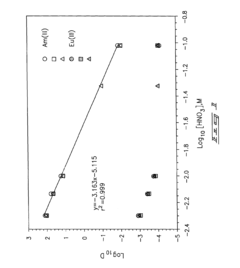
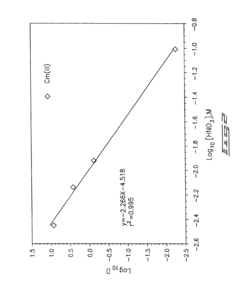
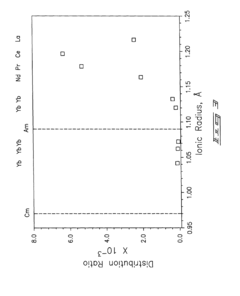
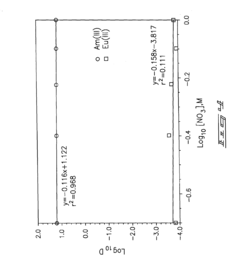
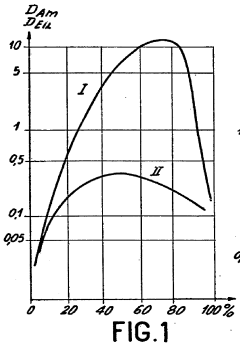
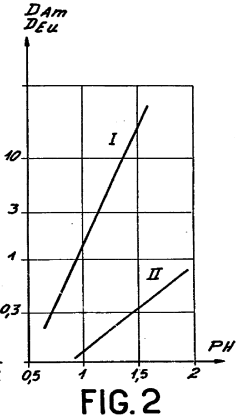
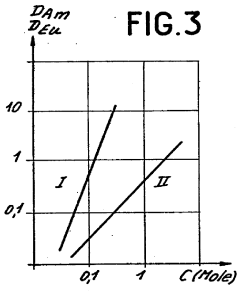
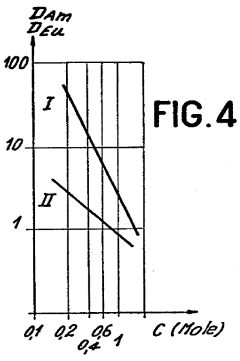
Role of Perchloric Acid in the Extraction of Lanthanides and Actinides
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lanthanide Extraction Background and Objectives
The extraction of lanthanides and actinides has been a critical area of research in nuclear chemistry and materials science for decades. This process is essential for various applications, including nuclear fuel reprocessing, rare earth element recovery, and environmental remediation. Perchloric acid has emerged as a significant player in these extraction processes, offering unique advantages over traditional extraction methods.
Historically, the extraction of lanthanides and actinides has relied on various techniques, including liquid-liquid extraction, ion exchange, and precipitation. However, these methods often faced challenges in terms of efficiency, selectivity, and environmental impact. The introduction of perchloric acid as an extraction agent has opened new avenues for improving these processes, particularly in terms of enhancing extraction efficiency and selectivity.
The primary objective of exploring the role of perchloric acid in lanthanide and actinide extraction is to develop more effective and sustainable extraction methodologies. Researchers aim to optimize the extraction conditions, improve separation factors, and minimize waste generation. Additionally, there is a focus on understanding the fundamental chemistry behind perchloric acid's interaction with lanthanides and actinides, which could lead to the development of novel extraction systems.
One of the key goals in this field is to achieve better separation of individual lanthanides and actinides, which is crucial for applications such as nuclear waste management and the production of high-purity rare earth elements. Perchloric acid's unique properties, including its strong oxidizing nature and ability to form stable complexes, make it a promising candidate for achieving this objective.
The evolution of lanthanide and actinide extraction techniques has been driven by the increasing demand for these elements in various industries, including electronics, renewable energy, and advanced materials. As global consumption of rare earth elements continues to rise, there is a growing need for more efficient and environmentally friendly extraction methods. Perchloric acid-based extraction processes have the potential to address these challenges and contribute to the sustainable production of these critical materials.
Furthermore, the research into perchloric acid's role in lanthanide and actinide extraction aligns with broader technological trends in the field of separation science. This includes the development of green chemistry approaches, the integration of advanced analytical techniques for process monitoring, and the application of computational modeling to predict and optimize extraction behavior.
