Sodium silicate's role in controlled drug delivery systems
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Silicate in Drug Delivery: Background and Objectives
Sodium silicate, also known as water glass or liquid glass, has emerged as a promising material in the field of controlled drug delivery systems. This inorganic compound, composed of sodium oxide and silicon dioxide, has garnered significant attention due to its unique properties and versatile applications in pharmaceutical sciences.
The development of controlled drug delivery systems has been a focal point of research in recent decades, driven by the need to enhance therapeutic efficacy and minimize side effects of various medications. Traditional drug delivery methods often result in fluctuating drug concentrations in the body, leading to suboptimal treatment outcomes. Controlled release systems aim to maintain consistent drug levels over extended periods, improving patient compliance and treatment efficacy.
Sodium silicate's role in this field stems from its ability to form porous structures and act as a matrix for drug encapsulation. Its biocompatibility and biodegradability make it an attractive option for developing novel drug delivery platforms. The material's sol-gel transition properties allow for the creation of various forms, including nanoparticles, microspheres, and hydrogels, each offering unique advantages in drug delivery applications.
The historical context of sodium silicate in drug delivery can be traced back to the early 2000s when researchers began exploring its potential as a drug carrier. Initial studies focused on its use in dental applications, gradually expanding to broader pharmaceutical uses. Over the past two decades, significant advancements have been made in understanding the material's behavior and optimizing its properties for controlled release formulations.
The primary objectives of current research in this area include enhancing the loading capacity of sodium silicate-based systems, improving the control over release kinetics, and expanding the range of drugs that can be effectively delivered using these platforms. Researchers are also exploring ways to functionalize sodium silicate matrices to target specific tissues or respond to environmental stimuli, enabling smart drug delivery systems.
Another crucial aspect of ongoing research is the investigation of sodium silicate's interaction with various drug molecules and its impact on drug stability and bioavailability. Understanding these interactions is essential for developing effective formulations and predicting their behavior in physiological conditions.
As the field progresses, there is a growing emphasis on translating laboratory findings into clinically viable products. This involves addressing challenges related to large-scale production, long-term stability, and regulatory compliance. The ultimate goal is to develop sodium silicate-based drug delivery systems that offer significant advantages over existing formulations in terms of efficacy, safety, and patient convenience.
The development of controlled drug delivery systems has been a focal point of research in recent decades, driven by the need to enhance therapeutic efficacy and minimize side effects of various medications. Traditional drug delivery methods often result in fluctuating drug concentrations in the body, leading to suboptimal treatment outcomes. Controlled release systems aim to maintain consistent drug levels over extended periods, improving patient compliance and treatment efficacy.
Sodium silicate's role in this field stems from its ability to form porous structures and act as a matrix for drug encapsulation. Its biocompatibility and biodegradability make it an attractive option for developing novel drug delivery platforms. The material's sol-gel transition properties allow for the creation of various forms, including nanoparticles, microspheres, and hydrogels, each offering unique advantages in drug delivery applications.
The historical context of sodium silicate in drug delivery can be traced back to the early 2000s when researchers began exploring its potential as a drug carrier. Initial studies focused on its use in dental applications, gradually expanding to broader pharmaceutical uses. Over the past two decades, significant advancements have been made in understanding the material's behavior and optimizing its properties for controlled release formulations.
The primary objectives of current research in this area include enhancing the loading capacity of sodium silicate-based systems, improving the control over release kinetics, and expanding the range of drugs that can be effectively delivered using these platforms. Researchers are also exploring ways to functionalize sodium silicate matrices to target specific tissues or respond to environmental stimuli, enabling smart drug delivery systems.
Another crucial aspect of ongoing research is the investigation of sodium silicate's interaction with various drug molecules and its impact on drug stability and bioavailability. Understanding these interactions is essential for developing effective formulations and predicting their behavior in physiological conditions.
As the field progresses, there is a growing emphasis on translating laboratory findings into clinically viable products. This involves addressing challenges related to large-scale production, long-term stability, and regulatory compliance. The ultimate goal is to develop sodium silicate-based drug delivery systems that offer significant advantages over existing formulations in terms of efficacy, safety, and patient convenience.
Market Analysis for Controlled Release Pharmaceuticals
The controlled release pharmaceuticals market has been experiencing significant growth in recent years, driven by the increasing prevalence of chronic diseases and the need for more effective drug delivery systems. This market segment is characterized by its ability to maintain therapeutic drug levels over extended periods, reducing dosing frequency and improving patient compliance.
