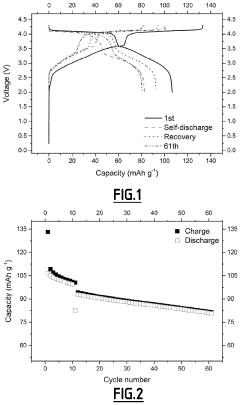
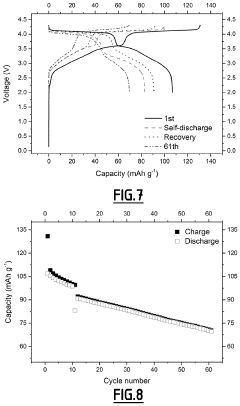
Role of Electrolyte Additives in Sodium Ion Batteries
AUG 7, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Additives in SIBs: Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. However, the development of high-performance SIBs faces several challenges, particularly in the electrolyte system. Electrolyte additives play a crucial role in addressing these challenges and improving the overall performance of SIBs.
The evolution of electrolyte additives in SIBs can be traced back to their initial application in lithium-ion batteries. As research in SIBs intensified, the focus shifted towards developing specific additives tailored to the unique chemistry of sodium-based systems. This transition marked the beginning of a new era in electrolyte engineering for SIBs, with the primary goal of enhancing battery performance, safety, and longevity.
The main objectives of incorporating electrolyte additives in SIBs are multifaceted. Firstly, they aim to stabilize the solid electrolyte interphase (SEI) formed on the electrode surfaces, particularly on the anode. A stable SEI is crucial for preventing continuous electrolyte decomposition and ensuring long-term cycling stability. Secondly, additives are employed to mitigate the dissolution of transition metals from cathode materials, which can lead to capacity fading and poor cycling performance.
Another key objective is to enhance the ionic conductivity of the electrolyte, facilitating faster sodium-ion transport and improving the rate capability of SIBs. Additionally, electrolyte additives are utilized to expand the electrochemical stability window of the electrolyte, allowing for the use of high-voltage cathode materials and consequently increasing the energy density of the battery.
Safety is a paramount concern in battery technology, and electrolyte additives play a vital role in this aspect. They are designed to improve the thermal stability of the electrolyte and suppress the generation of harmful gases during battery operation. Some additives also serve as flame retardants, reducing the risk of fire or explosion in case of battery failure.
The development of electrolyte additives for SIBs is driven by the need to overcome specific challenges inherent to sodium-based systems. These include the larger ionic radius of sodium compared to lithium, which affects ion transport and insertion kinetics, and the higher reactivity of sodium metal anodes. Consequently, the research in this field is focused on identifying compounds that can address these unique challenges while leveraging the advantages of sodium-ion technology.
As the field progresses, there is a growing trend towards developing multifunctional additives that can simultaneously address multiple aspects of battery performance. This approach aims to simplify the electrolyte formulation while maximizing the benefits of additive incorporation. The ultimate goal is to create a synergistic effect among various additives, leading to significant improvements in the overall performance and commercial viability of SIBs.
The evolution of electrolyte additives in SIBs can be traced back to their initial application in lithium-ion batteries. As research in SIBs intensified, the focus shifted towards developing specific additives tailored to the unique chemistry of sodium-based systems. This transition marked the beginning of a new era in electrolyte engineering for SIBs, with the primary goal of enhancing battery performance, safety, and longevity.
The main objectives of incorporating electrolyte additives in SIBs are multifaceted. Firstly, they aim to stabilize the solid electrolyte interphase (SEI) formed on the electrode surfaces, particularly on the anode. A stable SEI is crucial for preventing continuous electrolyte decomposition and ensuring long-term cycling stability. Secondly, additives are employed to mitigate the dissolution of transition metals from cathode materials, which can lead to capacity fading and poor cycling performance.