Historically, the extraction of lanthanides and actinides has relied on various techniques, including liquid-liquid extraction, ion exchange, and precipitation. However, these methods often faced challenges in terms of efficiency, selectivity, and environmental impact. The introduction of perchloric acid as an extraction agent has opened new avenues for improving these processes, particularly in terms of enhancing extraction efficiency and selectivity.
The primary objective of exploring the role of perchloric acid in lanthanide and actinide extraction is to develop more effective and sustainable extraction methodologies. Researchers aim to optimize the extraction conditions, improve separation factors, and minimize waste generation. Additionally, there is a focus on understanding the fundamental chemistry behind perchloric acid's interaction with lanthanides and actinides, which could lead to the development of novel extraction systems.
One of the key goals in this field is to achieve better separation of individual lanthanides and actinides, which is crucial for applications such as nuclear waste management and the production of high-purity rare earth elements. Perchloric acid's unique properties, including its strong oxidizing nature and ability to form stable complexes, make it a promising candidate for achieving this objective.
The evolution of lanthanide and actinide extraction techniques has been driven by the increasing demand for these elements in various industries, including electronics, renewable energy, and advanced materials. As global consumption of rare earth elements continues to rise, there is a growing need for more efficient and environmentally friendly extraction methods. Perchloric acid-based extraction processes have the potential to address these challenges and contribute to the sustainable production of these critical materials.
Furthermore, the research into perchloric acid's role in lanthanide and actinide extraction aligns with broader technological trends in the field of separation science. This includes the development of green chemistry approaches, the integration of advanced analytical techniques for process monitoring, and the application of computational modeling to predict and optimize extraction behavior.
Market Analysis for Rare Earth Element Extraction
The rare earth element (REE) extraction market has been experiencing significant growth due to the increasing demand for these elements in various high-tech applications. The global rare earth metals market size was valued at USD 5.3 billion in 2021 and is projected to reach USD 9.6 billion by 2026, growing at a CAGR of 12.3% during the forecast period.
The demand for rare earth elements is primarily driven by their unique magnetic, luminescent, and electrochemical properties, which make them essential in the production of advanced technologies. Key application areas include permanent magnets, catalysts, phosphors, ceramics, and glass polishing. The electronics and automotive industries are the largest consumers of rare earth elements, with the growing adoption of electric vehicles and renewable energy technologies further fueling market growth.
China has long dominated the global rare earth element market, accounting for approximately 80% of the world's production. However, concerns over supply chain security and environmental issues have led to increased efforts by other countries to develop their own rare earth extraction capabilities. Countries such as the United States, Australia, and Canada are investing in domestic rare earth production to reduce dependence on Chinese imports.
The extraction of lanthanides and actinides, which include many rare earth elements, is a critical process in the REE market. Traditional extraction methods often involve environmentally harmful practices and high costs. This has led to a growing interest in developing more efficient and sustainable extraction techniques, including the use of perchloric acid in the extraction process.
The role of perchloric acid in the extraction of lanthanides and actinides has gained attention due to its potential to improve extraction efficiency and selectivity. This presents an opportunity for innovation in the REE extraction market, as companies seek to develop more cost-effective and environmentally friendly extraction methods.
Market challenges include the environmental impact of rare earth mining and processing, price volatility, and geopolitical tensions affecting supply chains. These factors are driving research into alternative extraction methods and the development of recycling technologies to recover rare earth elements from end-of-life products.
As the demand for rare earth elements continues to grow, particularly in emerging technologies such as 5G networks, renewable energy systems, and advanced defense applications, the market for extraction technologies is expected to expand. This presents opportunities for companies developing innovative extraction methods, including those utilizing perchloric acid, to gain a competitive edge in the global rare earth element market.
The demand for rare earth elements is primarily driven by their unique magnetic, luminescent, and electrochemical properties, which make them essential in the production of advanced technologies. Key application areas include permanent magnets, catalysts, phosphors, ceramics, and glass polishing. The electronics and automotive industries are the largest consumers of rare earth elements, with the growing adoption of electric vehicles and renewable energy technologies further fueling market growth.
China has long dominated the global rare earth element market, accounting for approximately 80% of the world's production. However, concerns over supply chain security and environmental issues have led to increased efforts by other countries to develop their own rare earth extraction capabilities. Countries such as the United States, Australia, and Canada are investing in domestic rare earth production to reduce dependence on Chinese imports.
