Role of Calorimetry in Understanding Wood Pyrolysis Reactions
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calorimetry in Wood Pyrolysis: Background and Objectives
Calorimetry has emerged as a crucial tool in understanding the complex reactions involved in wood pyrolysis. The study of wood pyrolysis, which involves the thermal decomposition of wood in the absence of oxygen, has gained significant attention due to its relevance in various applications, including biofuel production, biomass conversion, and fire safety.
The historical development of calorimetry in wood pyrolysis research can be traced back to the early 20th century when scientists began to explore the thermal behavior of wood. Initially, simple calorimetric techniques were employed to measure the heat released during wood combustion. However, as the field progressed, more sophisticated calorimetric methods were developed to specifically study pyrolysis reactions.
In recent decades, the application of calorimetry in wood pyrolysis has expanded dramatically, driven by advancements in instrumentation and analytical techniques. Modern calorimetric methods, such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), have enabled researchers to obtain detailed information about the thermal events occurring during pyrolysis, including phase transitions, decomposition temperatures, and heat flow patterns.
The primary objective of using calorimetry in wood pyrolysis research is to gain a comprehensive understanding of the thermal behavior and reaction kinetics involved in the process. This includes quantifying the energy requirements for different stages of pyrolysis, identifying key reaction pathways, and elucidating the influence of various factors such as heating rate, particle size, and wood composition on the pyrolysis process.
Furthermore, calorimetric studies aim to provide insights into the complex interplay between the different components of wood (cellulose, hemicellulose, and lignin) during pyrolysis. By analyzing the thermal decomposition patterns of these individual components and comparing them to the behavior of whole wood, researchers can develop more accurate models of the pyrolysis process.
Another critical objective is to optimize the pyrolysis conditions for specific applications. For instance, in the context of biofuel production, calorimetry helps in determining the optimal temperature ranges and heating rates that maximize the yield of desired products while minimizing energy input. Similarly, in fire safety applications, calorimetric data are essential for predicting the fire behavior of wood-based materials and developing effective fire-retardant strategies.
As the field continues to evolve, emerging trends in calorimetric research for wood pyrolysis include the integration of calorimetry with other analytical techniques, such as mass spectrometry and infrared spectroscopy, to provide a more comprehensive characterization of the pyrolysis process. Additionally, there is a growing focus on in-situ calorimetric measurements, which allow for real-time monitoring of pyrolysis reactions under more realistic conditions.
The historical development of calorimetry in wood pyrolysis research can be traced back to the early 20th century when scientists began to explore the thermal behavior of wood. Initially, simple calorimetric techniques were employed to measure the heat released during wood combustion. However, as the field progressed, more sophisticated calorimetric methods were developed to specifically study pyrolysis reactions.
In recent decades, the application of calorimetry in wood pyrolysis has expanded dramatically, driven by advancements in instrumentation and analytical techniques. Modern calorimetric methods, such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), have enabled researchers to obtain detailed information about the thermal events occurring during pyrolysis, including phase transitions, decomposition temperatures, and heat flow patterns.
The primary objective of using calorimetry in wood pyrolysis research is to gain a comprehensive understanding of the thermal behavior and reaction kinetics involved in the process. This includes quantifying the energy requirements for different stages of pyrolysis, identifying key reaction pathways, and elucidating the influence of various factors such as heating rate, particle size, and wood composition on the pyrolysis process.
Furthermore, calorimetric studies aim to provide insights into the complex interplay between the different components of wood (cellulose, hemicellulose, and lignin) during pyrolysis. By analyzing the thermal decomposition patterns of these individual components and comparing them to the behavior of whole wood, researchers can develop more accurate models of the pyrolysis process.
Another critical objective is to optimize the pyrolysis conditions for specific applications. For instance, in the context of biofuel production, calorimetry helps in determining the optimal temperature ranges and heating rates that maximize the yield of desired products while minimizing energy input. Similarly, in fire safety applications, calorimetric data are essential for predicting the fire behavior of wood-based materials and developing effective fire-retardant strategies.