Another key objective is to enhance the ionic conductivity of the electrolyte, facilitating faster sodium-ion transport and improving the rate capability of SIBs. Additionally, electrolyte additives are utilized to expand the electrochemical stability window of the electrolyte, allowing for the use of high-voltage cathode materials and consequently increasing the energy density of the battery.
Safety is a paramount concern in battery technology, and electrolyte additives play a vital role in this aspect. They are designed to improve the thermal stability of the electrolyte and suppress the generation of harmful gases during battery operation. Some additives also serve as flame retardants, reducing the risk of fire or explosion in case of battery failure.
The development of electrolyte additives for SIBs is driven by the need to overcome specific challenges inherent to sodium-based systems. These include the larger ionic radius of sodium compared to lithium, which affects ion transport and insertion kinetics, and the higher reactivity of sodium metal anodes. Consequently, the research in this field is focused on identifying compounds that can address these unique challenges while leveraging the advantages of sodium-ion technology.
As the field progresses, there is a growing trend towards developing multifunctional additives that can simultaneously address multiple aspects of battery performance. This approach aims to simplify the electrolyte formulation while maximizing the benefits of additive incorporation. The ultimate goal is to create a synergistic effect among various additives, leading to significant improvements in the overall performance and commercial viability of SIBs.
Market Demand for SIB Technology
The market demand for sodium-ion battery (SIB) technology is experiencing significant growth, driven by the increasing need for sustainable and cost-effective energy storage solutions. As the world transitions towards renewable energy sources and electrification of transportation, the demand for large-scale energy storage systems has surged. SIBs are emerging as a promising alternative to lithium-ion batteries, particularly in applications where cost and resource availability are critical factors.
The global energy storage market is projected to expand rapidly in the coming years, with grid-scale storage and electric vehicles being the primary drivers. SIBs are well-positioned to capture a substantial portion of this market, especially in stationary storage applications. The abundance and widespread distribution of sodium resources contribute to the lower cost of SIBs compared to their lithium-ion counterparts, making them attractive for large-scale deployments.
In the electric vehicle sector, while lithium-ion batteries currently dominate, there is growing interest in SIBs for specific market segments. Electric buses, commercial vehicles, and low-speed electric vehicles are potential early adopters of SIB technology, particularly in regions where cost sensitivity is high. The ability of SIBs to operate efficiently at room temperature without the need for expensive thermal management systems further enhances their appeal in these applications.
The renewable energy sector presents another significant market opportunity for SIBs. As the share of intermittent renewable sources like solar and wind in the energy mix increases, the demand for grid-scale energy storage solutions grows proportionally. SIBs offer a cost-effective option for smoothing out supply fluctuations and providing grid stability, especially in developing countries where large-scale energy storage is crucial for expanding access to electricity.
Industrial and residential energy storage systems represent additional market segments with potential for SIB adoption. The technology's safety characteristics, particularly its lower risk of thermal runaway compared to lithium-ion batteries, make it an attractive option for indoor and densely populated areas. This safety aspect, combined with the potential for longer cycle life, positions SIBs as a compelling choice for backup power systems and off-grid applications.
The market demand for SIB technology is further bolstered by increasing environmental concerns and the push for sustainable technologies. The recyclability of sodium-ion batteries and the abundance of raw materials align well with circular economy principles, potentially giving SIBs an edge in markets with stringent environmental regulations.
The global energy storage market is projected to expand rapidly in the coming years, with grid-scale storage and electric vehicles being the primary drivers. SIBs are well-positioned to capture a substantial portion of this market, especially in stationary storage applications. The abundance and widespread distribution of sodium resources contribute to the lower cost of SIBs compared to their lithium-ion counterparts, making them attractive for large-scale deployments.
In the electric vehicle sector, while lithium-ion batteries currently dominate, there is growing interest in SIBs for specific market segments. Electric buses, commercial vehicles, and low-speed electric vehicles are potential early adopters of SIB technology, particularly in regions where cost sensitivity is high. The ability of SIBs to operate efficiently at room temperature without the need for expensive thermal management systems further enhances their appeal in these applications.