The global controlled release drug delivery market was valued at approximately $29 billion in 2020 and is projected to reach $41 billion by 2026, growing at a CAGR of around 7.5% during the forecast period. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high adoption rates of innovative drug delivery technologies.
Key factors driving market growth include the rising geriatric population, increasing incidence of chronic diseases such as diabetes and cardiovascular disorders, and growing demand for targeted drug delivery systems. Additionally, the pharmaceutical industry's focus on developing novel drug formulations and the need to extend product lifecycles are contributing to market expansion.
The market for controlled release pharmaceuticals is segmented based on technology, application, and route of administration. Oral controlled release systems currently dominate the market, accounting for the largest share due to their convenience and patient preference. However, transdermal and injectable systems are gaining traction, particularly in the treatment of neurological disorders and cancer.
Major players in the controlled release pharmaceuticals market include Johnson & Johnson, Pfizer, Merck & Co., Novartis, and AstraZeneca. These companies are investing heavily in research and development to create innovative drug delivery systems and maintain their competitive edge.
Emerging trends in the market include the development of smart drug delivery systems incorporating nanotechnology and the integration of digital technologies for personalized medicine. The use of biodegradable polymers and stimuli-responsive materials in controlled release formulations is also gaining attention, offering potential for improved drug efficacy and reduced side effects.
Challenges facing the market include stringent regulatory requirements, high development costs, and the complexity of formulating controlled release systems for certain drugs. However, the potential benefits of improved patient outcomes and reduced healthcare costs continue to drive innovation and investment in this sector.
The global controlled release drug delivery market was valued at approximately $29 billion in 2020 and is projected to reach $41 billion by 2026, growing at a CAGR of around 7.5% during the forecast period. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high adoption rates of innovative drug delivery technologies.
Key factors driving market growth include the rising geriatric population, increasing incidence of chronic diseases such as diabetes and cardiovascular disorders, and growing demand for targeted drug delivery systems. Additionally, the pharmaceutical industry's focus on developing novel drug formulations and the need to extend product lifecycles are contributing to market expansion.
The market for controlled release pharmaceuticals is segmented based on technology, application, and route of administration. Oral controlled release systems currently dominate the market, accounting for the largest share due to their convenience and patient preference. However, transdermal and injectable systems are gaining traction, particularly in the treatment of neurological disorders and cancer.
Major players in the controlled release pharmaceuticals market include Johnson & Johnson, Pfizer, Merck & Co., Novartis, and AstraZeneca. These companies are investing heavily in research and development to create innovative drug delivery systems and maintain their competitive edge.
Emerging trends in the market include the development of smart drug delivery systems incorporating nanotechnology and the integration of digital technologies for personalized medicine. The use of biodegradable polymers and stimuli-responsive materials in controlled release formulations is also gaining attention, offering potential for improved drug efficacy and reduced side effects.
Challenges facing the market include stringent regulatory requirements, high development costs, and the complexity of formulating controlled release systems for certain drugs. However, the potential benefits of improved patient outcomes and reduced healthcare costs continue to drive innovation and investment in this sector.
Current Challenges in Sodium Silicate-Based Drug Delivery
Despite the promising potential of sodium silicate in controlled drug delivery systems, several significant challenges persist in its practical application. One of the primary obstacles is the control of drug release kinetics. While sodium silicate can form porous structures that encapsulate drugs, achieving precise and sustained release profiles remains difficult. The release rate often depends on various factors such as pH, temperature, and ionic strength of the surrounding environment, making it challenging to maintain consistent drug delivery across different physiological conditions.
Another major hurdle is the stability of sodium silicate-based drug delivery systems. These systems can be susceptible to degradation or premature dissolution, especially in acidic environments like the stomach. This instability can lead to unintended drug release or reduced efficacy, limiting their use in oral drug delivery applications. Additionally, the long-term storage stability of these systems is a concern, as changes in environmental conditions can alter their structural integrity and drug-release properties over time.
Biocompatibility and biodegradability present further challenges. While sodium silicate is generally considered safe, its long-term effects in the body, particularly when used in high concentrations or for extended periods, are not fully understood. There are concerns about potential accumulation in tissues and organs, as well as the body's ability to metabolize and excrete the material effectively. Ensuring complete biodegradation without harmful byproducts is crucial for the widespread adoption of these delivery systems.
