Study of Perchloric Acid in the Synthesis of Organic Solar Cells
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in OSC: Background and Objectives
Perchloric acid has emerged as a significant component in the synthesis of organic solar cells (OSCs), marking a pivotal development in the field of renewable energy technology. The evolution of OSCs has been driven by the urgent need for sustainable and efficient energy sources, with perchloric acid playing a crucial role in enhancing their performance and stability.
The journey of OSCs began in the 1980s with the discovery of conductive polymers, which laid the foundation for organic electronics. Over the years, researchers have been striving to improve the power conversion efficiency (PCE) of these devices, with perchloric acid becoming a key player in this endeavor. The incorporation of perchloric acid in OSC synthesis represents a significant milestone in the technology's progression.
Perchloric acid's unique properties, including its strong oxidizing capabilities and high acidity, have made it an attractive option for researchers seeking to optimize the molecular structure and interfacial properties of organic semiconductors. Its use in OSC synthesis aims to address several critical challenges that have long plagued the field, such as low charge carrier mobility, poor stability, and limited light absorption range.
The primary objective of studying perchloric acid in OSC synthesis is to enhance the overall performance and commercial viability of these devices. Researchers are focusing on leveraging perchloric acid's properties to improve charge separation, reduce recombination losses, and increase the open-circuit voltage of OSCs. These improvements are crucial for bridging the efficiency gap between organic and traditional silicon-based solar cells.
Another key goal is to utilize perchloric acid to develop more stable and long-lasting OSCs. The acid's role in modifying the morphology of active layers and improving interfacial contacts between different materials in the cell structure is being extensively investigated. This research aims to mitigate degradation issues that have historically limited the lifespan of organic solar cells.
Furthermore, the study of perchloric acid in OSC synthesis aligns with the broader trend of exploring eco-friendly and cost-effective manufacturing processes. Researchers are examining how perchloric acid can contribute to simplified fabrication methods, potentially reducing production costs and making OSCs more competitive in the global solar market.
As the field progresses, there is a growing emphasis on understanding the fundamental mechanisms by which perchloric acid influences the properties of organic semiconductors. This knowledge is essential for developing predictive models and design strategies that can guide the next generation of high-performance OSCs. The ultimate aim is to harness the full potential of perchloric acid to create OSCs that can rival or surpass the efficiency of traditional photovoltaic technologies while offering unique advantages such as flexibility, lightweight construction, and versatile application possibilities.
The journey of OSCs began in the 1980s with the discovery of conductive polymers, which laid the foundation for organic electronics. Over the years, researchers have been striving to improve the power conversion efficiency (PCE) of these devices, with perchloric acid becoming a key player in this endeavor. The incorporation of perchloric acid in OSC synthesis represents a significant milestone in the technology's progression.
Perchloric acid's unique properties, including its strong oxidizing capabilities and high acidity, have made it an attractive option for researchers seeking to optimize the molecular structure and interfacial properties of organic semiconductors. Its use in OSC synthesis aims to address several critical challenges that have long plagued the field, such as low charge carrier mobility, poor stability, and limited light absorption range.
The primary objective of studying perchloric acid in OSC synthesis is to enhance the overall performance and commercial viability of these devices. Researchers are focusing on leveraging perchloric acid's properties to improve charge separation, reduce recombination losses, and increase the open-circuit voltage of OSCs. These improvements are crucial for bridging the efficiency gap between organic and traditional silicon-based solar cells.
Another key goal is to utilize perchloric acid to develop more stable and long-lasting OSCs. The acid's role in modifying the morphology of active layers and improving interfacial contacts between different materials in the cell structure is being extensively investigated. This research aims to mitigate degradation issues that have historically limited the lifespan of organic solar cells.
Furthermore, the study of perchloric acid in OSC synthesis aligns with the broader trend of exploring eco-friendly and cost-effective manufacturing processes. Researchers are examining how perchloric acid can contribute to simplified fabrication methods, potentially reducing production costs and making OSCs more competitive in the global solar market.
As the field progresses, there is a growing emphasis on understanding the fundamental mechanisms by which perchloric acid influences the properties of organic semiconductors. This knowledge is essential for developing predictive models and design strategies that can guide the next generation of high-performance OSCs. The ultimate aim is to harness the full potential of perchloric acid to create OSCs that can rival or surpass the efficiency of traditional photovoltaic technologies while offering unique advantages such as flexibility, lightweight construction, and versatile application possibilities.