The renewable energy sector presents another significant market opportunity for SIBs. As the share of intermittent renewable sources like solar and wind in the energy mix increases, the demand for grid-scale energy storage solutions grows proportionally. SIBs offer a cost-effective option for smoothing out supply fluctuations and providing grid stability, especially in developing countries where large-scale energy storage is crucial for expanding access to electricity.
Industrial and residential energy storage systems represent additional market segments with potential for SIB adoption. The technology's safety characteristics, particularly its lower risk of thermal runaway compared to lithium-ion batteries, make it an attractive option for indoor and densely populated areas. This safety aspect, combined with the potential for longer cycle life, positions SIBs as a compelling choice for backup power systems and off-grid applications.
The market demand for SIB technology is further bolstered by increasing environmental concerns and the push for sustainable technologies. The recyclability of sodium-ion batteries and the abundance of raw materials align well with circular economy principles, potentially giving SIBs an edge in markets with stringent environmental regulations.
Current Challenges in SIB Electrolyte Development
The development of sodium-ion batteries (SIBs) as a promising alternative to lithium-ion batteries has been hindered by several challenges, particularly in the realm of electrolyte development. One of the primary issues is the high reactivity of sodium metal with conventional organic electrolytes, leading to poor cycling stability and safety concerns. This reactivity often results in the formation of an unstable solid electrolyte interphase (SEI) layer, which is crucial for battery performance and longevity.
Another significant challenge is the limited choice of suitable solvents and salts for SIB electrolytes. Many conventional electrolyte components used in lithium-ion batteries are not directly transferable to SIBs due to differences in the chemical properties of sodium and lithium. This limitation restricts the optimization of electrolyte formulations for improved ionic conductivity, electrochemical stability, and compatibility with electrode materials.
The larger ionic radius of sodium compared to lithium also presents difficulties in electrolyte design. This size difference affects ion transport mechanisms and the formation of solvation structures, potentially leading to slower ion diffusion and reduced battery performance. Additionally, the larger sodium ions can cause more significant volume changes in electrode materials during cycling, putting stress on the electrolyte-electrode interface and potentially compromising the integrity of the SEI layer.
Electrolyte decomposition at high voltages is another critical challenge in SIB development. Many potential high-voltage cathode materials for SIBs require electrolytes with wider electrochemical stability windows. However, achieving this stability while maintaining other desirable properties such as high ionic conductivity and low viscosity remains a significant hurdle.
The issue of dendrite formation in sodium metal anodes is also a major concern for electrolyte development. Unlike lithium, sodium has a stronger tendency to form dendrites during cycling, which can lead to short circuits and safety hazards. Developing electrolytes that can effectively suppress dendrite growth without compromising other performance aspects is a key challenge in advancing SIB technology.
Furthermore, the environmental stability and safety of SIB electrolytes pose additional challenges. Many current electrolyte formulations are sensitive to moisture and air, requiring stringent manufacturing and handling conditions. Developing more robust and environmentally stable electrolytes is crucial for the practical implementation and widespread adoption of SIB technology.
Another significant challenge is the limited choice of suitable solvents and salts for SIB electrolytes. Many conventional electrolyte components used in lithium-ion batteries are not directly transferable to SIBs due to differences in the chemical properties of sodium and lithium. This limitation restricts the optimization of electrolyte formulations for improved ionic conductivity, electrochemical stability, and compatibility with electrode materials.
The larger ionic radius of sodium compared to lithium also presents difficulties in electrolyte design. This size difference affects ion transport mechanisms and the formation of solvation structures, potentially leading to slower ion diffusion and reduced battery performance. Additionally, the larger sodium ions can cause more significant volume changes in electrode materials during cycling, putting stress on the electrolyte-electrode interface and potentially compromising the integrity of the SEI layer.