As the field continues to evolve, emerging trends in calorimetric research for wood pyrolysis include the integration of calorimetry with other analytical techniques, such as mass spectrometry and infrared spectroscopy, to provide a more comprehensive characterization of the pyrolysis process. Additionally, there is a growing focus on in-situ calorimetric measurements, which allow for real-time monitoring of pyrolysis reactions under more realistic conditions.
Market Demand for Pyrolysis-Based Bioenergy
The market demand for pyrolysis-based bioenergy has been steadily growing in recent years, driven by increasing environmental concerns and the need for sustainable energy sources. Wood pyrolysis, in particular, has gained significant attention due to its potential to produce renewable fuels and chemicals from biomass waste.
The global bioenergy market, which includes pyrolysis-based products, is expected to experience substantial growth in the coming years. This growth is primarily attributed to government initiatives promoting renewable energy sources, rising energy demands, and the depletion of fossil fuel reserves. The wood pyrolysis sector is poised to benefit from this trend, as it offers a viable solution for converting abundant biomass resources into valuable energy products.
One of the key drivers for the market demand of pyrolysis-based bioenergy is the increasing focus on reducing greenhouse gas emissions. Pyrolysis technology allows for the efficient conversion of wood waste into bio-oil, biochar, and syngas, which can be used as cleaner alternatives to fossil fuels. This aligns well with global efforts to mitigate climate change and transition towards a low-carbon economy.
The agricultural and forestry industries generate significant amounts of wood waste, creating a readily available feedstock for pyrolysis processes. This abundance of raw material contributes to the growing interest in pyrolysis-based bioenergy solutions. Additionally, the versatility of pyrolysis products, such as bio-oil and biochar, opens up diverse market opportunities across various sectors, including energy, agriculture, and materials science.
In the energy sector, bio-oil produced through wood pyrolysis can be used as a renewable fuel for heat and power generation. The demand for such alternative fuels is increasing, particularly in regions with stringent emissions regulations. Biochar, another valuable product of wood pyrolysis, finds applications in soil amendment and carbon sequestration, addressing both agricultural productivity and climate change mitigation concerns.
The market for pyrolysis-based bioenergy is also driven by technological advancements in the field. Improved reactor designs, process optimization, and better understanding of pyrolysis reactions through calorimetry studies have enhanced the efficiency and economic viability of wood pyrolysis processes. These developments have made pyrolysis-based bioenergy more attractive to investors and industry stakeholders.
However, challenges remain in fully realizing the market potential of pyrolysis-based bioenergy. These include the need for further cost reductions, scaling up of technologies, and establishing robust supply chains. Despite these challenges, the overall market trend indicates a positive outlook for wood pyrolysis and its derived products, with growing interest from both public and private sectors in harnessing this technology for sustainable energy production.
The global bioenergy market, which includes pyrolysis-based products, is expected to experience substantial growth in the coming years. This growth is primarily attributed to government initiatives promoting renewable energy sources, rising energy demands, and the depletion of fossil fuel reserves. The wood pyrolysis sector is poised to benefit from this trend, as it offers a viable solution for converting abundant biomass resources into valuable energy products.
One of the key drivers for the market demand of pyrolysis-based bioenergy is the increasing focus on reducing greenhouse gas emissions. Pyrolysis technology allows for the efficient conversion of wood waste into bio-oil, biochar, and syngas, which can be used as cleaner alternatives to fossil fuels. This aligns well with global efforts to mitigate climate change and transition towards a low-carbon economy.
The agricultural and forestry industries generate significant amounts of wood waste, creating a readily available feedstock for pyrolysis processes. This abundance of raw material contributes to the growing interest in pyrolysis-based bioenergy solutions. Additionally, the versatility of pyrolysis products, such as bio-oil and biochar, opens up diverse market opportunities across various sectors, including energy, agriculture, and materials science.