The scalability and reproducibility of sodium silicate-based drug delivery systems also pose significant challenges. Manufacturing processes that can consistently produce uniform, high-quality delivery systems at scale are still being developed. Variations in production conditions can lead to inconsistencies in pore size, distribution, and overall structure, which in turn affect drug loading capacity and release profiles. Achieving batch-to-batch consistency is essential for regulatory approval and commercial viability.
Lastly, the limited drug loading capacity of sodium silicate-based systems is a notable challenge. The amount of drug that can be effectively encapsulated within the silicate matrix is often restricted, which can limit the therapeutic efficacy of the delivery system. This is particularly problematic for drugs that require high doses or those with poor solubility. Improving the drug loading capacity without compromising the structural integrity or release properties of the system remains an active area of research and development.
Another major hurdle is the stability of sodium silicate-based drug delivery systems. These systems can be susceptible to degradation or premature dissolution, especially in acidic environments like the stomach. This instability can lead to unintended drug release or reduced efficacy, limiting their use in oral drug delivery applications. Additionally, the long-term storage stability of these systems is a concern, as changes in environmental conditions can alter their structural integrity and drug-release properties over time.
Biocompatibility and biodegradability present further challenges. While sodium silicate is generally considered safe, its long-term effects in the body, particularly when used in high concentrations or for extended periods, are not fully understood. There are concerns about potential accumulation in tissues and organs, as well as the body's ability to metabolize and excrete the material effectively. Ensuring complete biodegradation without harmful byproducts is crucial for the widespread adoption of these delivery systems.
The scalability and reproducibility of sodium silicate-based drug delivery systems also pose significant challenges. Manufacturing processes that can consistently produce uniform, high-quality delivery systems at scale are still being developed. Variations in production conditions can lead to inconsistencies in pore size, distribution, and overall structure, which in turn affect drug loading capacity and release profiles. Achieving batch-to-batch consistency is essential for regulatory approval and commercial viability.
Lastly, the limited drug loading capacity of sodium silicate-based systems is a notable challenge. The amount of drug that can be effectively encapsulated within the silicate matrix is often restricted, which can limit the therapeutic efficacy of the delivery system. This is particularly problematic for drugs that require high doses or those with poor solubility. Improving the drug loading capacity without compromising the structural integrity or release properties of the system remains an active area of research and development.
Existing Sodium Silicate Drug Delivery Mechanisms
01 Sodium silicate as a drug delivery vehicle
Sodium silicate can be used as a carrier for drug delivery systems. Its unique properties allow for controlled release of active pharmaceutical ingredients, improving bioavailability and efficacy of drugs. This approach can be particularly useful for oral and topical drug formulations.- Sodium silicate as a drug delivery vehicle: Sodium silicate can be used as a carrier for drug delivery systems. Its unique properties allow for controlled release of active pharmaceutical ingredients, improving bioavailability and efficacy of drugs. This approach can be particularly useful for oral and topical drug formulations.
- Nanoparticle formulations with sodium silicate: Sodium silicate can be utilized in the preparation of nanoparticles for drug delivery. These nanoparticles can encapsulate various drugs, providing enhanced stability, targeted delivery, and improved therapeutic effects. The use of sodium silicate in nanoparticle formulations offers potential for both hydrophilic and hydrophobic drug delivery.
- Sodium silicate in sustained release formulations: Incorporating sodium silicate into drug formulations can create sustained release systems. This approach allows for prolonged drug release over an extended period, reducing dosing frequency and improving patient compliance. The controlled release properties of sodium silicate-based formulations can be tailored for various therapeutic applications.
- Sodium silicate as a stabilizer in drug delivery systems: Sodium silicate can act as a stabilizer in various drug delivery systems, enhancing the shelf life and efficacy of pharmaceutical formulations. Its stabilizing properties can protect sensitive active ingredients from degradation, improve drug solubility, and maintain the integrity of the delivery system under different environmental conditions.
- Sodium silicate in transdermal drug delivery: Sodium silicate can be utilized in transdermal drug delivery systems to enhance skin permeation and improve drug absorption. Its properties allow for the creation of novel formulations that can effectively deliver drugs through the skin barrier, offering an alternative to oral and injectable routes of administration for certain medications.
