Strategies For Mg-Li Separation To Achieve High Purity In Direct Lithium Extraction
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Li Separation Technology Background and Objectives
The evolution of lithium extraction technologies has undergone significant transformation over the past decades, shifting from traditional evaporation methods to more advanced direct lithium extraction (DLE) techniques. This technological progression has been primarily driven by increasing global demand for lithium, a critical component in rechargeable batteries for electric vehicles and energy storage systems. The lithium market has experienced unprecedented growth, with projections indicating a potential supply gap if extraction technologies do not advance to meet demand.
A persistent challenge in DLE processes is the separation of lithium from magnesium, as these elements share similar chemical properties due to their proximity in the periodic table. Historically, the presence of magnesium has significantly hindered the efficiency and economic viability of lithium extraction operations, often requiring complex and costly purification steps to achieve battery-grade lithium compounds.
The technical objective of Mg-Li separation technology development is to establish efficient, cost-effective, and environmentally sustainable methods to selectively extract lithium while minimizing magnesium contamination. This involves achieving high selectivity coefficients (typically >50) for lithium over magnesium, reducing energy consumption compared to traditional methods, and minimizing chemical reagent usage and waste generation.
Recent technological breakthroughs have focused on novel sorbent materials with enhanced Li/Mg selectivity, including ion-exchange resins, inorganic ion sieves, and metal-organic frameworks (MOFs). These materials exploit subtle differences in ionic radii, hydration energies, and coordination preferences between lithium and magnesium ions to achieve separation.
The development trajectory indicates a shift toward multi-stage separation processes that combine different technologies to overcome the limitations of single-method approaches. These hybrid systems often incorporate membrane technologies, electrochemical processes, and selective precipitation techniques working in synergy to achieve higher purity levels.
Environmental considerations have become increasingly important in technology development, with emphasis on reducing water consumption, minimizing chemical usage, and developing closed-loop systems that recycle process water and reagents. This aligns with broader sustainability goals in the mining and battery manufacturing sectors.
The ultimate technical goal is to develop separation technologies capable of producing battery-grade lithium compounds (>99.5% purity) directly from diverse lithium sources, including brines, geothermal waters, and recycled materials, with minimal environmental impact and competitive production costs compared to conventional methods.
A persistent challenge in DLE processes is the separation of lithium from magnesium, as these elements share similar chemical properties due to their proximity in the periodic table. Historically, the presence of magnesium has significantly hindered the efficiency and economic viability of lithium extraction operations, often requiring complex and costly purification steps to achieve battery-grade lithium compounds.
The technical objective of Mg-Li separation technology development is to establish efficient, cost-effective, and environmentally sustainable methods to selectively extract lithium while minimizing magnesium contamination. This involves achieving high selectivity coefficients (typically >50) for lithium over magnesium, reducing energy consumption compared to traditional methods, and minimizing chemical reagent usage and waste generation.
Recent technological breakthroughs have focused on novel sorbent materials with enhanced Li/Mg selectivity, including ion-exchange resins, inorganic ion sieves, and metal-organic frameworks (MOFs). These materials exploit subtle differences in ionic radii, hydration energies, and coordination preferences between lithium and magnesium ions to achieve separation.
The development trajectory indicates a shift toward multi-stage separation processes that combine different technologies to overcome the limitations of single-method approaches. These hybrid systems often incorporate membrane technologies, electrochemical processes, and selective precipitation techniques working in synergy to achieve higher purity levels.
Environmental considerations have become increasingly important in technology development, with emphasis on reducing water consumption, minimizing chemical usage, and developing closed-loop systems that recycle process water and reagents. This aligns with broader sustainability goals in the mining and battery manufacturing sectors.
The ultimate technical goal is to develop separation technologies capable of producing battery-grade lithium compounds (>99.5% purity) directly from diverse lithium sources, including brines, geothermal waters, and recycled materials, with minimal environmental impact and competitive production costs compared to conventional methods.
Market Analysis for High-Purity Lithium Products
The global high-purity lithium market is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Current market valuations place the high-purity lithium compounds sector at approximately $5.1 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 18.7% through 2030, potentially reaching $17.6 billion by the end of the decade.