In the energy sector, bio-oil produced through wood pyrolysis can be used as a renewable fuel for heat and power generation. The demand for such alternative fuels is increasing, particularly in regions with stringent emissions regulations. Biochar, another valuable product of wood pyrolysis, finds applications in soil amendment and carbon sequestration, addressing both agricultural productivity and climate change mitigation concerns.
The market for pyrolysis-based bioenergy is also driven by technological advancements in the field. Improved reactor designs, process optimization, and better understanding of pyrolysis reactions through calorimetry studies have enhanced the efficiency and economic viability of wood pyrolysis processes. These developments have made pyrolysis-based bioenergy more attractive to investors and industry stakeholders.
However, challenges remain in fully realizing the market potential of pyrolysis-based bioenergy. These include the need for further cost reductions, scaling up of technologies, and establishing robust supply chains. Despite these challenges, the overall market trend indicates a positive outlook for wood pyrolysis and its derived products, with growing interest from both public and private sectors in harnessing this technology for sustainable energy production.
Current Challenges in Wood Pyrolysis Calorimetry
Despite significant advancements in wood pyrolysis research, several challenges persist in the field of calorimetry for understanding these complex reactions. One of the primary difficulties lies in the accurate measurement of heat flow during the pyrolysis process. The heterogeneous nature of wood and the multitude of simultaneous reactions occurring during thermal decomposition make it challenging to isolate and quantify individual heat contributions.
The high temperatures involved in pyrolysis reactions pose another significant challenge. Many conventional calorimetric techniques are limited in their ability to operate effectively at the extreme temperatures required for wood pyrolysis, which can exceed 500°C. This limitation often necessitates the use of specialized equipment or indirect measurement methods, potentially introducing errors or reducing the resolution of thermal data.
Another critical issue is the dynamic nature of wood pyrolysis reactions. The rate and extent of thermal decomposition can vary significantly depending on factors such as heating rate, particle size, and the presence of catalysts or inhibitors. Capturing these dynamic changes accurately with calorimetric methods requires high temporal resolution and sensitivity, which can be technically demanding and expensive to achieve.
The formation of char and its insulating effect during pyrolysis further complicate calorimetric measurements. As char forms on the surface of wood particles, it can impede heat transfer and alter the thermal properties of the sample, potentially leading to inaccuracies in heat flow measurements. Accounting for these changes in thermal conductivity and heat capacity throughout the pyrolysis process remains a significant challenge.
Moreover, the release of volatile compounds during pyrolysis can interfere with calorimetric measurements. These volatiles can condense on sensor surfaces or escape from the measurement system, leading to underestimation of the total heat released during the process. Developing methods to capture and account for these volatile components without disrupting the calorimetric measurements is an ongoing area of research.
The complexity of wood composition, with its varying ratios of cellulose, hemicellulose, and lignin, adds another layer of difficulty to calorimetric studies. Different wood species and even different parts of the same tree can exhibit varying thermal behaviors, making it challenging to develop standardized calorimetric methods applicable across a wide range of wood types.
Lastly, the integration of calorimetric data with other analytical techniques, such as thermogravimetric analysis (TGA) and mass spectrometry, presents both opportunities and challenges. While combining these methods can provide a more comprehensive understanding of pyrolysis reactions, synchronizing and correlating data from multiple instruments introduces additional complexity and potential sources of error.
The high temperatures involved in pyrolysis reactions pose another significant challenge. Many conventional calorimetric techniques are limited in their ability to operate effectively at the extreme temperatures required for wood pyrolysis, which can exceed 500°C. This limitation often necessitates the use of specialized equipment or indirect measurement methods, potentially introducing errors or reducing the resolution of thermal data.
Another critical issue is the dynamic nature of wood pyrolysis reactions. The rate and extent of thermal decomposition can vary significantly depending on factors such as heating rate, particle size, and the presence of catalysts or inhibitors. Capturing these dynamic changes accurately with calorimetric methods requires high temporal resolution and sensitivity, which can be technically demanding and expensive to achieve.