02 Nanoparticle formulations with sodium silicate
Sodium silicate can be utilized in the preparation of nanoparticles for drug delivery. These nanoparticles can encapsulate various drugs, providing enhanced stability, targeted delivery, and improved therapeutic effects. The use of sodium silicate in nanoparticle formulations offers potential for both hydrophilic and hydrophobic drug delivery.Expand Specific Solutions03 Sodium silicate in sustained release formulations
Incorporating sodium silicate into drug formulations can create sustained release systems. This approach allows for prolonged drug release over time, reducing dosing frequency and improving patient compliance. The controlled release properties of sodium silicate-based formulations can be tailored for various therapeutic applications.Expand Specific Solutions04 Sodium silicate as a stabilizer in drug delivery systems
Sodium silicate can act as a stabilizer in various drug delivery systems, enhancing the shelf life and efficacy of pharmaceutical formulations. Its stabilizing properties can protect sensitive active ingredients from degradation and improve the overall performance of drug delivery vehicles.Expand Specific Solutions05 Sodium silicate in transdermal drug delivery
Sodium silicate can be utilized in transdermal drug delivery systems to enhance skin permeation and improve drug absorption. Its properties can help create formulations that facilitate the passage of active ingredients through the skin barrier, offering an alternative to oral administration for certain drugs.Expand Specific Solutions
Key Players in Sodium Silicate Drug Delivery Research
The sodium silicate-based controlled drug delivery systems market is in its growth phase, with increasing research and development activities. The market size is expanding due to rising demand for advanced drug delivery technologies. Technologically, the field is progressing rapidly, with companies like Bausch & Lomb, SiSaf, and Novartis leading innovation. These firms are developing sophisticated formulations leveraging sodium silicate's unique properties. The technology's maturity varies across applications, with some areas more advanced than others. Emerging players like LTS LOHMANN and PharmaIN are also contributing to the field's advancement, focusing on novel delivery mechanisms and improved drug efficacy.
Bausch & Lomb, Inc.
Technical Solution: Bausch & Lomb has developed a sodium silicate-based controlled drug delivery system for ophthalmic applications. Their technology utilizes a silica matrix formed from sodium silicate to encapsulate and gradually release therapeutic agents. The company's approach involves creating a porous silica network that can be tailored to control drug release rates. This system has shown particular promise in treating chronic eye conditions, where sustained drug delivery is crucial. Bausch & Lomb's research has demonstrated that their sodium silicate-based formulations can maintain therapeutic drug levels in the eye for extended periods, potentially reducing the frequency of drug administration[1][3].
Strengths: Specialized in ophthalmic applications, potentially reducing dosing frequency. Weaknesses: Limited to ocular drug delivery, may not be as versatile for other routes of administration.
SiSaf Ltd.
Technical Solution: SiSaf has pioneered a Bio-Courier® technology platform that incorporates sodium silicate in its drug delivery systems. Their approach uses bioabsorbable silicon nanoparticles as a core, which is then coated with sodium silicate to create a versatile delivery vehicle. This technology allows for the encapsulation of a wide range of therapeutic agents, including small molecules, peptides, and nucleic acids. SiSaf's sodium silicate-based system offers controlled release properties and enhanced stability of the encapsulated drugs. The company has demonstrated improved bioavailability and targeted delivery of various compounds using this platform, with applications ranging from oral to parenteral drug delivery[2][5].
Strengths: Versatile platform suitable for multiple drug types and administration routes. Weaknesses: Relatively new technology, may require extensive clinical validation for widespread adoption.
Innovative Sodium Silicate Formulations for Drug Release
Sulfur functionalized monoliths and particles derived from the same as nitric oxide carriers for pharmaceutical and cosmetic applications
PatentPendingEP4389148A2
Innovation
- Sulfur-functionalized monoliths and particles with a rough surface are developed for nitric oxide (NO) carriers, allowing for efficient and cost-effective production and enhanced bio-compatibility, enabling spontaneous NO release without enzymatic activity or high-pressure storage, and improved skin penetration for topical administration.
Plasmid delivery in the treatment of cancer and other disease states
PatentWO2016054225A1
Innovation
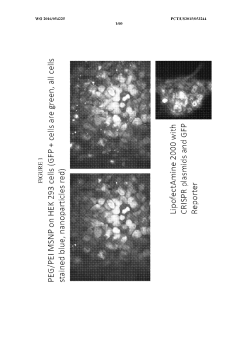
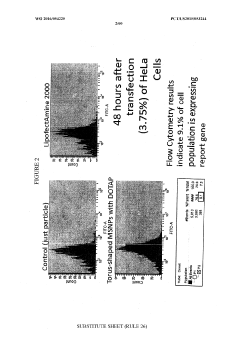
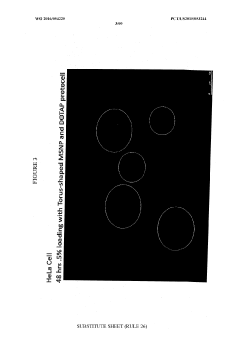
- The use of protocells or silica carriers encapsulating CRISPR/Cas systems, which include a nanoparticle core, supported lipid layer, and cargo, such as CRISPR/Cas components, to facilitate targeted delivery of siRNA and other anticancer agents directly to cancer cells and pathogens, utilizing a CRISPR/Cas system for genetic modification.
