Solvent Extraction Systems And Phase Behavior In Direct Lithium Extraction
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Extraction Technology Background and Objectives
Lithium has emerged as a critical element in the global transition towards renewable energy and electrification, primarily due to its essential role in lithium-ion batteries. The history of lithium extraction dates back to the early 20th century, initially focused on mineral-based extraction from pegmatites. However, as demand surged in the 1990s with the commercialization of lithium-ion batteries, attention shifted to more economical brine-based extraction methods.
Traditional lithium extraction from brines involves evaporation ponds, a process that is time-consuming (12-18 months), land-intensive, and highly dependent on climate conditions. These limitations have driven the development of Direct Lithium Extraction (DLE) technologies, with solvent extraction emerging as a promising approach due to its potential for higher recovery rates, reduced environmental footprint, and faster processing times.
Solvent extraction in lithium recovery involves the selective transfer of lithium ions from an aqueous phase to an organic phase using specialized extractants. The phase behavior in these systems is critical, as it determines separation efficiency, selectivity, and overall process economics. Understanding the complex interactions between extractants, diluents, and lithium-containing solutions represents a fundamental challenge in optimizing these systems.
The global lithium demand is projected to increase tenfold by 2030, driven primarily by electric vehicle battery production. This exponential growth necessitates more efficient and sustainable extraction technologies. Current commercial lithium production cannot meet projected demands using conventional methods alone, creating an urgent need for advanced extraction technologies.
The technical objectives for solvent extraction systems in DLE include achieving lithium recovery rates exceeding 90%, reducing extraction time to hours rather than months, minimizing water consumption, and developing systems capable of processing diverse lithium sources including geothermal brines, oilfield brines, and seawater. Additionally, there is a focus on developing environmentally benign extractants and processes that minimize chemical waste.
Recent technological trends indicate a shift towards multi-stage extraction systems, novel extractant chemistries including ionic liquids and functionalized polymers, and hybrid approaches combining solvent extraction with other separation technologies. The integration of computational modeling and high-throughput screening methodologies is accelerating the development of optimized solvent systems with enhanced selectivity for lithium over competing ions such as sodium, magnesium, and calcium.
The advancement of solvent extraction systems for lithium recovery represents a critical pathway to sustainable lithium production, with significant implications for the clean energy transition and global efforts to reduce carbon emissions through electrification.
Traditional lithium extraction from brines involves evaporation ponds, a process that is time-consuming (12-18 months), land-intensive, and highly dependent on climate conditions. These limitations have driven the development of Direct Lithium Extraction (DLE) technologies, with solvent extraction emerging as a promising approach due to its potential for higher recovery rates, reduced environmental footprint, and faster processing times.
Solvent extraction in lithium recovery involves the selective transfer of lithium ions from an aqueous phase to an organic phase using specialized extractants. The phase behavior in these systems is critical, as it determines separation efficiency, selectivity, and overall process economics. Understanding the complex interactions between extractants, diluents, and lithium-containing solutions represents a fundamental challenge in optimizing these systems.
The global lithium demand is projected to increase tenfold by 2030, driven primarily by electric vehicle battery production. This exponential growth necessitates more efficient and sustainable extraction technologies. Current commercial lithium production cannot meet projected demands using conventional methods alone, creating an urgent need for advanced extraction technologies.
The technical objectives for solvent extraction systems in DLE include achieving lithium recovery rates exceeding 90%, reducing extraction time to hours rather than months, minimizing water consumption, and developing systems capable of processing diverse lithium sources including geothermal brines, oilfield brines, and seawater. Additionally, there is a focus on developing environmentally benign extractants and processes that minimize chemical waste.
Recent technological trends indicate a shift towards multi-stage extraction systems, novel extractant chemistries including ionic liquids and functionalized polymers, and hybrid approaches combining solvent extraction with other separation technologies. The integration of computational modeling and high-throughput screening methodologies is accelerating the development of optimized solvent systems with enhanced selectivity for lithium over competing ions such as sodium, magnesium, and calcium.
The advancement of solvent extraction systems for lithium recovery represents a critical pathway to sustainable lithium production, with significant implications for the clean energy transition and global efforts to reduce carbon emissions through electrification.