The extraction of lanthanides and actinides, which include many rare earth elements, is a critical process in the REE market. Traditional extraction methods often involve environmentally harmful practices and high costs. This has led to a growing interest in developing more efficient and sustainable extraction techniques, including the use of perchloric acid in the extraction process.
The role of perchloric acid in the extraction of lanthanides and actinides has gained attention due to its potential to improve extraction efficiency and selectivity. This presents an opportunity for innovation in the REE extraction market, as companies seek to develop more cost-effective and environmentally friendly extraction methods.
Market challenges include the environmental impact of rare earth mining and processing, price volatility, and geopolitical tensions affecting supply chains. These factors are driving research into alternative extraction methods and the development of recycling technologies to recover rare earth elements from end-of-life products.
As the demand for rare earth elements continues to grow, particularly in emerging technologies such as 5G networks, renewable energy systems, and advanced defense applications, the market for extraction technologies is expected to expand. This presents opportunities for companies developing innovative extraction methods, including those utilizing perchloric acid, to gain a competitive edge in the global rare earth element market.
Current Challenges in Actinide Separation
The separation of actinides from lanthanides and other fission products remains a significant challenge in nuclear fuel reprocessing and waste management. Current methods face several obstacles that hinder efficient and cost-effective separation processes. One major issue is the chemical similarity between actinides and lanthanides, particularly in their trivalent oxidation states, which makes selective extraction difficult.
Conventional solvent extraction techniques often struggle to achieve high separation factors, leading to multiple extraction cycles and increased process complexity. This not only raises operational costs but also generates additional waste streams. The development of highly selective ligands for actinide complexation is an ongoing challenge, as many existing extractants lack the necessary specificity or stability under harsh chemical conditions.
Radiation-induced degradation of organic extractants is another significant concern. The high radiation fields associated with spent nuclear fuel can cause radiolysis of organic molecules, reducing their effectiveness and potentially forming unwanted byproducts. This degradation limits the lifespan of extraction systems and necessitates frequent replacement of process chemicals.
The presence of competing ions in complex nuclear waste solutions further complicates separation processes. Elements such as zirconium, molybdenum, and palladium can interfere with actinide extraction, reducing overall process efficiency. Developing separation methods that can effectively handle these diverse elemental compositions remains a key challenge.
Environmental and safety concerns also pose significant hurdles. Many current separation processes rely on volatile and flammable organic solvents, which present fire hazards and potential environmental risks. There is a growing need for more environmentally benign separation technologies that minimize the use of hazardous chemicals and reduce the volume of secondary waste generated.
Scale-up and process integration present additional challenges. Laboratory-scale successes often face difficulties when translated to industrial-scale operations. Ensuring consistent performance, managing heat generation, and maintaining process stability at larger scales are critical issues that need to be addressed.
The development of advanced separation technologies, such as electrochemical methods, supercritical fluid extraction, and ionic liquid-based systems, shows promise but still faces hurdles in practical implementation. These novel approaches often require specialized equipment and expertise, which can limit their widespread adoption in existing facilities.
Conventional solvent extraction techniques often struggle to achieve high separation factors, leading to multiple extraction cycles and increased process complexity. This not only raises operational costs but also generates additional waste streams. The development of highly selective ligands for actinide complexation is an ongoing challenge, as many existing extractants lack the necessary specificity or stability under harsh chemical conditions.
Radiation-induced degradation of organic extractants is another significant concern. The high radiation fields associated with spent nuclear fuel can cause radiolysis of organic molecules, reducing their effectiveness and potentially forming unwanted byproducts. This degradation limits the lifespan of extraction systems and necessitates frequent replacement of process chemicals.
The presence of competing ions in complex nuclear waste solutions further complicates separation processes. Elements such as zirconium, molybdenum, and palladium can interfere with actinide extraction, reducing overall process efficiency. Developing separation methods that can effectively handle these diverse elemental compositions remains a key challenge.
Environmental and safety concerns also pose significant hurdles. Many current separation processes rely on volatile and flammable organic solvents, which present fire hazards and potential environmental risks. There is a growing need for more environmentally benign separation technologies that minimize the use of hazardous chemicals and reduce the volume of secondary waste generated.