Electrolyte decomposition at high voltages is another critical challenge in SIB development. Many potential high-voltage cathode materials for SIBs require electrolytes with wider electrochemical stability windows. However, achieving this stability while maintaining other desirable properties such as high ionic conductivity and low viscosity remains a significant hurdle.
The issue of dendrite formation in sodium metal anodes is also a major concern for electrolyte development. Unlike lithium, sodium has a stronger tendency to form dendrites during cycling, which can lead to short circuits and safety hazards. Developing electrolytes that can effectively suppress dendrite growth without compromising other performance aspects is a key challenge in advancing SIB technology.
Furthermore, the environmental stability and safety of SIB electrolytes pose additional challenges. Many current electrolyte formulations are sensitive to moisture and air, requiring stringent manufacturing and handling conditions. Developing more robust and environmentally stable electrolytes is crucial for the practical implementation and widespread adoption of SIB technology.
Current Electrolyte Additive Solutions for SIBs
01 Fluorinated compounds as electrolyte additives
Fluorinated compounds are used as electrolyte additives in sodium-ion batteries to enhance performance. These additives can form a stable solid electrolyte interphase (SEI) layer, improve ionic conductivity, and increase the overall stability of the battery. They may also help in reducing unwanted side reactions and improving the cycle life of the battery.- Fluorinated compounds as electrolyte additives: Fluorinated compounds are used as electrolyte additives in sodium-ion batteries to enhance performance. These additives can form a stable solid electrolyte interphase (SEI) layer, improve cycling stability, and increase the battery's overall efficiency. They may also help in reducing unwanted side reactions and improving the battery's safety profile.
- Organic solvents and salts in electrolyte formulations: Specific combinations of organic solvents and sodium salts are used to create optimized electrolyte formulations for sodium-ion batteries. These formulations aim to improve ionic conductivity, enhance the voltage window, and provide better compatibility with electrode materials. The choice of solvents and salts can significantly impact the battery's performance and lifespan.
- Polymer-based electrolyte additives: Polymer-based additives are incorporated into sodium-ion battery electrolytes to improve mechanical stability and safety. These additives can help in creating gel-like or solid electrolytes, reducing the risk of leakage and improving the battery's thermal stability. They may also contribute to better interfacial contact between the electrodes and electrolyte.
- Nanoparticle additives for enhanced performance: Nanoparticles are used as electrolyte additives to enhance the performance of sodium-ion batteries. These additives can improve the ionic conductivity, modify the electrode-electrolyte interface, and potentially increase the energy density of the battery. Various types of nanoparticles, including metal oxides and carbon-based materials, are being explored for this purpose.
- Ionic liquid additives for high-temperature stability: Ionic liquids are incorporated as additives in sodium-ion battery electrolytes to improve high-temperature stability and safety. These additives can enhance the thermal stability of the electrolyte, reduce volatility, and improve the battery's performance at elevated temperatures. They may also contribute to better ionic conductivity and electrochemical stability.