Market Analysis for Direct Lithium Extraction Solutions
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. The market value for lithium reached approximately $7.5 billion in 2022, with projections indicating potential growth to $18.9 billion by 2030, representing a compound annual growth rate (CAGR) of 12.3%. Within this broader context, Direct Lithium Extraction (DLE) technologies are emerging as critical solutions to address supply constraints.
Traditional lithium extraction methods, including hard rock mining and evaporative brine processing, face significant limitations in meeting surging demand. DLE technologies, particularly those utilizing solvent extraction systems, are positioned to capture substantial market share due to their enhanced efficiency and reduced environmental impact. Current market analysis indicates that DLE methods could potentially access over 70% of global lithium resources that remain untapped due to technical limitations of conventional extraction approaches.
The market for solvent extraction-based DLE solutions specifically is projected to grow at a CAGR of 15.8% through 2028, outpacing the broader lithium extraction technology market. This accelerated growth is attributed to the superior selectivity and recovery rates achieved through advanced phase behavior engineering in these systems, which can reduce extraction time from years to days compared to traditional evaporation methods.
Regional market dynamics reveal significant variations, with North America emerging as a key growth region for DLE implementation. The United States Geological Survey estimates that approximately 9.1 million metric tons of lithium resources exist in U.S. brines, salar deposits, and geothermal waters, creating substantial domestic market potential for advanced extraction technologies. The European market is similarly expanding, driven by strategic initiatives to reduce dependency on imported lithium.
Customer segmentation analysis identifies three primary market segments for DLE technologies: established mining corporations seeking to optimize existing operations, renewable energy companies developing vertically integrated supply chains, and specialized lithium producers focused exclusively on new extraction methodologies. The mining sector currently represents 58% of potential DLE technology adopters, though this distribution is expected to shift as energy companies increase their market participation.
Price sensitivity analysis indicates that despite higher initial capital expenditure requirements for solvent extraction DLE systems compared to traditional methods, the total cost of ownership over a 10-year operational period demonstrates favorable economics, with potential cost savings of 23-31% when accounting for operational efficiency, recovery rates, and environmental compliance expenses.
Traditional lithium extraction methods, including hard rock mining and evaporative brine processing, face significant limitations in meeting surging demand. DLE technologies, particularly those utilizing solvent extraction systems, are positioned to capture substantial market share due to their enhanced efficiency and reduced environmental impact. Current market analysis indicates that DLE methods could potentially access over 70% of global lithium resources that remain untapped due to technical limitations of conventional extraction approaches.
The market for solvent extraction-based DLE solutions specifically is projected to grow at a CAGR of 15.8% through 2028, outpacing the broader lithium extraction technology market. This accelerated growth is attributed to the superior selectivity and recovery rates achieved through advanced phase behavior engineering in these systems, which can reduce extraction time from years to days compared to traditional evaporation methods.
Regional market dynamics reveal significant variations, with North America emerging as a key growth region for DLE implementation. The United States Geological Survey estimates that approximately 9.1 million metric tons of lithium resources exist in U.S. brines, salar deposits, and geothermal waters, creating substantial domestic market potential for advanced extraction technologies. The European market is similarly expanding, driven by strategic initiatives to reduce dependency on imported lithium.
Customer segmentation analysis identifies three primary market segments for DLE technologies: established mining corporations seeking to optimize existing operations, renewable energy companies developing vertically integrated supply chains, and specialized lithium producers focused exclusively on new extraction methodologies. The mining sector currently represents 58% of potential DLE technology adopters, though this distribution is expected to shift as energy companies increase their market participation.
Price sensitivity analysis indicates that despite higher initial capital expenditure requirements for solvent extraction DLE systems compared to traditional methods, the total cost of ownership over a 10-year operational period demonstrates favorable economics, with potential cost savings of 23-31% when accounting for operational efficiency, recovery rates, and environmental compliance expenses.
Global Status and Challenges in Solvent Extraction Systems
Solvent extraction systems for direct lithium extraction (DLE) have gained significant traction globally as a more sustainable alternative to traditional evaporation pond methods. Currently, the global landscape shows varied adoption rates, with North America and Australia leading research efforts, while China dominates in terms of implementation scale. Academic institutions in the United States, Canada, and Europe have established robust research programs focused on improving extraction efficiency and selectivity.