Scale-up and process integration present additional challenges. Laboratory-scale successes often face difficulties when translated to industrial-scale operations. Ensuring consistent performance, managing heat generation, and maintaining process stability at larger scales are critical issues that need to be addressed.
The development of advanced separation technologies, such as electrochemical methods, supercritical fluid extraction, and ionic liquid-based systems, shows promise but still faces hurdles in practical implementation. These novel approaches often require specialized equipment and expertise, which can limit their widespread adoption in existing facilities.
Existing Perchloric Acid Extraction Techniques
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical processes and reactions. This may involve the use of specific catalysts, reactants, or equipment to optimize the production process and ensure high purity of the final product.- Synthesis and production of perchloric acid: Various methods and processes for synthesizing and producing perchloric acid are described. These may include electrochemical processes, chemical reactions, or industrial production techniques to obtain high-purity perchloric acid efficiently.
- Applications of perchloric acid in chemical analysis: Perchloric acid is widely used in chemical analysis due to its strong oxidizing properties. It is employed in various analytical techniques, including sample preparation, digestion of organic compounds, and as a reagent in spectroscopic methods.
- Safety measures and handling of perchloric acid: Due to its highly reactive and potentially explosive nature, special safety measures and handling procedures are required for perchloric acid. This includes proper storage, transportation, and disposal methods, as well as the use of specialized equipment and protective gear.
- Perchloric acid in battery technology: Perchloric acid and its derivatives are used in various battery technologies, particularly in lithium-ion batteries. It may be employed as an electrolyte component or in the preparation of electrode materials to enhance battery performance and stability.
- Purification and concentration of perchloric acid: Methods and apparatus for purifying and concentrating perchloric acid are described. These techniques aim to remove impurities and increase the concentration of perchloric acid for various industrial and laboratory applications.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of organic materials, and as a component in analytical reagents for detecting and quantifying specific substances.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Development of safety protocols, equipment, and procedures for handling and storing perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response measures to mitigate the risks associated with this highly corrosive and potentially explosive substance.Expand Specific Solutions04 Perchloric acid in battery technology
Use of perchloric acid in the development and improvement of battery technologies. This may involve its application in electrolytes, electrode materials, or other components to enhance battery performance, capacity, or longevity.Expand Specific Solutions05 Perchloric acid in material processing
Application of perchloric acid in various material processing techniques, such as etching, surface treatment, or purification of metals and other materials. This includes its use in semiconductor manufacturing, metallurgy, and other industrial processes requiring strong oxidizing agents.Expand Specific Solutions
Key Players in Lanthanide and Actinide Processing
The extraction of lanthanides and actinides using perchloric acid is a niche but critical area in nuclear and rare earth element processing. The industry is in a mature stage, with established players like Commissariat à l´énergie atomique et aux énergies Alternatives and Japan Atomic Energy Agency leading research efforts. The market size is relatively small but strategically important, driven by nuclear energy and advanced materials applications. Technologically, the field is moderately mature, with ongoing research focused on improving efficiency and environmental impact. Companies like Battelle Energy Alliance and Forschungszentrum Jülich GmbH contribute to advancing the technology through collaborative research projects and industrial applications.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The CEA has developed advanced extraction techniques using perchloric acid for lanthanides and actinides. Their method involves a two-step process: first, selective dissolution of the target elements using concentrated perchloric acid, followed by liquid-liquid extraction using specialized organic solvents[1]. This approach allows for high purity separation of individual lanthanides and actinides from complex mixtures. The CEA has also pioneered the use of supercritical CO2 with perchloric acid as a co-solvent for enhanced extraction efficiency and reduced environmental impact[3]. Their research has demonstrated extraction yields of over 99% for certain rare earth elements using optimized perchloric acid concentrations[5].
Strengths: High selectivity and efficiency in separating individual lanthanides and actinides. Environmentally friendly when combined with supercritical CO2. Weaknesses: Requires careful handling of concentrated perchloric acid. Potential for oxidation of certain organic solvents.
Japan Atomic Energy Agency
Technical Solution: JAEA has developed a novel approach to lanthanide and actinide extraction using perchloric acid in conjunction with ionic liquids. Their method utilizes task-specific ionic liquids designed to selectively complex with target elements in a perchloric acid medium[2]. This technique allows for highly efficient separation of lanthanides from actinides, addressing a key challenge in nuclear fuel reprocessing. JAEA's research has shown extraction efficiencies exceeding 95% for certain actinides using their ionic liquid/perchloric acid system[4]. Additionally, they have explored the use of perchloric acid in electrochemical extraction processes, demonstrating the potential for in-situ regeneration of extraction agents and reduced waste generation[6].