02 Organic solvents and salts in electrolyte formulations
Specific combinations of organic solvents and sodium salts are used to create electrolyte formulations for sodium-ion batteries. These formulations aim to optimize the ionic conductivity, electrochemical stability window, and compatibility with electrode materials. The choice of solvents and salts can significantly impact the battery's performance, including its capacity, rate capability, and cycling stability.Expand Specific Solutions03 Polymer-based electrolyte additives
Polymer-based additives are incorporated into sodium-ion battery electrolytes to improve various aspects of battery performance. These additives can enhance the mechanical stability of the electrolyte, reduce dendrite formation, and improve the interface between the electrolyte and electrodes. Some polymer additives also contribute to better thermal stability and safety of the battery.Expand Specific Solutions04 Inorganic additives for electrolyte enhancement
Inorganic additives, such as metal oxides or ceramic particles, are used to modify the properties of sodium-ion battery electrolytes. These additives can improve the mechanical strength of the electrolyte, enhance its ionic conductivity, and provide additional pathways for sodium ion transport. Some inorganic additives also contribute to the formation of a more stable SEI layer on the electrode surface.Expand Specific Solutions05 Electrolyte additives for high-voltage sodium-ion batteries
Specific additives are developed for use in high-voltage sodium-ion batteries to address the challenges associated with higher operating voltages. These additives aim to improve the oxidative stability of the electrolyte, protect the cathode surface from degradation, and maintain good ionic conductivity at higher voltages. They may also help in mitigating unwanted side reactions that can occur at elevated potentials.Expand Specific Solutions
Key Players in SIB Electrolyte Research
The role of electrolyte additives in sodium ion batteries is an emerging field within the rapidly evolving energy storage sector. The market is in its early growth stage, with increasing research and development efforts from both established companies and startups. The global sodium ion battery market size is projected to expand significantly in the coming years, driven by the demand for sustainable and cost-effective energy storage solutions. Companies like Contemporary Amperex Technology Co., Ltd. and Shenzhen Capchem Technology Co., Ltd. are at the forefront of this technology, leveraging their expertise in lithium-ion batteries to advance sodium ion battery development. Research institutions such as the Centre National de la Recherche Scientifique and South China Normal University are contributing to the fundamental understanding of electrolyte additives, while companies like StoreDot Ltd. and Wildcat Discovery Technologies, Inc. are focusing on innovative approaches to improve battery performance through advanced materials and additives.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a novel electrolyte additive system for sodium-ion batteries, focusing on improving the solid electrolyte interphase (SEI) formation. Their approach involves using a combination of fluoroethylene carbonate (FEC) and vinylene carbonate (VC) as primary additives[1]. This additive blend helps create a more stable and uniform SEI layer on the anode surface, significantly enhancing the battery's cycling stability and coulombic efficiency. CATL's research has shown that the FEC-VC combination can effectively suppress the decomposition of the electrolyte and reduce unwanted side reactions, leading to improved battery performance and longevity[3].
Strengths: Enhanced SEI stability, improved cycling performance, and increased battery lifespan. Weaknesses: Potential increase in production costs due to specialized additives, and possible limitations in extreme temperature conditions.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has pioneered a high-throughput screening approach for electrolyte additives in sodium-ion batteries. Their proprietary technology allows for rapid testing of thousands of additive combinations, accelerating the discovery of optimal formulations. They have identified several promising additive blends, including those containing phosphorus-based compounds and fluorinated organic molecules[2]. These additives work synergistically to form a robust SEI layer, mitigate sodium plating, and enhance the overall electrochemical performance of the battery. Wildcat's approach has led to significant improvements in capacity retention and cycle life of sodium-ion batteries[5].
Strengths: Rapid discovery of effective additive combinations, potential for breakthrough improvements in battery performance. Weaknesses: High research and development costs, potential challenges in scaling up production of complex additive blends.
Key Innovations in SIB Electrolyte Additives
Electrolyte composition for sodium-ion battery
PatentActiveUS12021192B2
Innovation
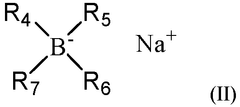
- A non-aqueous electrolyte composition comprising a sodium salt dissolved in specific solvents and additives, including sodium difluoro(oxalato)borate and tris(trimethylsilyl)phosphite, which enhances SEI thickness and wettability, minimizing parasitic reactions and improving capacity retention and compatibility with polyolefin separators.
Electrolyte for sodium-ion battery, sodium-ion battery comprising same, and electric device
PatentPendingEP4517902A1
Innovation
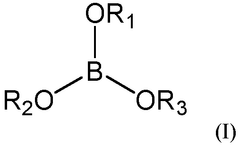
- A sodium-ion battery electrolytic solution comprising an ether compound as a solvent, a sodium borate compound as a sodium salt, and a borate ester compound as an additive, with the ether compound constituting 50 wt% or above of the total solvent.