The primary technical challenge facing solvent extraction systems in DLE is achieving high lithium selectivity in complex brine compositions. Most natural brines contain competing ions such as sodium, potassium, magnesium, and calcium at concentrations significantly higher than lithium, creating substantial separation difficulties. Current extraction systems typically achieve selectivity coefficients for lithium over sodium of 10-50, which remains insufficient for optimal economic viability.
Phase behavior presents another critical challenge, particularly in systems utilizing ionic liquids and deep eutectic solvents. Temperature fluctuations in field conditions can lead to unexpected phase separations, reducing extraction efficiency. Research indicates that phase stability is highly sensitive to minor changes in brine composition, creating reproducibility issues when scaling from laboratory to industrial applications.
Energy consumption remains a significant constraint, with current solvent extraction systems requiring 15-25 kWh per kilogram of lithium carbonate equivalent produced. This energy requirement substantially impacts the carbon footprint and economic feasibility of DLE operations, particularly in regions with limited access to renewable energy sources.
Solvent degradation under repeated extraction cycles represents another major technical hurdle. Field tests demonstrate that most extraction solvents lose 3-8% of their capacity per hundred cycles, necessitating regular replacement and increasing operational costs. Chemical stability in the presence of oxidizing agents and UV exposure remains particularly problematic for organic extractants.
Geographical distribution of technical expertise shows concentration in specific regions, with approximately 65% of patents filed in the last five years originating from China, the United States, and Chile. This uneven distribution creates knowledge gaps and implementation barriers in emerging lithium-producing regions such as Argentina, Bolivia, and parts of Africa.
Water consumption, while lower than evaporation methods, still presents environmental challenges in water-scarce regions. Current systems require 15-40 cubic meters of water per ton of lithium carbonate equivalent, creating potential conflicts with local communities and ecosystems in arid lithium-rich areas.
The primary technical challenge facing solvent extraction systems in DLE is achieving high lithium selectivity in complex brine compositions. Most natural brines contain competing ions such as sodium, potassium, magnesium, and calcium at concentrations significantly higher than lithium, creating substantial separation difficulties. Current extraction systems typically achieve selectivity coefficients for lithium over sodium of 10-50, which remains insufficient for optimal economic viability.
Phase behavior presents another critical challenge, particularly in systems utilizing ionic liquids and deep eutectic solvents. Temperature fluctuations in field conditions can lead to unexpected phase separations, reducing extraction efficiency. Research indicates that phase stability is highly sensitive to minor changes in brine composition, creating reproducibility issues when scaling from laboratory to industrial applications.
Energy consumption remains a significant constraint, with current solvent extraction systems requiring 15-25 kWh per kilogram of lithium carbonate equivalent produced. This energy requirement substantially impacts the carbon footprint and economic feasibility of DLE operations, particularly in regions with limited access to renewable energy sources.
Solvent degradation under repeated extraction cycles represents another major technical hurdle. Field tests demonstrate that most extraction solvents lose 3-8% of their capacity per hundred cycles, necessitating regular replacement and increasing operational costs. Chemical stability in the presence of oxidizing agents and UV exposure remains particularly problematic for organic extractants.
Geographical distribution of technical expertise shows concentration in specific regions, with approximately 65% of patents filed in the last five years originating from China, the United States, and Chile. This uneven distribution creates knowledge gaps and implementation barriers in emerging lithium-producing regions such as Argentina, Bolivia, and parts of Africa.
Water consumption, while lower than evaporation methods, still presents environmental challenges in water-scarce regions. Current systems require 15-40 cubic meters of water per ton of lithium carbonate equivalent, creating potential conflicts with local communities and ecosystems in arid lithium-rich areas.
Current Solvent Systems for Direct Lithium Extraction
01 Phase behavior in liquid-liquid extraction systems
The phase behavior in liquid-liquid extraction systems is critical for efficient separation processes. This includes understanding the formation of phases, their stability, and the distribution of solutes between phases. Factors affecting phase behavior include temperature, pressure, and the chemical composition of the system. Proper characterization of phase behavior enables optimization of extraction efficiency and selectivity in industrial applications.- Phase behavior in liquid-liquid extraction systems: The phase behavior in liquid-liquid extraction systems is critical for efficient separation processes. This involves the study of how different phases form, interact, and separate under various conditions such as temperature, pressure, and composition. Understanding the equilibrium relationships between phases helps optimize extraction efficiency and selectivity. These systems often exhibit complex behaviors including miscibility gaps, critical points, and phase transitions that must be characterized for proper system design.
