The Role of Calorimetry in Ice Nucleation Studies
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calorimetry in Ice Nucleation: Background and Objectives
Calorimetry has played a pivotal role in the study of ice nucleation, offering valuable insights into the thermodynamics and kinetics of this complex process. The field of ice nucleation research has evolved significantly over the past century, with calorimetry emerging as a crucial tool for understanding the fundamental mechanisms involved in the formation of ice crystals.
The primary objective of employing calorimetry in ice nucleation studies is to quantify the heat released or absorbed during the phase transition from liquid water to solid ice. This information is essential for elucidating the energetics of nucleation events and the subsequent growth of ice crystals. By precisely measuring the thermal changes associated with ice formation, researchers can gain a deeper understanding of the factors that influence nucleation rates and the overall freezing process.
Calorimetric techniques have undergone substantial advancements, from basic ice calorimeters to sophisticated differential scanning calorimetry (DSC) and isothermal microcalorimetry. These modern methods allow for high-precision measurements of heat flow, enabling researchers to detect even minute thermal events associated with ice nucleation. Such capabilities have proven invaluable in studying heterogeneous ice nucleation, where the presence of foreign particles or surfaces can significantly alter the nucleation process.
One of the key goals in applying calorimetry to ice nucleation research is to bridge the gap between theoretical models and experimental observations. By providing accurate thermodynamic data, calorimetric studies help refine existing nucleation theories and validate computational models. This synergy between experiment and theory is crucial for advancing our understanding of ice formation in various contexts, from atmospheric sciences to cryobiology.
In recent years, the integration of calorimetry with other analytical techniques has opened new avenues for ice nucleation research. For instance, combining calorimetry with spectroscopic methods or microscopy allows for simultaneous observation of thermal events and structural changes during ice formation. This multidisciplinary approach aims to provide a more comprehensive picture of the nucleation process, addressing longstanding questions about the molecular-level mechanisms involved.
As we look to the future, the role of calorimetry in ice nucleation studies is expected to expand further. Emerging technologies, such as nanocalorimetry and ultrafast calorimetry, promise to push the boundaries of what can be measured, potentially allowing for real-time observation of individual nucleation events. These advancements align with the overarching goal of unraveling the complexities of ice nucleation across different scales, from single molecules to bulk systems.
The primary objective of employing calorimetry in ice nucleation studies is to quantify the heat released or absorbed during the phase transition from liquid water to solid ice. This information is essential for elucidating the energetics of nucleation events and the subsequent growth of ice crystals. By precisely measuring the thermal changes associated with ice formation, researchers can gain a deeper understanding of the factors that influence nucleation rates and the overall freezing process.
Calorimetric techniques have undergone substantial advancements, from basic ice calorimeters to sophisticated differential scanning calorimetry (DSC) and isothermal microcalorimetry. These modern methods allow for high-precision measurements of heat flow, enabling researchers to detect even minute thermal events associated with ice nucleation. Such capabilities have proven invaluable in studying heterogeneous ice nucleation, where the presence of foreign particles or surfaces can significantly alter the nucleation process.
One of the key goals in applying calorimetry to ice nucleation research is to bridge the gap between theoretical models and experimental observations. By providing accurate thermodynamic data, calorimetric studies help refine existing nucleation theories and validate computational models. This synergy between experiment and theory is crucial for advancing our understanding of ice formation in various contexts, from atmospheric sciences to cryobiology.
In recent years, the integration of calorimetry with other analytical techniques has opened new avenues for ice nucleation research. For instance, combining calorimetry with spectroscopic methods or microscopy allows for simultaneous observation of thermal events and structural changes during ice formation. This multidisciplinary approach aims to provide a more comprehensive picture of the nucleation process, addressing longstanding questions about the molecular-level mechanisms involved.
As we look to the future, the role of calorimetry in ice nucleation studies is expected to expand further. Emerging technologies, such as nanocalorimetry and ultrafast calorimetry, promise to push the boundaries of what can be measured, potentially allowing for real-time observation of individual nucleation events. These advancements align with the overarching goal of unraveling the complexities of ice nucleation across different scales, from single molecules to bulk systems.
Market Demand for Ice Nucleation Research
The market demand for ice nucleation research has been steadily growing, driven by its critical applications in various industries and scientific fields. Climate science and atmospheric research heavily rely on understanding ice nucleation processes to improve climate models and predict weather patterns more accurately. This has led to increased funding for research projects and the development of advanced calorimetry techniques specifically tailored for ice nucleation studies.
In the pharmaceutical industry, ice nucleation research plays a crucial role in developing better cryopreservation methods for biological samples and organs. As the demand for organ transplants and cell-based therapies continues to rise, there is a growing need for improved freezing and thawing techniques that minimize damage to tissues. This has created a significant market for calorimetry instruments and expertise in ice nucleation studies within the biomedical sector.