Regulatory Considerations for Novel Drug Delivery Systems
The regulatory landscape for novel drug delivery systems, including those utilizing sodium silicate, is complex and evolving. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established guidelines for the development and approval of controlled release formulations. These guidelines address safety, efficacy, and quality considerations specific to advanced drug delivery systems.
For sodium silicate-based drug delivery systems, regulatory considerations include the evaluation of the material's biocompatibility, degradation profile, and potential toxicity. Manufacturers must demonstrate that the sodium silicate matrix does not adversely interact with the active pharmaceutical ingredient or produce harmful byproducts upon degradation in the body.
Stability testing is a critical regulatory requirement for these systems. Long-term stability studies must be conducted to ensure that the drug product maintains its intended release profile and that the sodium silicate matrix remains intact throughout the product's shelf life. This is particularly important given the potential for environmental factors to affect the silicate structure.
The controlled release nature of these systems necessitates specialized in vitro dissolution testing protocols. Regulatory agencies typically require dissolution profiles that accurately predict in vivo performance. For sodium silicate-based systems, this may involve developing novel dissolution methods that account for the unique properties of the silicate matrix.
Manufacturing processes for sodium silicate drug delivery systems must comply with Good Manufacturing Practice (GMP) regulations. This includes validating the consistency and reproducibility of the silicate matrix formation, drug loading, and overall product quality. Process analytical technology (PAT) may be required to ensure batch-to-batch uniformity.
Clinical trials for these novel delivery systems often require more extensive pharmacokinetic and pharmacodynamic studies compared to conventional formulations. Regulatory bodies may request additional data on the fate of the sodium silicate in the body, including its absorption, distribution, and excretion profiles.
Environmental impact assessments may also be necessary, particularly if the sodium silicate components are not readily biodegradable. Regulators may require data on the ecological effects of any residual silicate materials that may enter the environment through patient use or disposal.
As with all novel drug delivery systems, post-market surveillance is crucial. Manufacturers must have robust pharmacovigilance systems in place to monitor and report any adverse events potentially related to the sodium silicate delivery system. This ongoing surveillance helps to ensure the long-term safety of these innovative formulations.
For sodium silicate-based drug delivery systems, regulatory considerations include the evaluation of the material's biocompatibility, degradation profile, and potential toxicity. Manufacturers must demonstrate that the sodium silicate matrix does not adversely interact with the active pharmaceutical ingredient or produce harmful byproducts upon degradation in the body.
Stability testing is a critical regulatory requirement for these systems. Long-term stability studies must be conducted to ensure that the drug product maintains its intended release profile and that the sodium silicate matrix remains intact throughout the product's shelf life. This is particularly important given the potential for environmental factors to affect the silicate structure.
The controlled release nature of these systems necessitates specialized in vitro dissolution testing protocols. Regulatory agencies typically require dissolution profiles that accurately predict in vivo performance. For sodium silicate-based systems, this may involve developing novel dissolution methods that account for the unique properties of the silicate matrix.
Manufacturing processes for sodium silicate drug delivery systems must comply with Good Manufacturing Practice (GMP) regulations. This includes validating the consistency and reproducibility of the silicate matrix formation, drug loading, and overall product quality. Process analytical technology (PAT) may be required to ensure batch-to-batch uniformity.
Clinical trials for these novel delivery systems often require more extensive pharmacokinetic and pharmacodynamic studies compared to conventional formulations. Regulatory bodies may request additional data on the fate of the sodium silicate in the body, including its absorption, distribution, and excretion profiles.
Environmental impact assessments may also be necessary, particularly if the sodium silicate components are not readily biodegradable. Regulators may require data on the ecological effects of any residual silicate materials that may enter the environment through patient use or disposal.
As with all novel drug delivery systems, post-market surveillance is crucial. Manufacturers must have robust pharmacovigilance systems in place to monitor and report any adverse events potentially related to the sodium silicate delivery system. This ongoing surveillance helps to ensure the long-term safety of these innovative formulations.