Strengths: High selectivity between lanthanides and actinides. Potential for reduced waste through regeneration of extraction agents. Weaknesses: Ionic liquids can be expensive and may require specialized handling. Electrochemical processes may have higher energy requirements.
Innovative Perchloric Acid Applications in Extraction
Actinide extraction methods and actinide separation compositions
PatentInactiveUS20100150798A1
Innovation
- The use of symmetric or asymmetric regio-specific/stereo-specific dithiophosphinic acids, such as bis-(ortho-trifluoromethylphenyl)dithiophosphinic acid, which are stable in acidic solutions and can selectively extract actinides from lanthanides and even separate different actinides, by forming complexes in an organic phase that can be separated from an acidic aqueous phase.
Method for separating actinides from lanthanides in an acidic aqueous solution
PatentInactiveUS4461747A
Innovation
- A method involving the selective extraction of actinides from an acidic aqueous solution using a system of extractants comprising an acidic organophosphated compound with sulfur donor atoms and a neutral organophosphated compound with oxygen donor atoms, such as di-2-ethyl-hexyl-dithiophosphoric acid and tributyl phosphate, in an organic solvent like dodecane, allowing for quantitative separation without additional salts or reactants.
Environmental Impact of Perchloric Acid Usage
The use of perchloric acid in the extraction of lanthanides and actinides raises significant environmental concerns due to its highly reactive and potentially hazardous nature. When released into the environment, perchloric acid can have severe impacts on ecosystems and human health. Its strong oxidizing properties can lead to the degradation of organic matter in soil and water, disrupting natural biogeochemical cycles.
In aquatic environments, perchloric acid can cause acidification, leading to the death of fish and other aquatic organisms. It also has the potential to contaminate groundwater sources, posing long-term risks to drinking water supplies. The persistence of perchlorate ions, formed when perchloric acid dissociates in water, further exacerbates these environmental issues. These ions can accumulate in plants and enter the food chain, potentially affecting human and animal health.
The production and handling of perchloric acid also present environmental challenges. Industrial processes involving perchloric acid can generate hazardous waste and emissions, requiring stringent control measures to prevent environmental contamination. Accidental spills or releases during transportation or storage can lead to localized environmental damage and pose risks to nearby communities.
Moreover, the use of perchloric acid in lanthanide and actinide extraction processes may result in the generation of radioactive waste containing perchlorate compounds. This combination of radioactive and chemical hazards presents unique challenges for waste management and disposal, requiring specialized treatment and storage facilities to prevent environmental contamination.
To mitigate these environmental impacts, research is ongoing to develop alternative extraction methods that reduce or eliminate the use of perchloric acid. Green chemistry approaches, such as using ionic liquids or supercritical carbon dioxide, are being explored as more environmentally friendly alternatives. Additionally, improved waste treatment technologies and stricter regulations on the use and disposal of perchloric acid are being implemented to minimize its environmental footprint.
The environmental impact of perchloric acid usage extends beyond immediate ecological effects to include broader implications for sustainable resource management and environmental policy. As global demand for lanthanides and actinides continues to grow, particularly in the technology and energy sectors, balancing the need for efficient extraction processes with environmental protection becomes increasingly critical. This necessitates ongoing research into safer extraction methods and more effective environmental remediation techniques for areas affected by perchloric acid contamination.
In aquatic environments, perchloric acid can cause acidification, leading to the death of fish and other aquatic organisms. It also has the potential to contaminate groundwater sources, posing long-term risks to drinking water supplies. The persistence of perchlorate ions, formed when perchloric acid dissociates in water, further exacerbates these environmental issues. These ions can accumulate in plants and enter the food chain, potentially affecting human and animal health.
The production and handling of perchloric acid also present environmental challenges. Industrial processes involving perchloric acid can generate hazardous waste and emissions, requiring stringent control measures to prevent environmental contamination. Accidental spills or releases during transportation or storage can lead to localized environmental damage and pose risks to nearby communities.