The food industry also contributes to the market demand for ice nucleation research. Manufacturers of frozen foods and ice cream are constantly seeking ways to improve product texture and quality through better control of ice crystal formation. This has led to investments in calorimetry equipment and research collaborations with academic institutions to study ice nucleation in food systems.
Aviation and aerospace sectors have shown increasing interest in ice nucleation research to address safety concerns related to ice formation on aircraft surfaces. The development of anti-icing and de-icing technologies relies heavily on understanding the fundamental principles of ice nucleation, creating a niche market for specialized calorimetry instruments and research services.
Environmental protection agencies and water treatment facilities are another growing market segment for ice nucleation research. The study of ice nucleation processes is essential for developing more efficient water purification methods and understanding the impact of pollutants on cloud formation and precipitation patterns.
The materials science industry has also recognized the importance of ice nucleation research in developing new materials with enhanced properties. From self-cleaning surfaces to advanced thermal management systems, the applications of ice nucleation knowledge are expanding, driving demand for calorimetry-based research tools and expertise.
As the global focus on sustainability and climate change mitigation intensifies, the market demand for ice nucleation research is expected to grow further. This trend is likely to result in increased funding for academic research, as well as the development of more sophisticated calorimetry instruments specifically designed for ice nucleation studies across various industries and scientific disciplines.
In the pharmaceutical industry, ice nucleation research plays a crucial role in developing better cryopreservation methods for biological samples and organs. As the demand for organ transplants and cell-based therapies continues to rise, there is a growing need for improved freezing and thawing techniques that minimize damage to tissues. This has created a significant market for calorimetry instruments and expertise in ice nucleation studies within the biomedical sector.
The food industry also contributes to the market demand for ice nucleation research. Manufacturers of frozen foods and ice cream are constantly seeking ways to improve product texture and quality through better control of ice crystal formation. This has led to investments in calorimetry equipment and research collaborations with academic institutions to study ice nucleation in food systems.
Aviation and aerospace sectors have shown increasing interest in ice nucleation research to address safety concerns related to ice formation on aircraft surfaces. The development of anti-icing and de-icing technologies relies heavily on understanding the fundamental principles of ice nucleation, creating a niche market for specialized calorimetry instruments and research services.
Environmental protection agencies and water treatment facilities are another growing market segment for ice nucleation research. The study of ice nucleation processes is essential for developing more efficient water purification methods and understanding the impact of pollutants on cloud formation and precipitation patterns.
The materials science industry has also recognized the importance of ice nucleation research in developing new materials with enhanced properties. From self-cleaning surfaces to advanced thermal management systems, the applications of ice nucleation knowledge are expanding, driving demand for calorimetry-based research tools and expertise.
As the global focus on sustainability and climate change mitigation intensifies, the market demand for ice nucleation research is expected to grow further. This trend is likely to result in increased funding for academic research, as well as the development of more sophisticated calorimetry instruments specifically designed for ice nucleation studies across various industries and scientific disciplines.
Current Challenges in Calorimetric Ice Nucleation Studies
Calorimetric studies of ice nucleation face several significant challenges that hinder progress in this critical field of research. One of the primary obstacles is the inherent difficulty in accurately measuring the small heat changes associated with ice nucleation events. The heat released during nucleation is often minuscule, requiring highly sensitive calorimetric instruments to detect and quantify these thermal signatures reliably.
Another challenge lies in the stochastic nature of ice nucleation, which introduces variability in experimental results. This randomness makes it challenging to reproduce experiments and draw definitive conclusions from individual measurements. Researchers must conduct numerous trials to obtain statistically significant data, which can be time-consuming and resource-intensive.
The presence of impurities and heterogeneous nucleation sites in experimental setups further complicates calorimetric measurements. These factors can influence the nucleation process and potentially skew results, making it difficult to isolate and study homogeneous nucleation events. Developing methods to control or account for these variables remains an ongoing challenge in the field.
Temperature control and uniformity within the sample present additional hurdles. Achieving precise and consistent temperatures throughout the sample volume is crucial for accurate measurements, but thermal gradients and heat transfer issues can introduce uncertainties. This is particularly problematic when studying supercooled water, where even small temperature fluctuations can trigger unwanted nucleation events.
The time resolution of calorimetric measurements also poses a challenge, especially when investigating rapid nucleation processes. Many current calorimetric techniques struggle to capture the fast kinetics of ice nucleation, potentially missing critical information about the early stages of the process. Improving the temporal resolution of calorimetric methods is an area of active research and development.