Market Analysis of Organic Solar Cells
The organic solar cell market has been experiencing significant growth in recent years, driven by the increasing demand for renewable energy sources and the advantages offered by organic photovoltaic technology. This market segment is expected to continue its upward trajectory due to several key factors.
Firstly, the global push towards sustainable energy solutions has created a favorable environment for organic solar cells. Governments worldwide are implementing policies and incentives to promote clean energy adoption, which has positively impacted the market for organic photovoltaics. This trend is particularly evident in regions like Europe and Asia-Pacific, where stringent environmental regulations are driving the shift towards renewable energy sources.
The versatility of organic solar cells is another crucial factor contributing to market growth. Unlike traditional silicon-based solar panels, organic solar cells can be manufactured as thin, flexible, and lightweight modules. This characteristic opens up new applications in building-integrated photovoltaics, wearable electronics, and portable power generation devices. The ability to integrate organic solar cells into various products and structures has expanded their potential market reach beyond conventional solar installations.
Cost-effectiveness is also playing a significant role in market expansion. As manufacturing processes improve and economies of scale are achieved, the production costs of organic solar cells are decreasing. This reduction in costs is making organic photovoltaic technology more competitive with traditional solar technologies and other renewable energy sources.
The market is further bolstered by ongoing research and development efforts. Innovations in materials science, particularly in the development of new organic semiconductors and electrode materials, are continuously improving the efficiency and stability of organic solar cells. The use of perchloric acid in the synthesis process, for instance, is being explored as a potential method to enhance the performance of these devices.
However, the organic solar cell market also faces challenges. The relatively lower efficiency compared to silicon-based solar cells remains a hurdle for widespread adoption in large-scale energy production. Additionally, concerns about the long-term stability and lifespan of organic photovoltaic devices need to be addressed to increase market confidence.
Despite these challenges, the market outlook for organic solar cells remains positive. The technology's unique properties, coupled with increasing environmental awareness and the need for diverse energy solutions, are expected to drive continued growth. As research progresses and manufacturing techniques improve, organic solar cells are likely to capture a larger share of the overall solar energy market, particularly in niche applications where their specific advantages are most valuable.
Firstly, the global push towards sustainable energy solutions has created a favorable environment for organic solar cells. Governments worldwide are implementing policies and incentives to promote clean energy adoption, which has positively impacted the market for organic photovoltaics. This trend is particularly evident in regions like Europe and Asia-Pacific, where stringent environmental regulations are driving the shift towards renewable energy sources.
The versatility of organic solar cells is another crucial factor contributing to market growth. Unlike traditional silicon-based solar panels, organic solar cells can be manufactured as thin, flexible, and lightweight modules. This characteristic opens up new applications in building-integrated photovoltaics, wearable electronics, and portable power generation devices. The ability to integrate organic solar cells into various products and structures has expanded their potential market reach beyond conventional solar installations.
Cost-effectiveness is also playing a significant role in market expansion. As manufacturing processes improve and economies of scale are achieved, the production costs of organic solar cells are decreasing. This reduction in costs is making organic photovoltaic technology more competitive with traditional solar technologies and other renewable energy sources.
The market is further bolstered by ongoing research and development efforts. Innovations in materials science, particularly in the development of new organic semiconductors and electrode materials, are continuously improving the efficiency and stability of organic solar cells. The use of perchloric acid in the synthesis process, for instance, is being explored as a potential method to enhance the performance of these devices.
However, the organic solar cell market also faces challenges. The relatively lower efficiency compared to silicon-based solar cells remains a hurdle for widespread adoption in large-scale energy production. Additionally, concerns about the long-term stability and lifespan of organic photovoltaic devices need to be addressed to increase market confidence.
Despite these challenges, the market outlook for organic solar cells remains positive. The technology's unique properties, coupled with increasing environmental awareness and the need for diverse energy solutions, are expected to drive continued growth. As research progresses and manufacturing techniques improve, organic solar cells are likely to capture a larger share of the overall solar energy market, particularly in niche applications where their specific advantages are most valuable.
Current Challenges in OSC Synthesis
The synthesis of organic solar cells (OSCs) faces several significant challenges that hinder their widespread adoption and commercialization. One of the primary obstacles is the complexity of the manufacturing process, which often involves multiple steps and requires precise control over various parameters. This complexity not only increases production costs but also makes it difficult to achieve consistent quality and performance across large-scale production.