Battery-grade lithium compounds, particularly lithium hydroxide and lithium carbonate with purities exceeding 99.5%, command premium pricing in the market. As of early 2024, battery-grade lithium hydroxide trades at $15,000-$18,000 per metric ton, while high-purity lithium carbonate ranges between $12,000-$15,000 per metric ton. These prices reflect a significant premium over technical-grade lithium compounds, highlighting the market's willingness to pay for superior purity.
The demand for high-purity lithium is geographically concentrated, with China, South Korea, Japan, and increasingly Europe and North America representing the primary markets. China currently dominates global lithium processing, accounting for over 60% of refined lithium production, though diversification efforts are underway in other regions to reduce supply chain vulnerabilities.
End-user segmentation reveals that the EV battery sector consumes approximately 71% of high-purity lithium products, followed by consumer electronics (14%), grid storage systems (9%), and other applications including aerospace and medical devices (6%). Industry analysts project that by 2028, the EV sector alone may require over 1.5 million metric tons of lithium carbonate equivalent (LCE) annually.
The market demonstrates increasing sensitivity to purity levels, with magnesium contamination emerging as a critical concern. Battery manufacturers typically specify maximum Mg:Li ratios below 1:10,000 for premium battery applications, as higher magnesium content significantly impacts battery performance and longevity. This specification drives demand for advanced separation technologies that can achieve these stringent purity requirements.
Supply chain dynamics are evolving rapidly, with direct lithium extraction (DLE) technologies gaining prominence as alternatives to traditional evaporation pond methods. Market adoption of DLE is projected to grow from current levels of approximately 5% of global production to potentially 25-30% by 2030, contingent upon technological advancements in Mg-Li separation efficiency.
Regulatory frameworks are increasingly influencing market dynamics, with the EU Battery Regulation and similar initiatives in North America establishing carbon footprint requirements and responsible sourcing standards. These regulations create market premiums for lithium products with lower environmental impacts, potentially accelerating the adoption of DLE technologies that offer reduced water consumption and land use compared to conventional methods.
Battery-grade lithium compounds, particularly lithium hydroxide and lithium carbonate with purities exceeding 99.5%, command premium pricing in the market. As of early 2024, battery-grade lithium hydroxide trades at $15,000-$18,000 per metric ton, while high-purity lithium carbonate ranges between $12,000-$15,000 per metric ton. These prices reflect a significant premium over technical-grade lithium compounds, highlighting the market's willingness to pay for superior purity.
The demand for high-purity lithium is geographically concentrated, with China, South Korea, Japan, and increasingly Europe and North America representing the primary markets. China currently dominates global lithium processing, accounting for over 60% of refined lithium production, though diversification efforts are underway in other regions to reduce supply chain vulnerabilities.
End-user segmentation reveals that the EV battery sector consumes approximately 71% of high-purity lithium products, followed by consumer electronics (14%), grid storage systems (9%), and other applications including aerospace and medical devices (6%). Industry analysts project that by 2028, the EV sector alone may require over 1.5 million metric tons of lithium carbonate equivalent (LCE) annually.
The market demonstrates increasing sensitivity to purity levels, with magnesium contamination emerging as a critical concern. Battery manufacturers typically specify maximum Mg:Li ratios below 1:10,000 for premium battery applications, as higher magnesium content significantly impacts battery performance and longevity. This specification drives demand for advanced separation technologies that can achieve these stringent purity requirements.
Supply chain dynamics are evolving rapidly, with direct lithium extraction (DLE) technologies gaining prominence as alternatives to traditional evaporation pond methods. Market adoption of DLE is projected to grow from current levels of approximately 5% of global production to potentially 25-30% by 2030, contingent upon technological advancements in Mg-Li separation efficiency.
Regulatory frameworks are increasingly influencing market dynamics, with the EU Battery Regulation and similar initiatives in North America establishing carbon footprint requirements and responsible sourcing standards. These regulations create market premiums for lithium products with lower environmental impacts, potentially accelerating the adoption of DLE technologies that offer reduced water consumption and land use compared to conventional methods.
Current Challenges in Mg-Li Separation Technologies
Despite significant advancements in Direct Lithium Extraction (DLE) technologies, the separation of magnesium from lithium remains one of the most persistent technical challenges in achieving high-purity lithium products. The chemical similarity between lithium and magnesium ions, particularly their comparable ionic radii (Li⁺: 0.76 Å, Mg²⁺: 0.72 Å) and hydration behavior, makes selective extraction exceptionally difficult using conventional methods.