Furthermore, interpreting calorimetric data in the context of ice nucleation mechanisms remains challenging. Correlating heat signals with specific molecular-level events and distinguishing between different nucleation pathways require advanced data analysis techniques and theoretical frameworks. Bridging the gap between macroscopic calorimetric measurements and microscopic nucleation processes continues to be a significant challenge in the field.
Lastly, the integration of calorimetry with other analytical techniques presents both opportunities and challenges. While combining calorimetry with spectroscopic or imaging methods can provide more comprehensive insights, synchronizing these diverse techniques and interpreting the resulting multidimensional data sets introduce additional complexities that researchers must navigate.
Another challenge lies in the stochastic nature of ice nucleation, which introduces variability in experimental results. This randomness makes it challenging to reproduce experiments and draw definitive conclusions from individual measurements. Researchers must conduct numerous trials to obtain statistically significant data, which can be time-consuming and resource-intensive.
The presence of impurities and heterogeneous nucleation sites in experimental setups further complicates calorimetric measurements. These factors can influence the nucleation process and potentially skew results, making it difficult to isolate and study homogeneous nucleation events. Developing methods to control or account for these variables remains an ongoing challenge in the field.
Temperature control and uniformity within the sample present additional hurdles. Achieving precise and consistent temperatures throughout the sample volume is crucial for accurate measurements, but thermal gradients and heat transfer issues can introduce uncertainties. This is particularly problematic when studying supercooled water, where even small temperature fluctuations can trigger unwanted nucleation events.
The time resolution of calorimetric measurements also poses a challenge, especially when investigating rapid nucleation processes. Many current calorimetric techniques struggle to capture the fast kinetics of ice nucleation, potentially missing critical information about the early stages of the process. Improving the temporal resolution of calorimetric methods is an area of active research and development.
Furthermore, interpreting calorimetric data in the context of ice nucleation mechanisms remains challenging. Correlating heat signals with specific molecular-level events and distinguishing between different nucleation pathways require advanced data analysis techniques and theoretical frameworks. Bridging the gap between macroscopic calorimetric measurements and microscopic nucleation processes continues to be a significant challenge in the field.
Lastly, the integration of calorimetry with other analytical techniques presents both opportunities and challenges. While combining calorimetry with spectroscopic or imaging methods can provide more comprehensive insights, synchronizing these diverse techniques and interpreting the resulting multidimensional data sets introduce additional complexities that researchers must navigate.
Existing Calorimetric Methods for Ice Nucleation Analysis
01 Calorimetric methods for studying ice nucleation
Calorimetry techniques are used to measure the heat released or absorbed during ice nucleation processes. These methods provide insights into the thermodynamics of ice formation and can be used to study the effectiveness of various ice nucleating agents. Differential scanning calorimetry (DSC) is a common technique employed in this field.- Calorimetric methods for studying ice nucleation: Calorimetry techniques are used to measure the heat released or absorbed during ice nucleation processes. These methods can provide insights into the thermodynamics and kinetics of ice formation, helping to understand the mechanisms of ice nucleation in various systems.
- Ice nucleation in food preservation: Ice nucleation plays a crucial role in food preservation techniques. Controlling ice nucleation can improve the quality and texture of frozen foods, as well as extend their shelf life. Calorimetric studies help optimize freezing processes in the food industry.
- Ice nucleation agents and additives: Various substances can act as ice nucleation agents or additives, influencing the ice formation process. Calorimetric studies are used to evaluate the effectiveness of these agents and their impact on ice nucleation temperatures and rates.
- Ice nucleation in cryopreservation: Calorimetry is employed to study ice nucleation in cryopreservation applications, such as the preservation of biological samples and tissues. Understanding and controlling ice nucleation is crucial for maintaining the viability of preserved specimens.
- Ice nucleation in atmospheric and environmental studies: Calorimetric techniques are used to investigate ice nucleation processes in atmospheric and environmental contexts. These studies help understand cloud formation, precipitation, and the role of aerosols in ice nucleation, contributing to climate and weather research.