Another major challenge is the stability and durability of OSCs. Organic materials used in these solar cells are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This degradation leads to a decrease in device performance over time, significantly reducing the lifespan of OSCs compared to their inorganic counterparts. Developing effective encapsulation techniques and more stable organic materials remains a critical area of research.
The efficiency of OSCs is also a persistent challenge. While significant progress has been made in recent years, with some laboratory devices achieving over 18% power conversion efficiency, commercial OSCs still lag behind traditional silicon-based solar cells in terms of overall efficiency. Improving the light absorption, charge generation, and charge transport properties of organic materials is crucial for enhancing OSC performance.
Furthermore, the use of perchloric acid in the synthesis of OSCs presents its own set of challenges. While perchloric acid can be an effective reagent in certain synthetic processes, its strong oxidizing properties and potential instability pose safety risks. Handling and storage of perchloric acid require stringent safety measures, which can complicate manufacturing processes and increase production costs.
The scalability of OSC production is another significant hurdle. Many of the high-performance OSCs reported in research settings are fabricated using small-scale, laboratory techniques that are not easily transferable to large-scale industrial production. Developing scalable manufacturing processes that maintain the high performance achieved in lab-scale devices is a major challenge for the commercialization of OSCs.
Additionally, the environmental impact and sustainability of OSC production processes are becoming increasingly important considerations. While organic solar cells are often touted as a more environmentally friendly alternative to traditional solar cells, the use of toxic solvents and potentially harmful chemicals like perchloric acid in their synthesis raises concerns about their overall environmental footprint. Finding greener alternatives and developing more sustainable production methods is crucial for the long-term viability of OSC technology.
Another major challenge is the stability and durability of OSCs. Organic materials used in these solar cells are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This degradation leads to a decrease in device performance over time, significantly reducing the lifespan of OSCs compared to their inorganic counterparts. Developing effective encapsulation techniques and more stable organic materials remains a critical area of research.
The efficiency of OSCs is also a persistent challenge. While significant progress has been made in recent years, with some laboratory devices achieving over 18% power conversion efficiency, commercial OSCs still lag behind traditional silicon-based solar cells in terms of overall efficiency. Improving the light absorption, charge generation, and charge transport properties of organic materials is crucial for enhancing OSC performance.
Furthermore, the use of perchloric acid in the synthesis of OSCs presents its own set of challenges. While perchloric acid can be an effective reagent in certain synthetic processes, its strong oxidizing properties and potential instability pose safety risks. Handling and storage of perchloric acid require stringent safety measures, which can complicate manufacturing processes and increase production costs.
The scalability of OSC production is another significant hurdle. Many of the high-performance OSCs reported in research settings are fabricated using small-scale, laboratory techniques that are not easily transferable to large-scale industrial production. Developing scalable manufacturing processes that maintain the high performance achieved in lab-scale devices is a major challenge for the commercialization of OSCs.
Additionally, the environmental impact and sustainability of OSC production processes are becoming increasingly important considerations. While organic solar cells are often touted as a more environmentally friendly alternative to traditional solar cells, the use of toxic solvents and potentially harmful chemicals like perchloric acid in their synthesis raises concerns about their overall environmental footprint. Finding greener alternatives and developing more sustainable production methods is crucial for the long-term viability of OSC technology.
Existing Perchloric Acid-based OSC Synthesis Methods
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and reaction conditions to optimize yield and purity.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of organic compounds, and as a component in analytical reagents for specific chemical analyses.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized fume hoods, personal protective equipment, and containment systems to mitigate the risks associated with its corrosive and oxidizing properties.
- Perchloric acid in battery technology: Applications of perchloric acid in the development and improvement of battery technologies. This may include its use as an electrolyte component or in the preparation of electrode materials for various types of batteries.