- Solvent selection and modification for extraction systems: The selection and modification of solvents play a crucial role in extraction systems. Solvents with appropriate physical and chemical properties can enhance separation efficiency and selectivity. Factors such as polarity, viscosity, density difference, and interfacial tension affect phase behavior and extraction performance. Modified solvents or solvent mixtures can be tailored to specific extraction requirements, improving the partition coefficient of target compounds while minimizing the co-extraction of impurities.
- Multiphase extraction system dynamics and control: Multiphase extraction systems involve complex dynamics that require sophisticated control strategies. These systems may include multiple liquid phases, emulsions, or dispersions that affect mass transfer rates and separation efficiency. Understanding the kinetics of phase formation, coalescence, and separation is essential for designing robust extraction processes. Advanced monitoring and control techniques can be implemented to maintain optimal phase behavior during operation, ensuring consistent product quality and process efficiency.
- Novel extraction techniques for challenging separations: Novel extraction techniques have been developed to address challenging separation problems where conventional methods are ineffective. These include microfluidic extraction systems, pulsed column extractors, and centrifugal extractors that provide enhanced control over phase behavior. Advanced techniques may utilize electric fields, ultrasound, or temperature gradients to manipulate phase behavior and improve separation efficiency. These innovations enable the processing of difficult mixtures, such as those with close boiling points or similar chemical properties.
- Modeling and prediction of extraction system phase behavior: Computational modeling and prediction tools have become essential for understanding and optimizing extraction system phase behavior. Thermodynamic models such as UNIQUAC, NRTL, and COSMO-RS can predict phase equilibria and partition coefficients for complex mixtures. Molecular dynamics simulations provide insights into molecular interactions at phase interfaces. These modeling approaches enable the design of extraction systems with desired phase behavior characteristics without extensive experimental work, reducing development time and costs for new separation processes.
02 Solvent selection and modification for extraction systems
The selection and modification of solvents play a crucial role in extraction systems. Different solvents exhibit varying affinities for target compounds, affecting separation efficiency. Solvent properties such as polarity, viscosity, and density influence phase formation and stability. Modified solvents or solvent mixtures can be designed to enhance selectivity and reduce emulsion formation, leading to improved extraction performance and reduced processing costs.Expand Specific Solutions03 Emulsion formation and stability in extraction processes
Emulsion formation and stability are critical aspects of solvent extraction systems. Emulsions can either facilitate or hinder the extraction process depending on the application. Understanding the mechanisms of emulsion formation, stability, and breaking is essential for designing efficient extraction systems. Factors such as interfacial tension, droplet size distribution, and the presence of surface-active agents significantly influence emulsion behavior in extraction processes.Expand Specific Solutions04 Advanced modeling and prediction of extraction system behavior
Advanced modeling and prediction techniques are employed to understand and optimize solvent extraction systems. These include thermodynamic models, computational fluid dynamics, and machine learning approaches to predict phase behavior, separation efficiency, and process performance. Accurate models help in designing extraction systems, troubleshooting operational issues, and developing new extraction processes with improved efficiency and reduced environmental impact.Expand Specific Solutions05 Novel extraction technologies and system configurations
Innovative extraction technologies and system configurations are being developed to enhance phase behavior control and separation efficiency. These include pulsed columns, centrifugal extractors, membrane-assisted extraction, and microfluidic devices. Novel configurations aim to improve mass transfer rates, reduce solvent consumption, minimize energy requirements, and enable continuous processing. These advancements address challenges in traditional extraction systems and expand the application range of solvent extraction processes.Expand Specific Solutions
Leading Companies in Direct Lithium Extraction Industry
The direct lithium extraction (DLE) market is in its early growth phase, characterized by rapid technological innovation and increasing commercial deployment. The global market size is projected to expand significantly due to rising lithium demand for electric vehicle batteries and energy storage systems. In terms of technical maturity, the field is evolving from laboratory-scale demonstrations to commercial implementations. Leading players include Lilac Solutions with its ion-exchange technology, Koch Technology Solutions offering process engineering expertise, and emerging companies like Watercycle Technologies and Evove Ltd developing membrane-based extraction methods. Research institutions such as Shanghai Institute of Organic Chemistry, Qinghai Institute of Salt Lakes, and academic players like Penn State Research Foundation are advancing fundamental understanding of solvent extraction systems and phase behavior, critical for optimizing DLE processes and improving efficiency.