Traditional separation approaches such as precipitation and solvent extraction often suffer from low selectivity coefficients, typically ranging from 2:1 to 10:1 for Li/Mg, which is insufficient for producing battery-grade lithium compounds that require 99.5% purity or higher. This selectivity limitation necessitates multiple processing stages, significantly increasing operational costs and reducing overall lithium recovery rates.
Energy consumption presents another major challenge, with current Mg-Li separation processes requiring between 15-40 kWh per kilogram of lithium carbonate equivalent (LCE) produced. This energy intensity not only impacts economic viability but also contradicts the sustainability goals of the lithium industry, particularly as it relates to electric vehicle battery production.
Membrane-based separation technologies, while promising, face persistent issues with membrane fouling and degradation when processing brine solutions with high magnesium content. Field tests have shown that membrane performance can decrease by up to 30% within the first 500 hours of operation in high Mg/Li ratio brines (>15:1), necessitating frequent replacement and maintenance.
Ion-exchange materials designed for lithium selectivity often demonstrate diminished capacity and selectivity in the presence of competing magnesium ions. Current commercial adsorbents typically show a 40-60% reduction in lithium loading capacity when magnesium concentrations exceed 0.5 g/L, which is common in many natural brine sources.
Scalability remains problematic, with laboratory-proven technologies frequently failing to maintain separation efficiency at industrial scales. The gap between bench-scale performance (processing liters) and commercial requirements (processing thousands of cubic meters daily) has resulted in numerous promising technologies failing during scale-up phases.
Environmental considerations further complicate technology selection, as many effective separation agents contain toxic components or generate hazardous waste streams. Regulatory compliance increasingly demands more environmentally benign processes, limiting the application of certain chemical approaches that might otherwise offer superior separation performance.
The economic threshold for viable Mg-Li separation technologies continues to shift as lithium market prices fluctuate, creating uncertainty for technology developers and mining companies. Current separation costs typically represent 30-45% of total DLE operational expenses, making this step a critical focus for cost reduction efforts.
Traditional separation approaches such as precipitation and solvent extraction often suffer from low selectivity coefficients, typically ranging from 2:1 to 10:1 for Li/Mg, which is insufficient for producing battery-grade lithium compounds that require 99.5% purity or higher. This selectivity limitation necessitates multiple processing stages, significantly increasing operational costs and reducing overall lithium recovery rates.
Energy consumption presents another major challenge, with current Mg-Li separation processes requiring between 15-40 kWh per kilogram of lithium carbonate equivalent (LCE) produced. This energy intensity not only impacts economic viability but also contradicts the sustainability goals of the lithium industry, particularly as it relates to electric vehicle battery production.
Membrane-based separation technologies, while promising, face persistent issues with membrane fouling and degradation when processing brine solutions with high magnesium content. Field tests have shown that membrane performance can decrease by up to 30% within the first 500 hours of operation in high Mg/Li ratio brines (>15:1), necessitating frequent replacement and maintenance.
Ion-exchange materials designed for lithium selectivity often demonstrate diminished capacity and selectivity in the presence of competing magnesium ions. Current commercial adsorbents typically show a 40-60% reduction in lithium loading capacity when magnesium concentrations exceed 0.5 g/L, which is common in many natural brine sources.
Scalability remains problematic, with laboratory-proven technologies frequently failing to maintain separation efficiency at industrial scales. The gap between bench-scale performance (processing liters) and commercial requirements (processing thousands of cubic meters daily) has resulted in numerous promising technologies failing during scale-up phases.
Environmental considerations further complicate technology selection, as many effective separation agents contain toxic components or generate hazardous waste streams. Regulatory compliance increasingly demands more environmentally benign processes, limiting the application of certain chemical approaches that might otherwise offer superior separation performance.
The economic threshold for viable Mg-Li separation technologies continues to shift as lithium market prices fluctuate, creating uncertainty for technology developers and mining companies. Current separation costs typically represent 30-45% of total DLE operational expenses, making this step a critical focus for cost reduction efforts.