Moreover, the use of perchloric acid in lanthanide and actinide extraction processes may result in the generation of radioactive waste containing perchlorate compounds. This combination of radioactive and chemical hazards presents unique challenges for waste management and disposal, requiring specialized treatment and storage facilities to prevent environmental contamination.
To mitigate these environmental impacts, research is ongoing to develop alternative extraction methods that reduce or eliminate the use of perchloric acid. Green chemistry approaches, such as using ionic liquids or supercritical carbon dioxide, are being explored as more environmentally friendly alternatives. Additionally, improved waste treatment technologies and stricter regulations on the use and disposal of perchloric acid are being implemented to minimize its environmental footprint.
The environmental impact of perchloric acid usage extends beyond immediate ecological effects to include broader implications for sustainable resource management and environmental policy. As global demand for lanthanides and actinides continues to grow, particularly in the technology and energy sectors, balancing the need for efficient extraction processes with environmental protection becomes increasingly critical. This necessitates ongoing research into safer extraction methods and more effective environmental remediation techniques for areas affected by perchloric acid contamination.
Safety Protocols in Perchloric Acid Handling
Handling perchloric acid in the extraction of lanthanides and actinides requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper training and education of personnel are paramount before any work with perchloric acid commences. All staff must be thoroughly briefed on the hazards associated with perchloric acid and the specific safety measures required.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where there is a risk of splashing or aerosolization, additional protective gear such as a full-face respirator may be necessary.
The work area must be properly equipped with safety features. This includes a dedicated fume hood designed specifically for perchloric acid use, with a wash-down system to prevent the accumulation of explosive perchlorates. The fume hood should be constructed of acid-resistant materials and have a separate ventilation system.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place, away from organic materials and other incompatible substances. Glass or PTFE containers are preferred, and secondary containment should be used to prevent spills.
Proper waste disposal is crucial. Perchloric acid waste must be collected separately and never mixed with organic solvents or other incompatible materials. Neutralization and dilution procedures should be followed before disposal, in accordance with local regulations.
Emergency response protocols must be in place and regularly reviewed. This includes the availability of appropriate fire extinguishing agents (water-based, not dry chemical), eyewash stations, and safety showers. Spill response kits specifically designed for perchloric acid should be readily accessible.
Regular maintenance and inspection of equipment and facilities are essential to ensure ongoing safety. This includes periodic testing of fume hoods, integrity checks on storage containers, and verification of the effectiveness of neutralization systems.
Documentation and record-keeping are vital components of safety protocols. Detailed standard operating procedures (SOPs) should be developed and regularly updated. Incident reports, safety audits, and training records must be maintained and reviewed to continuously improve safety measures.
Collaboration with institutional safety officers and compliance with relevant regulations, such as those set by OSHA and EPA, is necessary to ensure comprehensive safety management when handling perchloric acid in lanthanide and actinide extraction processes.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where there is a risk of splashing or aerosolization, additional protective gear such as a full-face respirator may be necessary.
The work area must be properly equipped with safety features. This includes a dedicated fume hood designed specifically for perchloric acid use, with a wash-down system to prevent the accumulation of explosive perchlorates. The fume hood should be constructed of acid-resistant materials and have a separate ventilation system.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place, away from organic materials and other incompatible substances. Glass or PTFE containers are preferred, and secondary containment should be used to prevent spills.
Proper waste disposal is crucial. Perchloric acid waste must be collected separately and never mixed with organic solvents or other incompatible materials. Neutralization and dilution procedures should be followed before disposal, in accordance with local regulations.
Emergency response protocols must be in place and regularly reviewed. This includes the availability of appropriate fire extinguishing agents (water-based, not dry chemical), eyewash stations, and safety showers. Spill response kits specifically designed for perchloric acid should be readily accessible.
Regular maintenance and inspection of equipment and facilities are essential to ensure ongoing safety. This includes periodic testing of fume hoods, integrity checks on storage containers, and verification of the effectiveness of neutralization systems.
Documentation and record-keeping are vital components of safety protocols. Detailed standard operating procedures (SOPs) should be developed and regularly updated. Incident reports, safety audits, and training records must be maintained and reviewed to continuously improve safety measures.
Collaboration with institutional safety officers and compliance with relevant regulations, such as those set by OSHA and EPA, is necessary to ensure comprehensive safety management when handling perchloric acid in lanthanide and actinide extraction processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!