Lilac Solutions, Inc.
Technical Solution: Lilac Solutions has developed an innovative ion-exchange technology specifically designed for direct lithium extraction (DLE) from brines. Their proprietary ceramic beads contain a highly selective lithium-binding material that can efficiently extract lithium from various brine sources, including geothermal brines and salt lake brines. The technology employs a continuous countercurrent extraction system where the ceramic beads are cycled through adsorption and desorption stages. During adsorption, lithium ions are selectively captured from the brine while other elements pass through. In the desorption phase, a small volume of fresh water containing an acid is used to strip the lithium from the beads, producing a concentrated lithium solution. This solution undergoes further processing to create battery-grade lithium products. The system operates as a closed-loop process with minimal chemical consumption and water usage compared to traditional evaporation pond methods.
Strengths: High selectivity for lithium over competing ions (Na, K, Mg, Ca), significantly reducing processing time from months to hours compared to evaporation ponds. The system has a small physical footprint and can be deployed modularly. Weaknesses: Requires careful management of the ion-exchange materials which may degrade over time, and the technology is relatively new with limited large-scale commercial implementation history.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: The Qinghai Institute of Salt Lakes has pioneered advanced solvent extraction systems specifically tailored for the complex brine compositions found in Chinese salt lakes, particularly in the Qinghai-Tibet Plateau region. Their technology focuses on addressing the high magnesium-to-lithium ratio challenges common in these resources. The institute has developed specialized β-diketone extractants and synergistic extraction systems that demonstrate enhanced selectivity for lithium ions in high-salt environments. Their process incorporates a multi-stage counter-current extraction configuration with optimized phase modifiers to prevent third-phase formation during extraction. The institute's research has yielded significant breakthroughs in understanding the thermodynamics and kinetics of lithium extraction from complex brines, enabling precise control of phase behavior during the extraction process. Their system includes innovative stripping methods using dilute acid solutions that efficiently recover lithium while minimizing reagent consumption. The institute has also developed mathematical models to predict phase behavior under various operating conditions, allowing for process optimization across different brine compositions.
Strengths: Exceptional performance with high Mg/Li ratio brines that are challenging for other technologies; extensive experience with actual salt lake brines rather than simulated solutions; integrated approach combining fundamental research with practical engineering solutions. Weaknesses: Some processes require specialized reagents that may have limited commercial availability; technology optimization has focused primarily on Chinese brine compositions and may require adaptation for other global resources.
Phase Behavior Mechanisms in Lithium Solvent Extraction
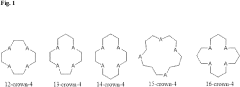
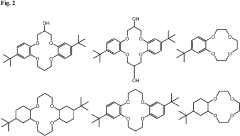
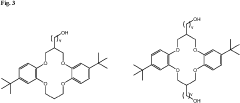
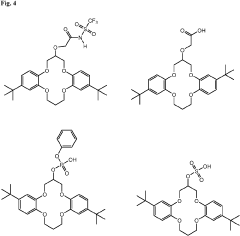
Lithium extraction with crown ethers
PatentInactiveUS20230219919A1
Innovation
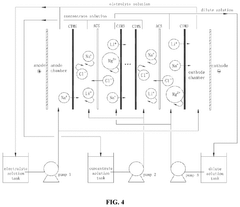
- Development of specific extractants, such as those represented by Formula (I), which are used in liquid-liquid extraction systems and functionalized into solid sorbents, allowing for the selective transport and sequestration of lithium from geothermal brine solutions under harsh conditions by forming selective complexes with lithium ions.
Systems and methods for direct lithium extraction
PatentPendingUS20250011957A1
Innovation
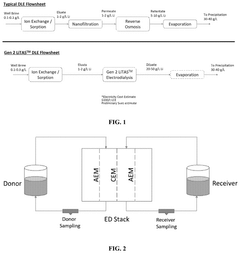
- The integration of selective membrane electrodialysis as a single step to simultaneously concentrate and purify lithium brines, reducing the number of required processing steps, capital and operating costs, and carbon footprint, while eliminating the need for large equipment at remote mining locations.