Current Mg-Li Separation Techniques and Processes
01 Electrochemical separation methods for Mg-Li alloys
Electrochemical processes can be used to separate magnesium and lithium from alloys to achieve high purity products. These methods typically involve electrolysis in molten salt systems where controlled potential differences allow selective deposition of either magnesium or lithium. The processes often utilize specialized electrolytes and electrode materials to enhance separation efficiency and product purity, with some approaches achieving purity levels exceeding 99.9%.- Electrochemical separation methods for Mg-Li alloys: Electrochemical processes can be used to separate magnesium and lithium from alloys to achieve high purity products. These methods typically involve electrolysis in molten salt systems where controlled potential differences allow for selective deposition of either magnesium or lithium. The processes often utilize specialized electrolytes and electrode materials to enhance separation efficiency and product purity. Electrochemical separation is advantageous for producing high-purity metals while minimizing environmental impact compared to traditional chemical methods.
- Chemical extraction and precipitation techniques: Chemical methods for separating magnesium and lithium involve selective extraction and precipitation processes. These techniques typically use specific reagents that react preferentially with either magnesium or lithium to form compounds that can be easily separated. Sequential precipitation steps with controlled pH and temperature conditions allow for high purity separation. Solvent extraction methods may also be employed where organic solvents selectively bind to one metal over the other, enabling effective separation of the two elements.
- Vacuum distillation and thermal separation processes: Vacuum distillation and thermal separation techniques exploit the different vapor pressures and melting points of magnesium and lithium to achieve separation. These processes typically involve heating Mg-Li alloys under controlled vacuum conditions, allowing the more volatile component to evaporate first. By carefully controlling temperature and pressure parameters, high purity metals can be obtained. Multiple distillation stages may be employed to progressively increase the purity of the separated metals, making this method particularly effective for producing ultra-high purity materials.
- Ion exchange and membrane separation technologies: Ion exchange resins and specialized membrane technologies can be used to separate magnesium and lithium ions from solutions. These methods utilize selective ion exchange materials that have different affinities for magnesium versus lithium ions. Membrane separation processes, including nanofiltration and electrodialysis, can effectively separate the two elements based on differences in ionic size and charge. These technologies are particularly useful for processing brine solutions and can achieve high separation efficiency while maintaining high purity of the final products.
- Combined and integrated purification systems: Integrated purification systems combine multiple separation techniques to achieve ultra-high purity magnesium and lithium. These systems typically involve sequential processing steps such as initial chemical separation followed by electrorefining or vacuum distillation. Advanced process control and monitoring ensure optimal separation conditions throughout the purification process. Some integrated systems also incorporate recycling loops to reprocess intermediate products, maximizing yield and purity. These comprehensive approaches are particularly effective for industrial-scale production of high-purity magnesium and lithium for specialized applications.
02 Vacuum distillation techniques for high-purity separation
Vacuum distillation leverages the different vapor pressures of magnesium and lithium to achieve separation. By controlling temperature and pressure in vacuum conditions, one metal can be selectively vaporized while the other remains in solid or liquid form. This technique is particularly effective for producing high-purity magnesium from Mg-Li alloys, as magnesium typically has a higher vapor pressure than lithium at processing temperatures. Multiple distillation stages can be implemented to progressively increase the purity of the separated metals.Expand Specific Solutions03 Chemical extraction and precipitation methods
Chemical processes for Mg-Li separation involve selective dissolution, extraction, and precipitation techniques. These methods typically use specific reagents that preferentially react with either magnesium or lithium, allowing for their separation. Common approaches include acid leaching followed by selective precipitation using pH control, solvent extraction with specialized extractants, and ion exchange processes. These chemical methods can be optimized to achieve high purity levels while minimizing reagent consumption and environmental impact.Expand Specific Solutions04 Combined physical-chemical separation processes
Hybrid separation approaches combine multiple physical and chemical techniques to achieve high-purity Mg-Li separation. These integrated processes often involve sequential steps such as preliminary mechanical separation, chemical leaching, electrochemical refining, and final purification through crystallization or zone refining. The combination of different separation mechanisms allows for more efficient removal of impurities and higher final product purity than single-method approaches. These integrated systems can be optimized for specific feed materials and desired purity specifications.Expand Specific Solutions05 Advanced purification techniques for ultra-high purity products
For applications requiring ultra-high purity magnesium or lithium, specialized purification techniques are employed after initial separation. These include zone refining, sublimation, advanced filtration systems, and getter processes that can remove trace impurities down to parts-per-billion levels. Some methods utilize specialized atmospheres or vacuum conditions to prevent contamination during processing. These techniques are particularly important for electronic, battery, and aerospace applications where metal purity directly impacts performance and reliability.Expand Specific Solutions
Leading Companies in Lithium Extraction Industry
The direct lithium extraction (DLE) market is currently in an early growth phase, characterized by rapid technological innovation and increasing commercial interest. The global market for DLE technologies is projected to expand significantly as demand for high-purity lithium continues to surge for battery applications. The Mg-Li separation challenge represents a critical technical hurdle in achieving economically viable DLE processes. Academic institutions like Central South University and Tianjin University are advancing fundamental research, while commercial players demonstrate varying levels of technological maturity. Companies such as Energy Exploration Technologies, Pure Lithium, and Albemarle are developing proprietary solutions, with established industrial players like POSCO Holdings and Eramet investing heavily in pilot projects. The competitive landscape shows a mix of specialized startups, established mining companies, and research institutions collaborating to overcome selectivity challenges in complex brine environments.