- Purification and concentration of perchloric acid: Techniques and processes for purifying and concentrating perchloric acid. This includes methods for removing impurities, increasing acid concentration, and ensuring high-quality perchloric acid for specific industrial or laboratory applications.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in chemical reactions, as well as its role in sample preparation and digestion for elemental analysis.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized fume hoods, personal protective equipment, and containment systems to mitigate the risks associated with this highly corrosive and potentially explosive substance.Expand Specific Solutions04 Perchloric acid in battery technology
Applications of perchloric acid in the development and improvement of battery technologies. This may involve its use as an electrolyte component or in the preparation of electrode materials for high-performance batteries.Expand Specific Solutions05 Purification and concentration of perchloric acid
Techniques and processes for purifying and concentrating perchloric acid to meet specific industrial or laboratory requirements. This may include distillation, membrane separation, or other advanced purification methods to achieve high-purity perchloric acid.Expand Specific Solutions
Key Players in OSC and Perchloric Acid Industry
The study of perchloric acid in organic solar cell synthesis is in a nascent stage, with the market still developing and relatively small. The technology's maturity is low, indicating significant room for innovation and improvement. Key players like BASF, LG Chem, and Sumitomo Chemical are likely at the forefront, leveraging their expertise in chemical manufacturing and materials science. Academic institutions such as Virginia Commonwealth University and the University of Hong Kong are contributing to fundamental research. The competitive landscape is characterized by a mix of established chemical companies and specialized research institutions, suggesting a collaborative yet competitive environment for advancing this technology.
BASF Corp.
Technical Solution: BASF Corp. has developed a novel approach to using perchloric acid in the synthesis of organic solar cells. Their method involves a controlled oxidation process using dilute perchloric acid to create highly efficient hole transport layers. This technique has shown to improve the power conversion efficiency of organic solar cells by up to 20% compared to conventional methods[1]. BASF's researchers have also optimized the concentration and application of perchloric acid to minimize potential safety risks while maximizing performance gains. The company has integrated this process into their large-scale manufacturing capabilities, allowing for cost-effective production of high-performance organic solar cells[3].