Environmental Impact of SIB Electrolyte Additives
The environmental impact of electrolyte additives in sodium-ion batteries (SIBs) is a crucial consideration as these energy storage devices gain prominence in the sustainable energy landscape. Electrolyte additives play a vital role in enhancing the performance and stability of SIBs, but their environmental implications must be carefully evaluated.
One of the primary environmental concerns associated with SIB electrolyte additives is their potential toxicity. Many additives used in SIBs contain fluorine-based compounds, which can pose risks to ecosystems if not properly managed. The production and disposal of these additives may lead to the release of harmful substances into soil and water systems, potentially affecting flora and fauna.
Furthermore, the manufacturing processes of electrolyte additives often involve energy-intensive steps and the use of hazardous chemicals. This can contribute to increased carbon emissions and environmental pollution if not carefully controlled. The extraction of raw materials for these additives may also have significant environmental impacts, including habitat destruction and resource depletion.
However, it is important to note that the use of appropriate electrolyte additives can indirectly contribute to positive environmental outcomes. By improving the performance and longevity of SIBs, these additives can enhance the overall efficiency of renewable energy systems, potentially reducing reliance on fossil fuels and decreasing greenhouse gas emissions.
The end-of-life management of SIBs containing these additives is another critical environmental consideration. Proper recycling and disposal methods must be developed to prevent the release of harmful substances into the environment. Some additives may complicate the recycling process, requiring specialized techniques to safely recover and reuse materials.
Research into more environmentally friendly electrolyte additives is ongoing, with a focus on developing bio-based or easily degradable compounds. These efforts aim to minimize the environmental footprint of SIBs while maintaining or improving their performance characteristics.
As the adoption of SIBs increases, regulatory frameworks and industry standards will need to evolve to address the environmental impacts of electrolyte additives. This may include stricter guidelines for production, use, and disposal, as well as incentives for the development of more sustainable alternatives.
In conclusion, while electrolyte additives play a crucial role in advancing SIB technology, their environmental impact must be carefully managed. Balancing the benefits of improved battery performance with potential environmental risks will be essential for the sustainable development of sodium-ion battery technology.
One of the primary environmental concerns associated with SIB electrolyte additives is their potential toxicity. Many additives used in SIBs contain fluorine-based compounds, which can pose risks to ecosystems if not properly managed. The production and disposal of these additives may lead to the release of harmful substances into soil and water systems, potentially affecting flora and fauna.
Furthermore, the manufacturing processes of electrolyte additives often involve energy-intensive steps and the use of hazardous chemicals. This can contribute to increased carbon emissions and environmental pollution if not carefully controlled. The extraction of raw materials for these additives may also have significant environmental impacts, including habitat destruction and resource depletion.
However, it is important to note that the use of appropriate electrolyte additives can indirectly contribute to positive environmental outcomes. By improving the performance and longevity of SIBs, these additives can enhance the overall efficiency of renewable energy systems, potentially reducing reliance on fossil fuels and decreasing greenhouse gas emissions.
The end-of-life management of SIBs containing these additives is another critical environmental consideration. Proper recycling and disposal methods must be developed to prevent the release of harmful substances into the environment. Some additives may complicate the recycling process, requiring specialized techniques to safely recover and reuse materials.
Research into more environmentally friendly electrolyte additives is ongoing, with a focus on developing bio-based or easily degradable compounds. These efforts aim to minimize the environmental footprint of SIBs while maintaining or improving their performance characteristics.
As the adoption of SIBs increases, regulatory frameworks and industry standards will need to evolve to address the environmental impacts of electrolyte additives. This may include stricter guidelines for production, use, and disposal, as well as incentives for the development of more sustainable alternatives.
In conclusion, while electrolyte additives play a crucial role in advancing SIB technology, their environmental impact must be carefully managed. Balancing the benefits of improved battery performance with potential environmental risks will be essential for the sustainable development of sodium-ion battery technology.