02 Ice nucleation in food preservation
Ice nucleation plays a crucial role in food preservation techniques, particularly in freezing processes. Controlled ice nucleation can improve the quality and texture of frozen foods by promoting the formation of smaller ice crystals. This approach is applied in various food industries to enhance product quality and extend shelf life.Expand Specific Solutions03 Environmental applications of ice nucleation studies
Ice nucleation research has significant environmental applications, particularly in atmospheric science and climate studies. Understanding ice nucleation processes helps in predicting cloud formation, precipitation patterns, and climate change effects. Calorimetric methods are used to study ice nucleation in atmospheric aerosols and other environmental samples.Expand Specific Solutions04 Biological ice nucleation agents
Certain biological entities, such as bacteria and proteins, can act as efficient ice nucleation agents. These biological ice nucleators are studied using calorimetric techniques to understand their mechanisms and potential applications. This research has implications in various fields, including agriculture, biotechnology, and cryopreservation.Expand Specific Solutions05 Ice nucleation in material science and engineering
Ice nucleation studies are important in material science and engineering applications, such as the development of anti-icing surfaces, cryogenic materials, and thermal energy storage systems. Calorimetric methods are used to evaluate the ice nucleation properties of various materials and coatings, aiding in the design of more efficient and durable products.Expand Specific Solutions
Key Players in Calorimetry and Ice Nucleation Research
The field of calorimetry in ice nucleation studies is in a growth phase, with increasing market size and technological advancements. The competitive landscape is characterized by a mix of academic institutions, research laboratories, and private companies. Key players like Northeastern University, Palo Alto Research Center, and Integrated DNA Technologies are driving innovation in this niche area. The technology is maturing, with organizations like The Charles Stark Draper Laboratory and Xi'an Jiaotong University contributing to its development. However, the field still has room for further refinement and application expansion, indicating a dynamic and evolving competitive environment.
Northeastern University
Technical Solution: Northeastern University has developed a novel approach to studying ice nucleation using nanocalorimetry combined with in situ electron microscopy. Their technique involves fabricating ultra-thin film calorimeters that can be integrated into a transmission electron microscope (TEM)[13]. This allows for simultaneous thermal measurements and direct visualization of ice crystal formation at the nanoscale. The researchers have achieved femtojoule sensitivity in their calorimetric measurements, enabling the detection of individual nucleation events in nanoliter-sized water droplets[14]. Additionally, they have implemented machine learning algorithms to analyze the TEM images in real-time, correlating structural changes with the calorimetric data. This approach has provided unprecedented insights into the early stages of ice nucleation and the role of surface defects and impurities in the process[15].
Strengths: Direct visualization of nucleation events at the nanoscale, extremely high sensitivity for thermal measurements. Weaknesses: Limited to very small sample volumes, potential electron beam effects on the nucleation process.
Penn State Research Foundation
Technical Solution: Penn State has developed a unique approach to calorimetry in ice nucleation studies, focusing on environmental and atmospheric applications. Their method combines differential scanning calorimetry with a custom-built environmental chamber that can simulate various atmospheric conditions[7]. This setup allows researchers to study ice nucleation on aerosol particles under realistic temperature, pressure, and humidity conditions. The team has also integrated spectroscopic techniques, such as Raman spectroscopy, with their calorimetric measurements to provide chemical information about the nucleating particles[8]. Additionally, Penn State researchers have developed advanced data analysis techniques, including machine learning algorithms, to interpret the complex calorimetric signals and identify different modes of ice nucleation[9].
Strengths: Ability to simulate realistic atmospheric conditions, integration of spectroscopic techniques for chemical analysis. Weaknesses: Complex experimental setup may limit sample throughput, challenges in maintaining precise control over all environmental parameters.
Innovative Calorimetry Approaches in Ice Nucleation
DSC Electrode System Capable of Applying Electric Field
PatentActiveUS20210037812A1
Innovation
- A DSC electrode system that applies an electric field using a differential scanning calorimeter to monitor heat flow changes in biological materials during freezing and reheating, comparing different electric field parameters to inhibit ice crystal formation and improve cryopreservation efficiency.