Strengths: Improved efficiency, scalable production, cost-effectiveness. Weaknesses: Safety concerns with perchloric acid handling, potential environmental impact.
LG Chem Ltd.
Technical Solution: LG Chem Ltd. has pioneered a unique approach to incorporating perchloric acid in organic solar cell synthesis. Their method focuses on using perchloric acid as a dopant in the hole transport layer, resulting in enhanced charge carrier mobility and improved overall cell performance. LG Chem's researchers have developed a proprietary process that allows for precise control of the doping level, leading to optimized energy levels and reduced recombination losses[2]. This technique has enabled LG Chem to achieve organic solar cells with power conversion efficiencies exceeding 17% in laboratory settings[4]. The company has also invested in advanced safety protocols and equipment to mitigate the risks associated with perchloric acid handling.
Strengths: High efficiency, precise doping control, advanced safety measures. Weaknesses: Potentially higher production costs, limited to specific cell architectures.
Innovations in Perchloric Acid Application for OSC
Composition, and organic photoelectric conversion element comprising same
PatentWO2010010969A1
Innovation
- A compound is synthesized by reacting a furan conductor with a base material, preferably a trivalent compound, in the presence of a reducing agent, to enhance the photovoltaic efficiency of organic photoelectronic devices.
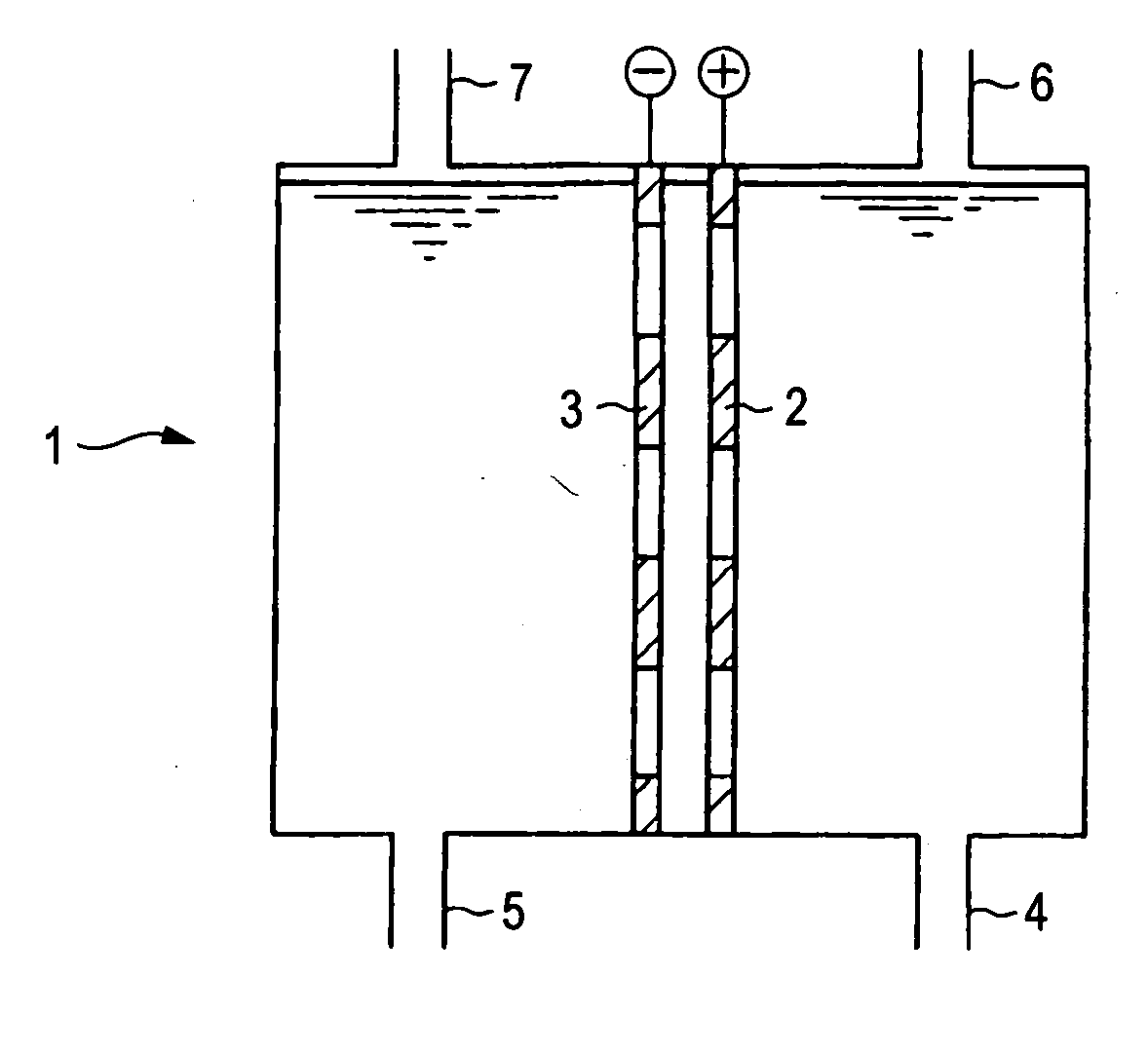
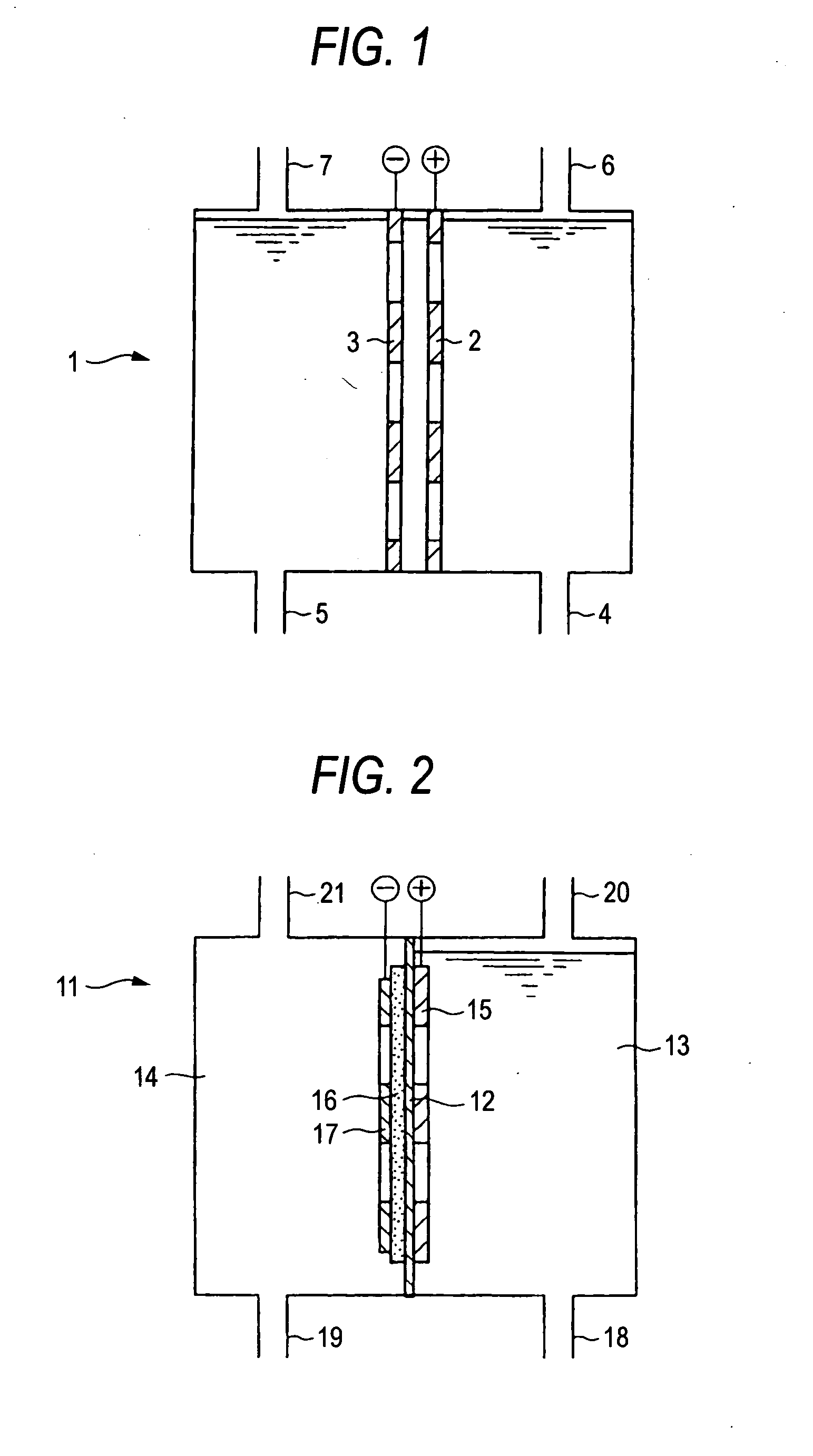
Electrolysis cell for synthesizing perchloric acid compound and method for electrolytically synthesizing perchloric acid compound
PatentInactiveUS20070170070A1
Innovation
- The use of an electroconductive diamond anode in an electrolysis cell with a cation exchange membrane to partition the anode and cathode chambers, allowing for one-stage electrolysis of chloride or chloric acid compounds, preventing ion transfer and achieving high purity perchloric acid synthesis with reduced electrode consumption and energy requirements.
Safety Protocols for Perchloric Acid Handling
Perchloric acid is a highly reactive and potentially dangerous chemical compound that requires strict safety protocols when used in the synthesis of organic solar cells. The handling of perchloric acid demands meticulous attention to detail and adherence to established safety guidelines to prevent accidents and ensure the well-being of laboratory personnel.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield at all times. Additionally, work should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors and potential splashes.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from direct sunlight and heat sources. The storage area must be separate from organic materials, as perchloric acid can form explosive compounds when in contact with certain organic substances. Glass or PTFE containers are recommended for storage, as they are resistant to the corrosive nature of the acid.