The formation of char and its insulating effect during pyrolysis further complicate calorimetric measurements. As char forms on the surface of wood particles, it can impede heat transfer and alter the thermal properties of the sample, potentially leading to inaccuracies in heat flow measurements. Accounting for these changes in thermal conductivity and heat capacity throughout the pyrolysis process remains a significant challenge.
Moreover, the release of volatile compounds during pyrolysis can interfere with calorimetric measurements. These volatiles can condense on sensor surfaces or escape from the measurement system, leading to underestimation of the total heat released during the process. Developing methods to capture and account for these volatile components without disrupting the calorimetric measurements is an ongoing area of research.
The complexity of wood composition, with its varying ratios of cellulose, hemicellulose, and lignin, adds another layer of difficulty to calorimetric studies. Different wood species and even different parts of the same tree can exhibit varying thermal behaviors, making it challenging to develop standardized calorimetric methods applicable across a wide range of wood types.
Lastly, the integration of calorimetric data with other analytical techniques, such as thermogravimetric analysis (TGA) and mass spectrometry, presents both opportunities and challenges. While combining these methods can provide a more comprehensive understanding of pyrolysis reactions, synchronizing and correlating data from multiple instruments introduces additional complexity and potential sources of error.
Existing Calorimetric Methods for Wood Pyrolysis
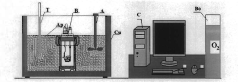
01 Calorimetry measurement techniques
Various techniques are employed in calorimetry to measure heat transfer and energy changes in chemical or physical processes. These methods may include differential scanning calorimetry, isothermal titration calorimetry, and bomb calorimetry. Advanced sensors and precise temperature control systems are utilized to ensure accurate measurements of heat flow and thermal properties.- Calorimetry measurement techniques: Various techniques are employed in calorimetry to measure heat changes in chemical or physical processes. These methods include differential scanning calorimetry, isothermal titration calorimetry, and bomb calorimetry. Each technique is suited for specific applications and provides valuable data on thermodynamic properties of materials.
- Data analysis and interpretation in calorimetry: Advanced algorithms and software tools are used to analyze and interpret calorimetric data. These methods involve signal processing, peak detection, and curve fitting to extract meaningful information from raw calorimetric measurements. Machine learning techniques are increasingly applied to improve data analysis accuracy and efficiency.
- Applications of calorimetry in various fields: Calorimetry finds applications in diverse fields such as materials science, pharmaceuticals, food industry, and biochemistry. It is used for studying phase transitions, determining binding affinities, assessing food quality, and investigating metabolic processes. The versatility of calorimetric techniques makes them valuable tools in research and development.
- Miniaturization and automation of calorimetric devices: Recent advancements focus on developing miniaturized and automated calorimetric devices. These innovations aim to reduce sample size requirements, increase throughput, and improve measurement precision. Microfluidic calorimetry and lab-on-a-chip devices are examples of such developments, enabling more efficient and cost-effective analyses.
- Integration of calorimetry with other analytical techniques: Calorimetry is increasingly integrated with other analytical techniques to provide comprehensive characterization of materials and processes. Combining calorimetry with spectroscopy, chromatography, or microscopy allows for simultaneous measurement of multiple parameters, enhancing the understanding of complex systems and their behavior under various conditions.