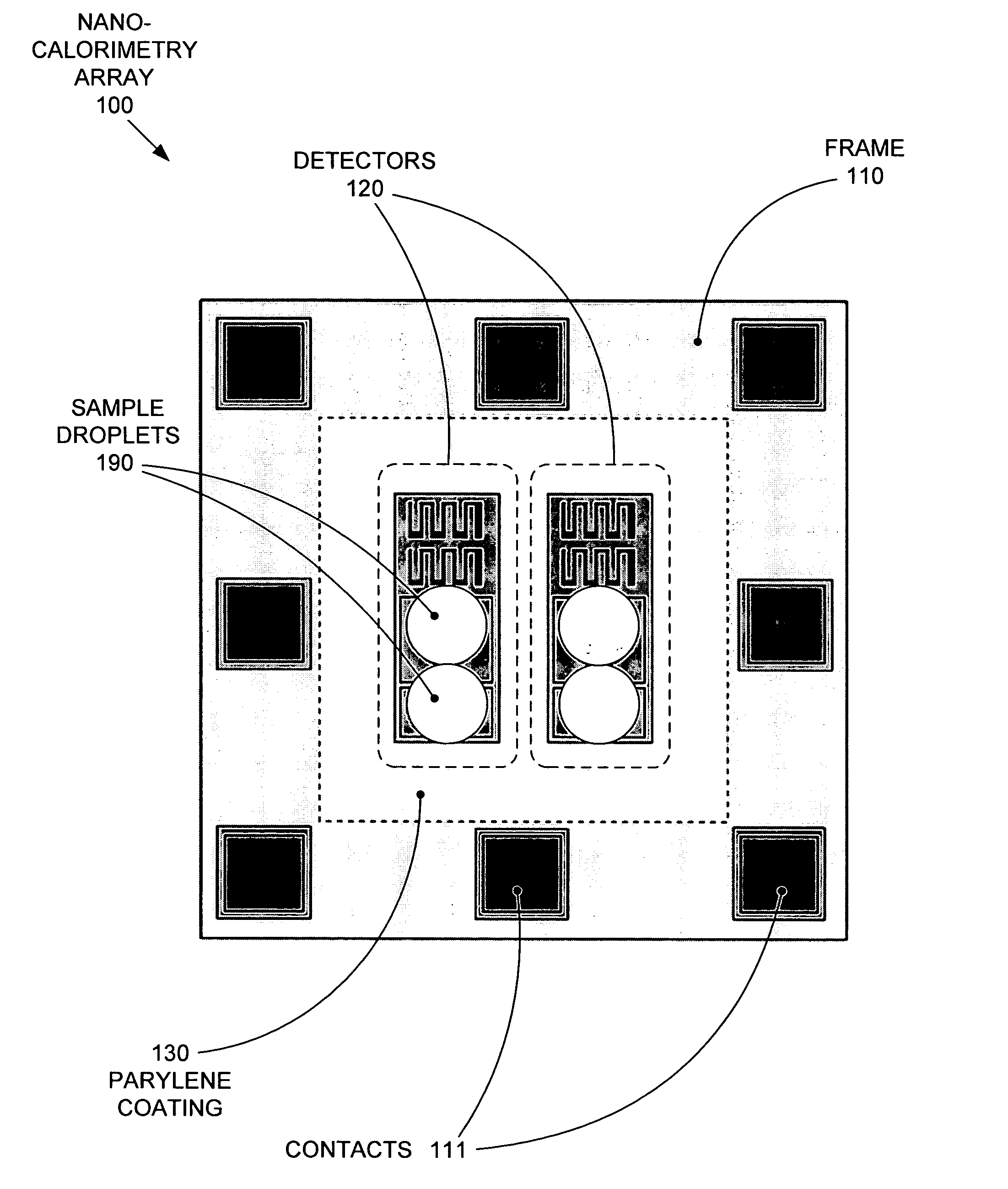
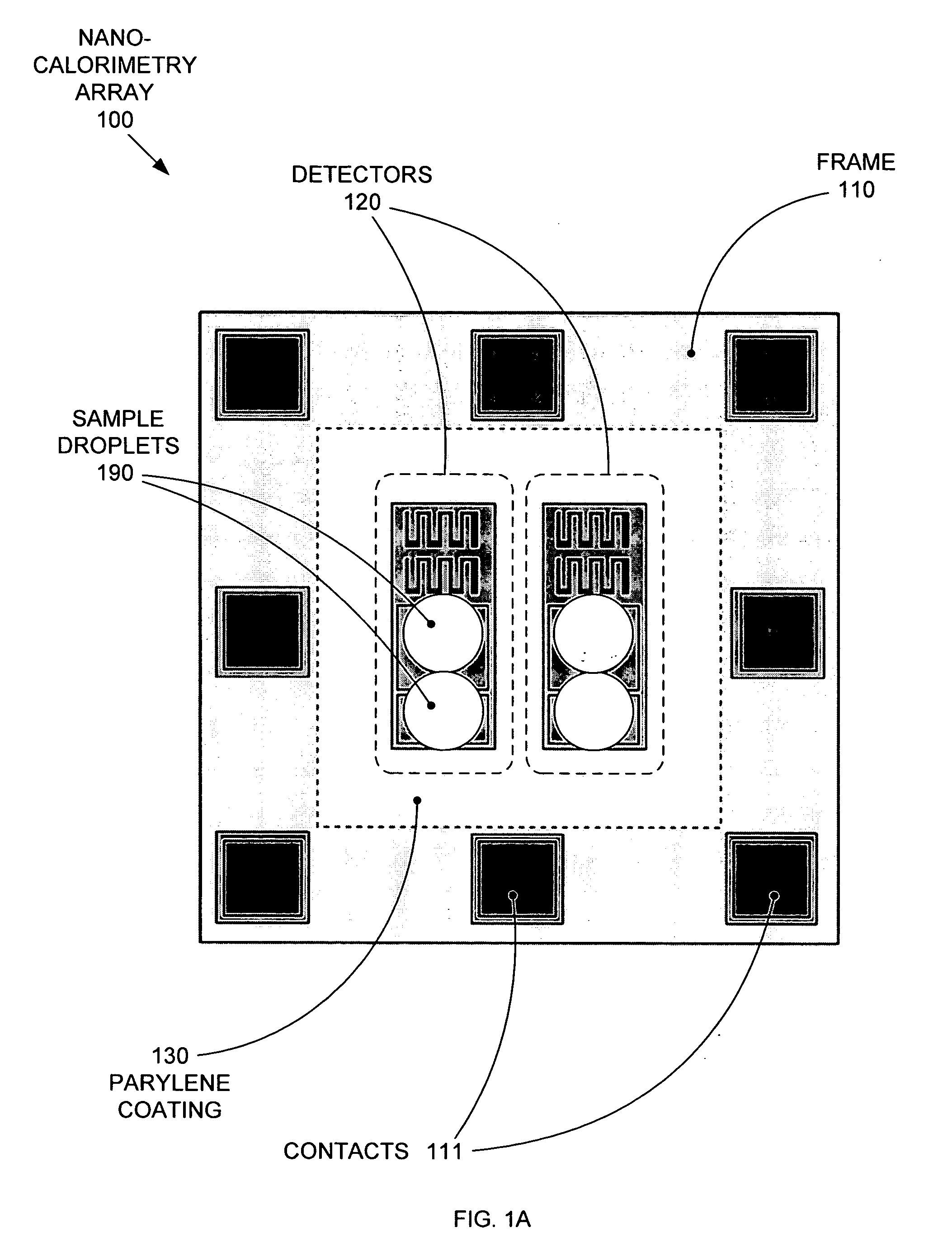
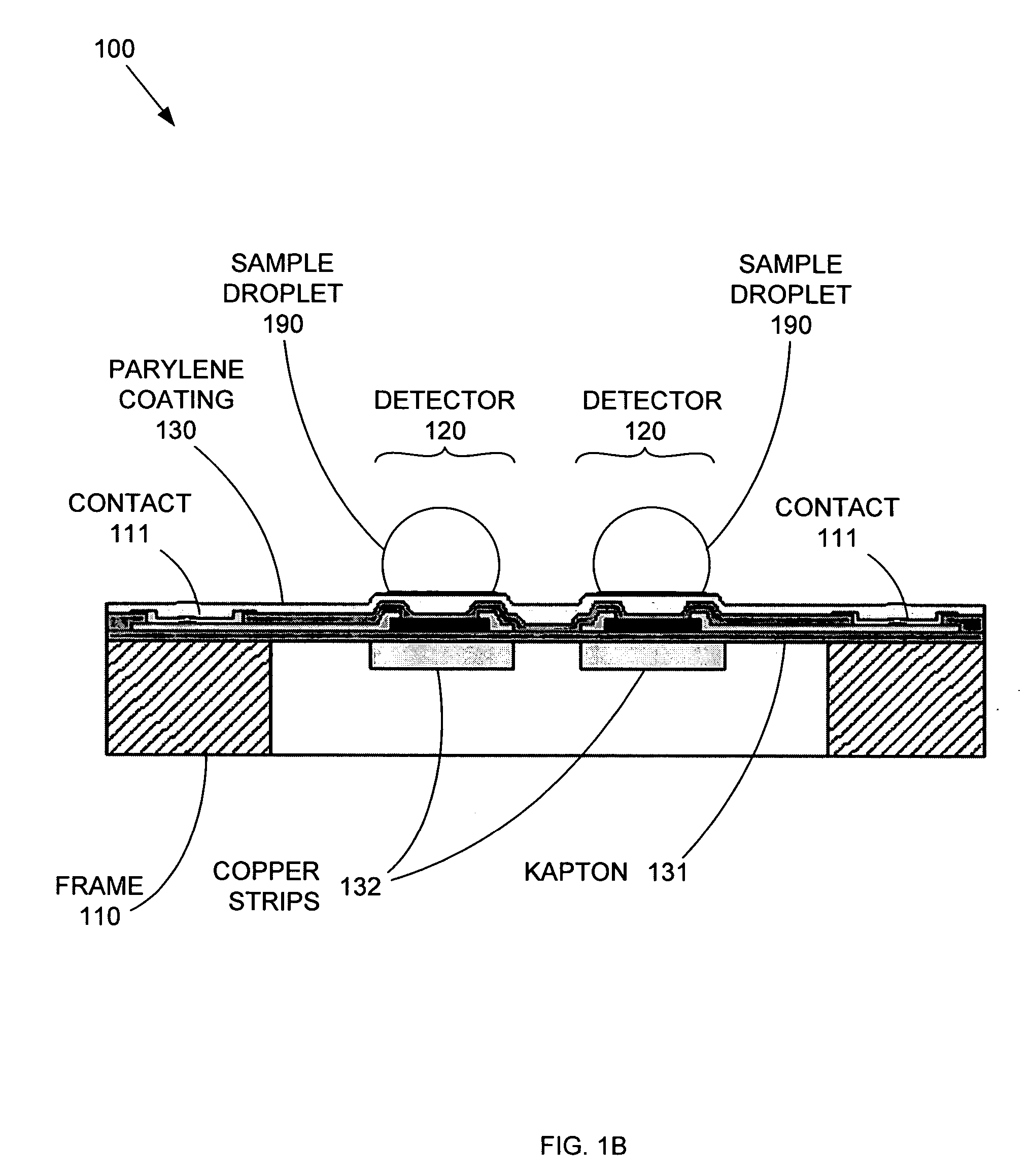
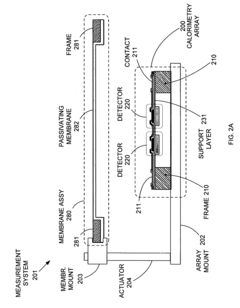
Replaceable parylene membranes for nanocalorimeter
PatentActiveUS20050135455A1
Innovation
- A replaceable passivation membrane is used to cover the measurement elements of a calorimeter array, allowing for reuse and reducing costs by simplifying the replacement process, while maintaining measurement efficiency and preventing contamination.
Environmental Impact of Ice Nucleation Research
Ice nucleation research, particularly through calorimetry, has significant environmental implications that extend beyond laboratory settings. The study of ice formation processes contributes to our understanding of various atmospheric phenomena, including cloud formation, precipitation patterns, and climate change dynamics. As researchers delve deeper into the mechanisms of ice nucleation, they uncover valuable insights that can be applied to environmental modeling and prediction.
One of the primary environmental impacts of ice nucleation research lies in its potential to improve climate models. By understanding how ice crystals form and grow under different conditions, scientists can refine their predictions of cloud behavior and its effects on global temperature patterns. This knowledge is crucial for developing more accurate climate change projections and informing policy decisions aimed at mitigating environmental risks.