Central South University
Technical Solution: Central South University has developed a comprehensive approach to Mg-Li separation in direct lithium extraction processes. Their technology combines selective adsorption with advanced electrochemical methods to achieve high-purity lithium products. The university's research team has engineered novel lithium-selective adsorbent materials based on manganese oxide structures with modified surface chemistry that enhances lithium selectivity while minimizing magnesium uptake. Their process incorporates a multi-stage treatment system where initial selective adsorption is followed by electrochemical separation techniques that exploit the different electrochemical potentials of lithium and magnesium ions. The university has also developed innovative elution strategies using carefully controlled pH gradients that maximize lithium recovery while maintaining magnesium rejection. Their approach includes a final purification stage utilizing membrane separation technology to achieve ultra-high purity lithium products. Central South University's research has demonstrated effective separation in brines with Mg:Li ratios exceeding 70:1, achieving lithium purities above 99.9% with minimal magnesium contamination.
Strengths: Highly effective for challenging high Mg:Li ratio brines; achieves exceptional final product purity; combines multiple separation mechanisms for enhanced performance; environmentally sustainable approach with reduced chemical consumption. Weaknesses: Complex multi-stage process may increase operational complexity; higher energy requirements for electrochemical separation stages; requires specialized expertise for operation and maintenance.
Koch Technology Solutions LLC
Technical Solution: Koch Technology Solutions has developed an integrated membrane-based approach for Mg-Li separation in direct lithium extraction processes. Their technology combines selective nanofiltration membranes with proprietary pre-treatment systems to address the challenging separation of lithium from magnesium in brine resources. The process begins with a specialized pre-treatment stage that modifies the ionic species' hydration shells, enhancing the effective size difference between lithium and magnesium ions. This is followed by a multi-stage nanofiltration system utilizing Koch's proprietary membrane technology that exploits differences in ion size, charge density, and hydration energy to achieve separation. Their system incorporates pH and temperature optimization to maximize separation efficiency, as these parameters significantly affect ion hydration and membrane selectivity. Koch's approach also includes an innovative concentrate management system that reduces waste and improves overall lithium recovery rates. The technology can process diverse brine compositions and has demonstrated effective separation even in brines with high Mg:Li ratios exceeding 50:1.
Strengths: Modular and scalable design; lower energy consumption than thermal processes; minimal chemical requirements; continuous operation capability; applicable to diverse brine chemistries. Weaknesses: Membrane fouling can occur with certain brine compositions; requires careful pre-treatment; performance may degrade at extremely high Mg:Li ratios.
Key Patents and Innovations in Selective Ion Extraction
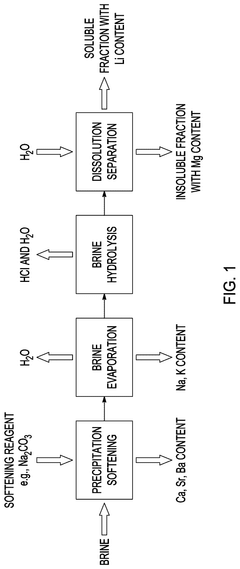
Method of separating mixed magnesium and lithium salts
PatentPendingUS20250066206A1
Innovation
- A method involving the hydrolysis of a hydrolysis composition containing MgCl2 and LiCl, treated with heat and water to form HCl, water, and a hydrolyzed mixture including LiCl and MgO, followed by dissolution separation to effectively separate LiCl from Mg-containing products.