02 Data analysis and interpretation in calorimetry
Sophisticated algorithms and software tools are developed to analyze and interpret calorimetric data. These systems can process complex thermodynamic information, identify patterns, and extract meaningful insights from raw calorimetric measurements. Machine learning and artificial intelligence techniques may be applied to enhance data interpretation and predictive capabilities.Expand Specific Solutions03 Applications of calorimetry in various fields
Calorimetry finds applications in diverse fields such as materials science, pharmaceuticals, food industry, and biochemistry. It is used for studying phase transitions, determining binding affinities of biomolecules, assessing food quality, and characterizing the thermal properties of materials. The versatility of calorimetric techniques makes it a valuable tool in research and development across multiple industries.Expand Specific Solutions04 Miniaturization and portability of calorimetric devices
Efforts are being made to develop miniaturized and portable calorimetric devices. These innovations aim to bring calorimetry capabilities to field applications, point-of-care diagnostics, and on-site quality control. Microfluidic technologies and advanced manufacturing techniques are employed to create compact, yet highly sensitive calorimetric systems.Expand Specific Solutions05 Integration of calorimetry with other analytical techniques
Calorimetry is being integrated with other analytical techniques to provide comprehensive characterization of materials and processes. This may include coupling calorimetry with spectroscopy, chromatography, or microscopy. Such integrated systems offer synergistic benefits, allowing for simultaneous measurement of thermal properties along with structural, chemical, or morphological information.Expand Specific Solutions
Key Players in Wood Pyrolysis and Calorimetry
The calorimetry in wood pyrolysis reactions field is in a growth phase, with increasing market size driven by renewable energy and biorefinery applications. The technology is moderately mature, with ongoing research to improve efficiency and scalability. Key players like Beijing Forestry University, Nanjing Forestry University, and UOP LLC are advancing the understanding of pyrolysis kinetics and heat transfer mechanisms. Companies such as Ensyn Renewables and Anaergia are commercializing pyrolysis technologies, while research institutions like The Energy & Resources Institute are exploring novel applications. The competitive landscape is diverse, with academic institutions, established energy companies, and innovative startups all contributing to technological advancements in this field.
Beijing Forestry University
Technical Solution: Beijing Forestry University has developed advanced calorimetry techniques to study wood pyrolysis reactions. Their approach combines Differential Scanning Calorimetry (DSC) with Thermogravimetric Analysis (TGA) to provide comprehensive insights into the thermal behavior of wood during pyrolysis[1]. This method allows for the simultaneous measurement of heat flow and mass loss, enabling researchers to identify key reaction stages and quantify the energy requirements for different pyrolysis processes. The university has also pioneered the use of high-pressure DSC to simulate industrial pyrolysis conditions more accurately, leading to improved understanding of reaction kinetics and product formation pathways[2].
Strengths: Comprehensive thermal analysis, simulation of industrial conditions. Weaknesses: Potential limitations in scaling up laboratory findings to industrial applications.
Nanjing Forestry University
Technical Solution: Nanjing Forestry University has developed a novel approach to studying wood pyrolysis using micro-calorimetry techniques. Their method focuses on the use of microscale combustion calorimetry (MCC) to analyze the heat release rate and total heat release during wood pyrolysis[3]. This technique allows for the characterization of different wood species and their components (cellulose, hemicellulose, and lignin) with minimal sample sizes. The university has also integrated this approach with spectroscopic methods like FTIR to correlate thermal events with chemical changes during pyrolysis[4]. Their research has led to improved understanding of the influence of wood composition on pyrolysis behavior and product distribution.
Strengths: High-resolution analysis of wood components, correlation of thermal and chemical events. Weaknesses: Limited to small sample sizes, which may not fully represent bulk material behavior.
Innovative Calorimetric Approaches in Pyrolysis Studies
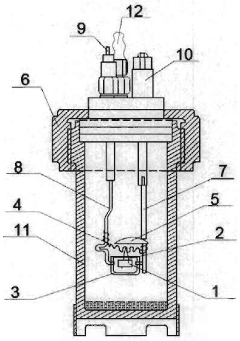
Process for determining the caloric power of wooden biomass using a calorimeter bomb provided with its own software
PatentUndeterminedRO129020A0
Innovation
- A calibrated calorimetric pump system with proprietary software that standardizes the preparation and testing of wood samples, using benzoic acid calibration and Pre-Control method validation to ensure accurate and efficient determination of higher and lower calorific power, facilitating precise and labor-saving calculations.
Method for conducting a pyrolysis process of woody biomass
PatentWO2023175384A1
Innovation
- A pyrolysis process that involves grinding woody biomass into particles less than 3cm³, drying to less than 10% humidity, and heating in a horizontal reactor with heat transfer balls preheated to 400°C-650°C, ensuring consistent heat transfer and prolonged heating duration to achieve temperatures of 550°C-660°C within the reactor, maximizing thermal energy storage and contact surface area.