Furthermore, ice nucleation studies have implications for understanding and potentially mitigating the effects of atmospheric aerosols. These tiny particles, both natural and anthropogenic, play a significant role in ice formation in the atmosphere. Research in this area can help identify the sources and impacts of various aerosols, leading to more effective strategies for reducing air pollution and its environmental consequences.
In the realm of agriculture, ice nucleation research has the potential to revolutionize frost protection methods. By understanding the mechanisms that trigger ice formation on crops, scientists can develop more efficient and environmentally friendly ways to protect plants from frost damage. This could lead to reduced use of chemical sprays and energy-intensive heating methods, resulting in more sustainable agricultural practices.
The environmental impact of ice nucleation research also extends to the field of water resource management. As climate change alters precipitation patterns, understanding ice formation processes becomes increasingly important for predicting and managing water availability. This knowledge can inform strategies for water conservation, flood prevention, and drought mitigation, all of which have significant environmental implications.
Moreover, ice nucleation studies contribute to our understanding of polar and alpine ecosystems. These environments are particularly sensitive to changes in ice formation patterns, and research in this area can help predict and potentially mitigate the impacts of climate change on these delicate ecosystems. This includes understanding the effects on wildlife habitats, glacial stability, and sea level rise.
One of the primary environmental impacts of ice nucleation research lies in its potential to improve climate models. By understanding how ice crystals form and grow under different conditions, scientists can refine their predictions of cloud behavior and its effects on global temperature patterns. This knowledge is crucial for developing more accurate climate change projections and informing policy decisions aimed at mitigating environmental risks.
Furthermore, ice nucleation studies have implications for understanding and potentially mitigating the effects of atmospheric aerosols. These tiny particles, both natural and anthropogenic, play a significant role in ice formation in the atmosphere. Research in this area can help identify the sources and impacts of various aerosols, leading to more effective strategies for reducing air pollution and its environmental consequences.
In the realm of agriculture, ice nucleation research has the potential to revolutionize frost protection methods. By understanding the mechanisms that trigger ice formation on crops, scientists can develop more efficient and environmentally friendly ways to protect plants from frost damage. This could lead to reduced use of chemical sprays and energy-intensive heating methods, resulting in more sustainable agricultural practices.
The environmental impact of ice nucleation research also extends to the field of water resource management. As climate change alters precipitation patterns, understanding ice formation processes becomes increasingly important for predicting and managing water availability. This knowledge can inform strategies for water conservation, flood prevention, and drought mitigation, all of which have significant environmental implications.