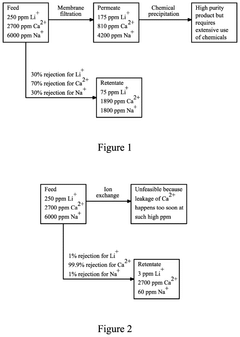
Apparatus and process for monovalent ion extraction
PatentPendingUS20250041806A1
Innovation
- The use of a membrane-based separation process, including a nanofiltration membrane with a coating, to reduce the ratio of divalent ions to a target monovalent ion in an aqueous solution, improving the efficiency and reducing the number of stages required in the extraction process.
Environmental Impact Assessment of Extraction Methods
The environmental impact of direct lithium extraction (DLE) methods, particularly those focused on Mg-Li separation, requires comprehensive assessment to ensure sustainable resource development. Traditional lithium extraction methods, including evaporation ponds and hard rock mining, have significant environmental footprints through land use, water consumption, and chemical pollution. DLE technologies promise reduced environmental impacts, but their specific Mg-Li separation strategies still present various ecological considerations.
Water usage represents a critical environmental factor in DLE operations. Ion exchange and adsorption-based separation methods typically require substantial water volumes for regeneration cycles, though significantly less than evaporation ponds. Advanced membrane technologies and electrochemical systems have demonstrated up to 70% reduction in water consumption compared to conventional methods, presenting a promising direction for water conservation in arid lithium-rich regions.
Chemical consumption patterns differ markedly across separation technologies. Solvent extraction methods often employ organic compounds that pose contamination risks if improperly managed. Conversely, selective adsorption materials and ion-sieves generally require fewer hazardous chemicals but may necessitate acidic regeneration solutions. Recent innovations in green chemistry approaches have yielded biodegradable complexing agents that reduce environmental persistence of processing chemicals while maintaining effective Mg-Li selectivity.
Energy requirements constitute another significant environmental dimension. Electrochemical separation techniques typically demand higher energy inputs, potentially increasing carbon footprints unless powered by renewable sources. Passive adsorption systems generally require less operational energy but may involve energy-intensive material production. Life cycle assessments indicate that despite higher initial energy investments, advanced separation technologies can achieve 30-45% lower lifetime carbon emissions compared to traditional methods.
Waste management challenges vary considerably across separation technologies. Precipitation-based methods generate solid waste requiring disposal, while membrane systems produce concentrated brine streams that must be managed to prevent salinization of surrounding ecosystems. Closed-loop systems that recycle process solutions show promise in minimizing discharge volumes, though they typically increase operational complexity and energy demands.
Land disturbance metrics favor most DLE technologies, which typically require 50-80% less surface area than evaporation ponds. However, infrastructure requirements for processing facilities and waste management can still present significant ecological disruption in sensitive environments. Site restoration protocols and minimally invasive installation techniques have demonstrated effectiveness in reducing long-term habitat impacts in pilot projects across various geographical contexts.
Water usage represents a critical environmental factor in DLE operations. Ion exchange and adsorption-based separation methods typically require substantial water volumes for regeneration cycles, though significantly less than evaporation ponds. Advanced membrane technologies and electrochemical systems have demonstrated up to 70% reduction in water consumption compared to conventional methods, presenting a promising direction for water conservation in arid lithium-rich regions.
Chemical consumption patterns differ markedly across separation technologies. Solvent extraction methods often employ organic compounds that pose contamination risks if improperly managed. Conversely, selective adsorption materials and ion-sieves generally require fewer hazardous chemicals but may necessitate acidic regeneration solutions. Recent innovations in green chemistry approaches have yielded biodegradable complexing agents that reduce environmental persistence of processing chemicals while maintaining effective Mg-Li selectivity.
Energy requirements constitute another significant environmental dimension. Electrochemical separation techniques typically demand higher energy inputs, potentially increasing carbon footprints unless powered by renewable sources. Passive adsorption systems generally require less operational energy but may involve energy-intensive material production. Life cycle assessments indicate that despite higher initial energy investments, advanced separation technologies can achieve 30-45% lower lifetime carbon emissions compared to traditional methods.