Environmental Impact of Wood Pyrolysis Technologies
Wood pyrolysis technologies, while offering potential benefits in bioenergy production and waste management, also pose significant environmental concerns that require careful consideration. The environmental impact of these technologies encompasses various aspects, including air emissions, soil and water contamination, and greenhouse gas emissions.
One of the primary environmental concerns associated with wood pyrolysis is air pollution. The process releases a range of volatile organic compounds (VOCs), particulate matter, and other potentially harmful substances into the atmosphere. These emissions can contribute to local air quality issues and may have adverse effects on human health and ecosystems. The specific composition and quantity of emissions depend on factors such as feedstock characteristics, pyrolysis conditions, and the presence of pollution control measures.
Soil and water contamination are additional environmental risks associated with wood pyrolysis technologies. The production of biochar, a byproduct of pyrolysis, can lead to the accumulation of heavy metals and other contaminants in soil when applied as a soil amendment. Furthermore, improper handling or disposal of pyrolysis residues may result in the leaching of pollutants into groundwater or surface water bodies, potentially impacting aquatic ecosystems and water resources.
The carbon footprint of wood pyrolysis technologies is a complex issue that requires careful assessment. While these processes can potentially contribute to carbon sequestration through biochar production, they also generate greenhouse gas emissions during the pyrolysis process itself. The net carbon balance depends on various factors, including feedstock sourcing, energy efficiency of the pyrolysis system, and the ultimate fate of the pyrolysis products.
Land use changes associated with the sourcing of wood feedstock for pyrolysis can have significant environmental implications. Increased demand for wood biomass may lead to deforestation or the conversion of natural habitats, potentially resulting in biodiversity loss and ecosystem degradation. Sustainable forest management practices and the use of waste wood or agricultural residues as feedstock can help mitigate these impacts.
To address these environmental concerns, ongoing research and development efforts are focused on improving the efficiency and sustainability of wood pyrolysis technologies. This includes the development of advanced emission control systems, optimization of pyrolysis conditions to minimize pollutant formation, and the exploration of innovative approaches to utilize pyrolysis byproducts in environmentally beneficial ways. Additionally, life cycle assessments and environmental impact studies are crucial for evaluating the overall sustainability of wood pyrolysis technologies and informing policy decisions regarding their implementation and regulation.
One of the primary environmental concerns associated with wood pyrolysis is air pollution. The process releases a range of volatile organic compounds (VOCs), particulate matter, and other potentially harmful substances into the atmosphere. These emissions can contribute to local air quality issues and may have adverse effects on human health and ecosystems. The specific composition and quantity of emissions depend on factors such as feedstock characteristics, pyrolysis conditions, and the presence of pollution control measures.
Soil and water contamination are additional environmental risks associated with wood pyrolysis technologies. The production of biochar, a byproduct of pyrolysis, can lead to the accumulation of heavy metals and other contaminants in soil when applied as a soil amendment. Furthermore, improper handling or disposal of pyrolysis residues may result in the leaching of pollutants into groundwater or surface water bodies, potentially impacting aquatic ecosystems and water resources.
The carbon footprint of wood pyrolysis technologies is a complex issue that requires careful assessment. While these processes can potentially contribute to carbon sequestration through biochar production, they also generate greenhouse gas emissions during the pyrolysis process itself. The net carbon balance depends on various factors, including feedstock sourcing, energy efficiency of the pyrolysis system, and the ultimate fate of the pyrolysis products.
Land use changes associated with the sourcing of wood feedstock for pyrolysis can have significant environmental implications. Increased demand for wood biomass may lead to deforestation or the conversion of natural habitats, potentially resulting in biodiversity loss and ecosystem degradation. Sustainable forest management practices and the use of waste wood or agricultural residues as feedstock can help mitigate these impacts.