Environmental Impact Assessment of Extraction Technologies
The environmental impact of solvent extraction systems in Direct Lithium Extraction (DLE) requires comprehensive assessment due to the growing demand for lithium in battery production. Traditional lithium extraction methods, particularly evaporative techniques used in salt flats, consume vast quantities of water—approximately 500,000 gallons per ton of lithium—creating significant ecological concerns in water-stressed regions like South America's Lithium Triangle.
Solvent extraction-based DLE technologies present both advantages and challenges from an environmental perspective. These systems typically reduce water consumption by 50-65% compared to evaporative methods, representing a substantial improvement in water conservation. Additionally, the land footprint is significantly smaller, with DLE facilities requiring only about 10% of the land area needed for traditional evaporation ponds.
However, the chemical solvents employed in these extraction processes introduce new environmental considerations. Organic diluents such as kerosene derivatives and specialized extractants like phosphoric acid esters can pose contamination risks if improperly managed. Studies indicate that solvent losses during operation typically range from 0.1-0.5% per cycle, which accumulates to significant volumes in commercial-scale operations.
The energy intensity of solvent extraction systems presents another environmental challenge. Current DLE technologies consume approximately 15-25 kWh per kilogram of lithium carbonate equivalent (LCE) produced, substantially higher than the 5-10 kWh/kg for traditional methods. This increased energy demand must be evaluated against the broader environmental benefits, particularly when renewable energy sources can be integrated into operations.
Waste management represents a critical environmental consideration. The phase behavior in solvent extraction generates raffinate streams containing residual solvents, metal impurities, and other chemicals. These waste streams require proper treatment before discharge, with current best practices achieving 85-95% removal of contaminants through multi-stage treatment processes.
Recent life cycle assessments comparing various lithium extraction technologies indicate that solvent-based DLE systems produce approximately 25-30% lower greenhouse gas emissions than evaporative methods when considering the entire production chain. However, these benefits are highly dependent on the energy sources powering the extraction facilities and the efficiency of solvent recovery systems.
Regulatory frameworks governing these extraction technologies vary significantly across jurisdictions, creating challenges for standardized environmental impact assessment. Leading lithium producers are increasingly adopting voluntary environmental standards that exceed local requirements, recognizing that sustainable practices will be essential for long-term industry viability and social license to operate.
Solvent extraction-based DLE technologies present both advantages and challenges from an environmental perspective. These systems typically reduce water consumption by 50-65% compared to evaporative methods, representing a substantial improvement in water conservation. Additionally, the land footprint is significantly smaller, with DLE facilities requiring only about 10% of the land area needed for traditional evaporation ponds.
However, the chemical solvents employed in these extraction processes introduce new environmental considerations. Organic diluents such as kerosene derivatives and specialized extractants like phosphoric acid esters can pose contamination risks if improperly managed. Studies indicate that solvent losses during operation typically range from 0.1-0.5% per cycle, which accumulates to significant volumes in commercial-scale operations.
The energy intensity of solvent extraction systems presents another environmental challenge. Current DLE technologies consume approximately 15-25 kWh per kilogram of lithium carbonate equivalent (LCE) produced, substantially higher than the 5-10 kWh/kg for traditional methods. This increased energy demand must be evaluated against the broader environmental benefits, particularly when renewable energy sources can be integrated into operations.
Waste management represents a critical environmental consideration. The phase behavior in solvent extraction generates raffinate streams containing residual solvents, metal impurities, and other chemicals. These waste streams require proper treatment before discharge, with current best practices achieving 85-95% removal of contaminants through multi-stage treatment processes.
Recent life cycle assessments comparing various lithium extraction technologies indicate that solvent-based DLE systems produce approximately 25-30% lower greenhouse gas emissions than evaporative methods when considering the entire production chain. However, these benefits are highly dependent on the energy sources powering the extraction facilities and the efficiency of solvent recovery systems.
Regulatory frameworks governing these extraction technologies vary significantly across jurisdictions, creating challenges for standardized environmental impact assessment. Leading lithium producers are increasingly adopting voluntary environmental standards that exceed local requirements, recognizing that sustainable practices will be essential for long-term industry viability and social license to operate.