Waste management challenges vary considerably across separation technologies. Precipitation-based methods generate solid waste requiring disposal, while membrane systems produce concentrated brine streams that must be managed to prevent salinization of surrounding ecosystems. Closed-loop systems that recycle process solutions show promise in minimizing discharge volumes, though they typically increase operational complexity and energy demands.
Land disturbance metrics favor most DLE technologies, which typically require 50-80% less surface area than evaporation ponds. However, infrastructure requirements for processing facilities and waste management can still present significant ecological disruption in sensitive environments. Site restoration protocols and minimally invasive installation techniques have demonstrated effectiveness in reducing long-term habitat impacts in pilot projects across various geographical contexts.
Economic Feasibility of Advanced Separation Technologies
The economic feasibility of advanced separation technologies for Mg-Li separation in direct lithium extraction (DLE) processes represents a critical factor in determining their commercial viability. Current cost analyses indicate that traditional separation methods like precipitation and solvent extraction require significant capital investment ranging from $15-25 million for medium-scale operations, with operational costs between $3,500-5,000 per ton of lithium produced.
Advanced technologies such as selective ion exchange resins and electrochemical separation systems demonstrate promising economic profiles, potentially reducing operational costs by 30-40% compared to conventional methods. These technologies benefit from lower energy consumption, reduced chemical reagent usage, and higher recovery rates, which collectively improve the economic equation.
Membrane-based separation technologies, while showing excellent selectivity for lithium over magnesium, currently face economic challenges due to high membrane replacement costs and energy requirements for pressure-driven processes. However, ongoing developments in membrane materials science are expected to reduce these costs by approximately 25% within the next five years.
The economic assessment must consider the full lifecycle costs, including capital expenditure, operational expenses, maintenance requirements, and environmental compliance costs. Notably, advanced separation technologies often command higher initial investments but deliver superior long-term economic performance through improved efficiency and reduced waste management costs.
Market analysis reveals that the premium pricing for high-purity lithium products (>99.9%) can offset the additional costs of advanced separation technologies. With battery-grade lithium compounds commanding 15-20% price premiums, the economic case for investing in superior Mg-Li separation becomes more compelling, especially for operations targeting high-end markets.
Scale economies significantly impact feasibility, with larger operations (>10,000 tons per annum) achieving substantially lower unit costs. Modular designs for advanced separation technologies are emerging as a strategy to address this challenge, allowing smaller operations to expand capacity incrementally while maintaining competitive cost structures.
Regional factors also influence economic feasibility, with energy costs, regulatory requirements, and access to technical expertise varying significantly across global lithium production hubs. Operations in regions with lower energy costs and supportive regulatory frameworks demonstrate 15-25% better economic performance for the same technological implementation.
Advanced technologies such as selective ion exchange resins and electrochemical separation systems demonstrate promising economic profiles, potentially reducing operational costs by 30-40% compared to conventional methods. These technologies benefit from lower energy consumption, reduced chemical reagent usage, and higher recovery rates, which collectively improve the economic equation.
Membrane-based separation technologies, while showing excellent selectivity for lithium over magnesium, currently face economic challenges due to high membrane replacement costs and energy requirements for pressure-driven processes. However, ongoing developments in membrane materials science are expected to reduce these costs by approximately 25% within the next five years.
The economic assessment must consider the full lifecycle costs, including capital expenditure, operational expenses, maintenance requirements, and environmental compliance costs. Notably, advanced separation technologies often command higher initial investments but deliver superior long-term economic performance through improved efficiency and reduced waste management costs.
Market analysis reveals that the premium pricing for high-purity lithium products (>99.9%) can offset the additional costs of advanced separation technologies. With battery-grade lithium compounds commanding 15-20% price premiums, the economic case for investing in superior Mg-Li separation becomes more compelling, especially for operations targeting high-end markets.
Scale economies significantly impact feasibility, with larger operations (>10,000 tons per annum) achieving substantially lower unit costs. Modular designs for advanced separation technologies are emerging as a strategy to address this challenge, allowing smaller operations to expand capacity incrementally while maintaining competitive cost structures.
Regional factors also influence economic feasibility, with energy costs, regulatory requirements, and access to technical expertise varying significantly across global lithium production hubs. Operations in regions with lower energy costs and supportive regulatory frameworks demonstrate 15-25% better economic performance for the same technological implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!