Moreover, ice nucleation studies contribute to our understanding of polar and alpine ecosystems. These environments are particularly sensitive to changes in ice formation patterns, and research in this area can help predict and potentially mitigate the impacts of climate change on these delicate ecosystems. This includes understanding the effects on wildlife habitats, glacial stability, and sea level rise.
Applications of Calorimetry in Climate Science
Calorimetry plays a crucial role in climate science, particularly in the study of ice nucleation processes. This technique provides valuable insights into the thermodynamics and kinetics of phase transitions, which are fundamental to understanding cloud formation and precipitation mechanisms in the atmosphere.
One of the primary applications of calorimetry in climate science is the investigation of ice nucleation in cloud droplets. By measuring the heat released during the freezing process, researchers can determine the temperature at which ice formation occurs and the rate of nucleation. This information is essential for improving climate models and predicting cloud behavior under various atmospheric conditions.
Calorimetric methods are also employed to study the effects of aerosols on ice nucleation. Aerosols, such as dust particles and organic compounds, can serve as ice nuclei, influencing the formation of ice crystals in clouds. By using calorimetry to analyze the freezing behavior of water droplets containing different types of aerosols, scientists can better understand how these particles impact cloud microphysics and, consequently, global climate patterns.
Furthermore, calorimetry is utilized in the examination of ice nucleation in biological systems, which has implications for both terrestrial and marine ecosystems. For instance, studying the freezing characteristics of plant tissues and microorganisms can provide insights into how different species adapt to cold environments and how climate change may affect their survival and distribution.
In the context of paleoclimatology, calorimetric techniques are applied to analyze ice cores and other frozen samples. By measuring the energy required to melt these samples and examining their composition, researchers can reconstruct past climate conditions and track long-term changes in atmospheric composition and temperature.
Calorimetry also contributes to the development and evaluation of cloud seeding technologies. By assessing the effectiveness of various ice nucleating agents through calorimetric measurements, scientists can optimize cloud seeding strategies for weather modification and precipitation enhancement in arid regions.
Moreover, calorimetric studies aid in understanding the role of ice nucleation in the formation of polar stratospheric clouds. These clouds play a significant role in ozone depletion, and calorimetric data on their formation processes are crucial for predicting and mitigating ozone loss in polar regions.
In conclusion, calorimetry serves as a powerful tool in climate science, enabling researchers to unravel the complexities of ice nucleation and its far-reaching effects on atmospheric processes, ecosystem dynamics, and global climate patterns. The insights gained from calorimetric studies continue to refine our understanding of climate systems and inform strategies for addressing climate change challenges.
One of the primary applications of calorimetry in climate science is the investigation of ice nucleation in cloud droplets. By measuring the heat released during the freezing process, researchers can determine the temperature at which ice formation occurs and the rate of nucleation. This information is essential for improving climate models and predicting cloud behavior under various atmospheric conditions.
Calorimetric methods are also employed to study the effects of aerosols on ice nucleation. Aerosols, such as dust particles and organic compounds, can serve as ice nuclei, influencing the formation of ice crystals in clouds. By using calorimetry to analyze the freezing behavior of water droplets containing different types of aerosols, scientists can better understand how these particles impact cloud microphysics and, consequently, global climate patterns.
Furthermore, calorimetry is utilized in the examination of ice nucleation in biological systems, which has implications for both terrestrial and marine ecosystems. For instance, studying the freezing characteristics of plant tissues and microorganisms can provide insights into how different species adapt to cold environments and how climate change may affect their survival and distribution.
In the context of paleoclimatology, calorimetric techniques are applied to analyze ice cores and other frozen samples. By measuring the energy required to melt these samples and examining their composition, researchers can reconstruct past climate conditions and track long-term changes in atmospheric composition and temperature.
Calorimetry also contributes to the development and evaluation of cloud seeding technologies. By assessing the effectiveness of various ice nucleating agents through calorimetric measurements, scientists can optimize cloud seeding strategies for weather modification and precipitation enhancement in arid regions.
Moreover, calorimetric studies aid in understanding the role of ice nucleation in the formation of polar stratospheric clouds. These clouds play a significant role in ozone depletion, and calorimetric data on their formation processes are crucial for predicting and mitigating ozone loss in polar regions.
In conclusion, calorimetry serves as a powerful tool in climate science, enabling researchers to unravel the complexities of ice nucleation and its far-reaching effects on atmospheric processes, ecosystem dynamics, and global climate patterns. The insights gained from calorimetric studies continue to refine our understanding of climate systems and inform strategies for addressing climate change challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!