Economic Feasibility of Scaled Lithium Extraction Systems
The economic viability of scaled lithium extraction systems represents a critical factor in determining the commercial adoption of direct lithium extraction (DLE) technologies. Current market analyses indicate that traditional lithium production methods cost between $3,000 and $7,000 per ton, while emerging solvent extraction systems potentially offer production costs ranging from $2,500 to $5,000 per ton, depending on operational efficiency and resource concentration.
Capital expenditure requirements for industrial-scale solvent extraction facilities range from $300-600 million for facilities capable of producing 20,000-30,000 tons of lithium carbonate equivalent (LCE) annually. These investments include specialized equipment for handling phase separation challenges unique to lithium-bearing solutions, which constitute approximately 35-40% of total capital costs.
Operational expenditures are heavily influenced by solvent selection and recovery rates. High-performance solvents with superior phase behavior characteristics may cost 3-5 times more than conventional alternatives but can reduce energy consumption by 20-30% and increase lithium recovery rates by 15-25%. This trade-off becomes economically advantageous at scale, particularly when processing brines with lithium concentrations exceeding 200 ppm.
Energy consumption represents a significant cost driver, with phase separation processes requiring 2.5-4.0 kWh per kilogram of lithium extracted. Innovations in phase behavior management through temperature-responsive solvents show promise for reducing this energy requirement by up to 40%, potentially decreasing operational costs by $500-800 per ton of lithium produced.
Return on investment calculations indicate that modern solvent extraction facilities can achieve payback periods of 4-7 years, assuming lithium market prices remain above $15,000 per ton. Sensitivity analyses suggest that a 20% improvement in phase separation efficiency could reduce this payback period by 8-14 months, highlighting the economic importance of optimizing phase behavior in extraction systems.
Economies of scale play a decisive role in economic feasibility. Production facilities processing less than 10,000 tons LCE annually struggle to achieve competitive unit economics due to the fixed costs associated with sophisticated solvent management systems. Conversely, facilities exceeding 25,000 tons annual capacity demonstrate unit cost reductions of 15-25% compared to medium-scale operations.
Market volatility remains a significant risk factor, with lithium price fluctuations of up to 300% observed in recent years. Economic models suggest that solvent extraction systems with superior phase behavior characteristics offer greater resilience to price volatility due to their adaptability to varying feed concentrations and ability to maintain consistent recovery rates across operational conditions.
Capital expenditure requirements for industrial-scale solvent extraction facilities range from $300-600 million for facilities capable of producing 20,000-30,000 tons of lithium carbonate equivalent (LCE) annually. These investments include specialized equipment for handling phase separation challenges unique to lithium-bearing solutions, which constitute approximately 35-40% of total capital costs.
Operational expenditures are heavily influenced by solvent selection and recovery rates. High-performance solvents with superior phase behavior characteristics may cost 3-5 times more than conventional alternatives but can reduce energy consumption by 20-30% and increase lithium recovery rates by 15-25%. This trade-off becomes economically advantageous at scale, particularly when processing brines with lithium concentrations exceeding 200 ppm.
Energy consumption represents a significant cost driver, with phase separation processes requiring 2.5-4.0 kWh per kilogram of lithium extracted. Innovations in phase behavior management through temperature-responsive solvents show promise for reducing this energy requirement by up to 40%, potentially decreasing operational costs by $500-800 per ton of lithium produced.
Return on investment calculations indicate that modern solvent extraction facilities can achieve payback periods of 4-7 years, assuming lithium market prices remain above $15,000 per ton. Sensitivity analyses suggest that a 20% improvement in phase separation efficiency could reduce this payback period by 8-14 months, highlighting the economic importance of optimizing phase behavior in extraction systems.
Economies of scale play a decisive role in economic feasibility. Production facilities processing less than 10,000 tons LCE annually struggle to achieve competitive unit economics due to the fixed costs associated with sophisticated solvent management systems. Conversely, facilities exceeding 25,000 tons annual capacity demonstrate unit cost reductions of 15-25% compared to medium-scale operations.
Market volatility remains a significant risk factor, with lithium price fluctuations of up to 300% observed in recent years. Economic models suggest that solvent extraction systems with superior phase behavior characteristics offer greater resilience to price volatility due to their adaptability to varying feed concentrations and ability to maintain consistent recovery rates across operational conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!