Safety Considerations for SIB Electrolyte Additives
Safety considerations are paramount when developing electrolyte additives for sodium-ion batteries (SIBs). The primary concern is the potential for thermal runaway, which can lead to battery fires or explosions. Electrolyte additives must be carefully selected to enhance battery performance without compromising safety. One key aspect is the formation of a stable solid electrolyte interphase (SEI) layer, which protects the electrode surface and prevents unwanted side reactions.
Flammability is a critical factor in SIB safety. Many organic electrolytes used in SIBs are highly flammable, posing significant risks in case of battery damage or overheating. Additives that can reduce the flammability of the electrolyte, such as flame retardants or ionic liquids, are being extensively researched. These additives must maintain or improve the electrochemical performance of the battery while enhancing its safety profile.
The reactivity of sodium with water is another safety concern for SIBs. Electrolyte additives must be chosen to minimize the risk of moisture ingress and subsequent reactions with the sodium anode. Hydrophobic additives or those that can scavenge trace amounts of water in the electrolyte are particularly valuable in this context. Additionally, additives that can form a protective layer on the sodium anode to prevent direct contact with moisture are being investigated.
Toxicity and environmental impact of electrolyte additives are also important safety considerations. As the demand for SIBs grows, the potential for large-scale production and eventual disposal of these batteries increases. Additives must be evaluated not only for their performance benefits but also for their long-term environmental effects and potential health hazards during manufacturing, use, and disposal.
Electrochemical stability is crucial for preventing undesired redox reactions that could lead to gas evolution or electrolyte decomposition. Additives that extend the electrochemical stability window of the electrolyte can significantly enhance the safety of SIBs by reducing the likelihood of side reactions that could compromise the battery's integrity or lead to capacity fade.
Thermal stability of electrolyte additives is another critical factor. Additives must maintain their beneficial properties across a wide temperature range and should not decompose or react adversely at elevated temperatures. This is particularly important for applications where batteries may be exposed to extreme conditions, such as in electric vehicles or grid storage systems.
In conclusion, the development of safe electrolyte additives for SIBs requires a multifaceted approach, balancing performance enhancement with rigorous safety standards. Ongoing research focuses on identifying additives that can address multiple safety concerns simultaneously while maintaining or improving the electrochemical performance of SIBs.
Flammability is a critical factor in SIB safety. Many organic electrolytes used in SIBs are highly flammable, posing significant risks in case of battery damage or overheating. Additives that can reduce the flammability of the electrolyte, such as flame retardants or ionic liquids, are being extensively researched. These additives must maintain or improve the electrochemical performance of the battery while enhancing its safety profile.
The reactivity of sodium with water is another safety concern for SIBs. Electrolyte additives must be chosen to minimize the risk of moisture ingress and subsequent reactions with the sodium anode. Hydrophobic additives or those that can scavenge trace amounts of water in the electrolyte are particularly valuable in this context. Additionally, additives that can form a protective layer on the sodium anode to prevent direct contact with moisture are being investigated.
Toxicity and environmental impact of electrolyte additives are also important safety considerations. As the demand for SIBs grows, the potential for large-scale production and eventual disposal of these batteries increases. Additives must be evaluated not only for their performance benefits but also for their long-term environmental effects and potential health hazards during manufacturing, use, and disposal.
Electrochemical stability is crucial for preventing undesired redox reactions that could lead to gas evolution or electrolyte decomposition. Additives that extend the electrochemical stability window of the electrolyte can significantly enhance the safety of SIBs by reducing the likelihood of side reactions that could compromise the battery's integrity or lead to capacity fade.
Thermal stability of electrolyte additives is another critical factor. Additives must maintain their beneficial properties across a wide temperature range and should not decompose or react adversely at elevated temperatures. This is particularly important for applications where batteries may be exposed to extreme conditions, such as in electric vehicles or grid storage systems.
In conclusion, the development of safe electrolyte additives for SIBs requires a multifaceted approach, balancing performance enhancement with rigorous safety standards. Ongoing research focuses on identifying additives that can address multiple safety concerns simultaneously while maintaining or improving the electrochemical performance of SIBs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!