To address these environmental concerns, ongoing research and development efforts are focused on improving the efficiency and sustainability of wood pyrolysis technologies. This includes the development of advanced emission control systems, optimization of pyrolysis conditions to minimize pollutant formation, and the exploration of innovative approaches to utilize pyrolysis byproducts in environmentally beneficial ways. Additionally, life cycle assessments and environmental impact studies are crucial for evaluating the overall sustainability of wood pyrolysis technologies and informing policy decisions regarding their implementation and regulation.
Standardization of Calorimetric Methods in Pyrolysis
Standardization of calorimetric methods in pyrolysis is crucial for ensuring consistent and comparable results across different research studies and industrial applications. The lack of standardized procedures has been a significant challenge in the field, leading to difficulties in comparing data from various sources and replicating experimental results.
To address this issue, several international organizations and research institutions have been working towards developing standardized protocols for calorimetric measurements in pyrolysis. These efforts focus on establishing uniform sample preparation techniques, calibration procedures, and data analysis methods.
One key aspect of standardization is the selection of appropriate reference materials for calibration. Benzoic acid has been widely adopted as a primary standard due to its well-defined thermodynamic properties. However, for wood pyrolysis specifically, cellulose and lignin standards are being considered to better represent the complex composition of wood biomass.
Standardized sample preparation methods are essential for obtaining reproducible results. This includes guidelines for particle size distribution, moisture content, and sample mass. For wood samples, a uniform particle size range of 0.5-1 mm is often recommended to ensure consistent heat and mass transfer during pyrolysis.
Temperature calibration and control are critical factors in calorimetric measurements. Standardized procedures typically involve the use of high-purity metal standards with known melting points to verify and calibrate temperature readings across different instruments and laboratories.
Data analysis and reporting protocols are being developed to ensure consistency in interpreting calorimetric results. This includes standardized methods for baseline correction, peak integration, and the calculation of reaction enthalpies and kinetic parameters.
Interlaboratory comparison studies are being conducted to validate the proposed standardized methods. These studies involve multiple laboratories performing identical experiments on the same set of samples, allowing for the assessment of reproducibility and the identification of potential sources of variability.
The implementation of standardized calorimetric methods in pyrolysis is expected to significantly enhance the reliability and comparability of research findings. This will facilitate more accurate modeling of pyrolysis processes, leading to improved design and optimization of biomass conversion technologies.
To address this issue, several international organizations and research institutions have been working towards developing standardized protocols for calorimetric measurements in pyrolysis. These efforts focus on establishing uniform sample preparation techniques, calibration procedures, and data analysis methods.
One key aspect of standardization is the selection of appropriate reference materials for calibration. Benzoic acid has been widely adopted as a primary standard due to its well-defined thermodynamic properties. However, for wood pyrolysis specifically, cellulose and lignin standards are being considered to better represent the complex composition of wood biomass.
Standardized sample preparation methods are essential for obtaining reproducible results. This includes guidelines for particle size distribution, moisture content, and sample mass. For wood samples, a uniform particle size range of 0.5-1 mm is often recommended to ensure consistent heat and mass transfer during pyrolysis.
Temperature calibration and control are critical factors in calorimetric measurements. Standardized procedures typically involve the use of high-purity metal standards with known melting points to verify and calibrate temperature readings across different instruments and laboratories.
Data analysis and reporting protocols are being developed to ensure consistency in interpreting calorimetric results. This includes standardized methods for baseline correction, peak integration, and the calculation of reaction enthalpies and kinetic parameters.
Interlaboratory comparison studies are being conducted to validate the proposed standardized methods. These studies involve multiple laboratories performing identical experiments on the same set of samples, allowing for the assessment of reproducibility and the identification of potential sources of variability.
The implementation of standardized calorimetric methods in pyrolysis is expected to significantly enhance the reliability and comparability of research findings. This will facilitate more accurate modeling of pyrolysis processes, leading to improved design and optimization of biomass conversion technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!