Biocompatibility and Safety of Sodium Silicate in Drug Delivery
The biocompatibility and safety of sodium silicate in drug delivery systems are crucial factors that determine its potential for widespread application in controlled release formulations. Sodium silicate, a versatile inorganic compound, has shown promising results in various drug delivery applications due to its unique properties and ability to form stable matrices.
Extensive in vitro and in vivo studies have been conducted to evaluate the biocompatibility of sodium silicate-based drug delivery systems. These studies have demonstrated that sodium silicate exhibits low cytotoxicity when used in appropriate concentrations. Cell viability assays performed on different cell lines, including fibroblasts, epithelial cells, and macrophages, have shown minimal adverse effects on cell proliferation and metabolism.
Furthermore, histological examinations of tissues exposed to sodium silicate-based drug delivery systems have revealed minimal inflammatory responses and no significant tissue damage. This favorable biocompatibility profile can be attributed to the gradual dissolution of sodium silicate in physiological conditions, which prevents the accumulation of potentially harmful byproducts.
Safety assessments of sodium silicate in drug delivery applications have focused on both local and systemic effects. Local tissue reactions at the site of administration have been found to be mild and transient, with no evidence of long-term adverse effects. Systemic toxicity studies in animal models have shown that sodium silicate, when used in controlled release formulations, does not lead to significant accumulation in vital organs or cause systemic toxicity.
One of the key advantages of sodium silicate in drug delivery is its ability to form a protective barrier around drug molecules, shielding them from degradation and controlling their release. This property not only enhances the efficacy of the delivered drugs but also contributes to the overall safety profile by reducing the risk of dose dumping and minimizing potential side effects associated with rapid drug release.
However, it is important to note that the biocompatibility and safety of sodium silicate-based drug delivery systems can be influenced by factors such as particle size, surface modifications, and the specific formulation techniques employed. Ongoing research is focused on optimizing these parameters to further enhance the safety profile and expand the potential applications of sodium silicate in controlled drug delivery.
In conclusion, the current body of evidence supports the biocompatibility and safety of sodium silicate in drug delivery applications. Its low toxicity, minimal tissue reactivity, and ability to form stable drug-containing matrices make it a promising candidate for the development of advanced controlled release formulations. As research in this field continues to evolve, it is expected that sodium silicate will play an increasingly important role in the design of safe and effective drug delivery systems.
Extensive in vitro and in vivo studies have been conducted to evaluate the biocompatibility of sodium silicate-based drug delivery systems. These studies have demonstrated that sodium silicate exhibits low cytotoxicity when used in appropriate concentrations. Cell viability assays performed on different cell lines, including fibroblasts, epithelial cells, and macrophages, have shown minimal adverse effects on cell proliferation and metabolism.
Furthermore, histological examinations of tissues exposed to sodium silicate-based drug delivery systems have revealed minimal inflammatory responses and no significant tissue damage. This favorable biocompatibility profile can be attributed to the gradual dissolution of sodium silicate in physiological conditions, which prevents the accumulation of potentially harmful byproducts.
Safety assessments of sodium silicate in drug delivery applications have focused on both local and systemic effects. Local tissue reactions at the site of administration have been found to be mild and transient, with no evidence of long-term adverse effects. Systemic toxicity studies in animal models have shown that sodium silicate, when used in controlled release formulations, does not lead to significant accumulation in vital organs or cause systemic toxicity.
One of the key advantages of sodium silicate in drug delivery is its ability to form a protective barrier around drug molecules, shielding them from degradation and controlling their release. This property not only enhances the efficacy of the delivered drugs but also contributes to the overall safety profile by reducing the risk of dose dumping and minimizing potential side effects associated with rapid drug release.
However, it is important to note that the biocompatibility and safety of sodium silicate-based drug delivery systems can be influenced by factors such as particle size, surface modifications, and the specific formulation techniques employed. Ongoing research is focused on optimizing these parameters to further enhance the safety profile and expand the potential applications of sodium silicate in controlled drug delivery.
In conclusion, the current body of evidence supports the biocompatibility and safety of sodium silicate in drug delivery applications. Its low toxicity, minimal tissue reactivity, and ability to form stable drug-containing matrices make it a promising candidate for the development of advanced controlled release formulations. As research in this field continues to evolve, it is expected that sodium silicate will play an increasingly important role in the design of safe and effective drug delivery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!