while revolutionizing drug delivery and formulation, pose challenges in terms of production, handling, and disposal.
One of the primary environmental issues associated with isocyanates is their persistence in the environment. When released into water bodies or soil, these chemicals can remain stable for extended periods, potentially accumulating in the food chain. This bioaccumulation can lead to long-term ecological effects, affecting aquatic life and terrestrial organisms.
Air pollution is another critical concern. During the manufacturing process of isocyanate-based pharmaceuticals, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions contribute to smog formation and can have adverse effects on air quality, particularly in industrial areas where production facilities are located.
The disposal of isocyanate-containing pharmaceutical waste presents additional environmental challenges. Improper disposal methods can lead to soil and groundwater contamination, potentially affecting drinking water sources and agricultural land. This necessitates the development of specialized waste management protocols to ensure safe handling and disposal of these materials.
Furthermore, the production of isocyanates often involves the use of fossil fuel-derived raw materials, contributing to carbon emissions and climate change. As the pharmaceutical industry seeks to reduce its carbon footprint, finding more sustainable sources and production methods for isocyanates becomes increasingly important.
To address these environmental concerns, researchers and pharmaceutical companies are exploring various mitigation strategies. These include the development of green chemistry approaches to isocyanate synthesis, utilizing renewable resources as raw materials, and implementing closed-loop manufacturing systems to minimize waste and emissions.
Additionally, efforts are being made to improve the biodegradability of isocyanate-based pharmaceutical products. This involves designing molecules that can break down more readily in the environment without compromising their therapeutic efficacy. Such innovations could significantly reduce the long-term environmental impact of these compounds.
Regulatory bodies are also playing a crucial role in addressing the environmental impact of isocyanates in pharmaceuticals. Stricter guidelines for production, handling, and disposal are being implemented to ensure environmental protection. These regulations often require pharmaceutical companies to conduct thorough environmental impact assessments and implement robust monitoring systems.
In conclusion, while isocyanates offer significant benefits in pharmaceutical applications, their environmental impact cannot be overlooked. Balancing the revolutionary potential of these compounds with sustainable practices and environmental stewardship remains a key challenge for the pharmaceutical industry. Ongoing research and technological advancements will be crucial in minimizing the ecological footprint of isocyanate-based pharmaceuticals while maximizing their therapeutic benefits.
One of the primary environmental issues associated with isocyanates is their persistence in the environment. When released into water bodies or soil, these chemicals can remain stable for extended periods, potentially accumulating in the food chain. This bioaccumulation can lead to long-term ecological effects, affecting aquatic life and terrestrial organisms.
Air pollution is another critical concern. During the manufacturing process of isocyanate-based pharmaceuticals, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions contribute to smog formation and can have adverse effects on air quality, particularly in industrial areas where production facilities are located.
The disposal of isocyanate-containing pharmaceutical waste presents additional environmental challenges. Improper disposal methods can lead to soil and groundwater contamination, potentially affecting drinking water sources and agricultural land. This necessitates the development of specialized waste management protocols to ensure safe handling and disposal of these materials.
Furthermore, the production of isocyanates often involves the use of fossil fuel-derived raw materials, contributing to carbon emissions and climate change. As the pharmaceutical industry seeks to reduce its carbon footprint, finding more sustainable sources and production methods for isocyanates becomes increasingly important.
To address these environmental concerns, researchers and pharmaceutical companies are exploring various mitigation strategies. These include the development of green chemistry approaches to isocyanate synthesis, utilizing renewable resources as raw materials, and implementing closed-loop manufacturing systems to minimize waste and emissions.
Additionally, efforts are being made to improve the biodegradability of isocyanate-based pharmaceutical products. This involves designing molecules that can break down more readily in the environment without compromising their therapeutic efficacy. Such innovations could significantly reduce the long-term environmental impact of these compounds.
Regulatory bodies are also playing a crucial role in addressing the environmental impact of isocyanates in pharmaceuticals. Stricter guidelines for production, handling, and disposal are being implemented to ensure environmental protection. These regulations often require pharmaceutical companies to conduct thorough environmental impact assessments and implement robust monitoring systems.
In conclusion, while isocyanates offer significant benefits in pharmaceutical applications, their environmental impact cannot be overlooked. Balancing the revolutionary potential of these compounds with sustainable practices and environmental stewardship remains a key challenge for the pharmaceutical industry. Ongoing research and technological advancements will be crucial in minimizing the ecological footprint of isocyanate-based pharmaceuticals while maximizing their therapeutic benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!