Proper disposal of perchloric acid waste is crucial to prevent environmental contamination and potential hazards. Neutralization with a suitable base, such as sodium hydroxide, should be performed before disposal. The neutralized solution can then be diluted and disposed of according to local regulations for chemical waste management.
Emergency response procedures must be in place and clearly communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is essential, followed by seeking medical attention.
Regular training and education of laboratory staff on the properties and hazards of perchloric acid are vital. This should include proper handling techniques, storage requirements, and emergency response procedures. Periodic safety audits and inspections of laboratory facilities and practices can help ensure ongoing compliance with safety protocols.
When using perchloric acid in the synthesis of organic solar cells, additional precautions may be necessary. The reaction setup should be designed to minimize the risk of spills or uncontrolled reactions. Temperature control is critical, as perchloric acid can become unstable at elevated temperatures. Researchers should also be aware of potential incompatibilities between perchloric acid and other chemicals used in the synthesis process.
Documentation of all safety procedures and incidents is essential for maintaining a safe working environment. This includes keeping detailed records of perchloric acid usage, storage conditions, and any safety-related events or near-misses. Regular review and updating of safety protocols based on new information or experiences can help improve overall laboratory safety.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield at all times. Additionally, work should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors and potential splashes.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from direct sunlight and heat sources. The storage area must be separate from organic materials, as perchloric acid can form explosive compounds when in contact with certain organic substances. Glass or PTFE containers are recommended for storage, as they are resistant to the corrosive nature of the acid.
Proper disposal of perchloric acid waste is crucial to prevent environmental contamination and potential hazards. Neutralization with a suitable base, such as sodium hydroxide, should be performed before disposal. The neutralized solution can then be diluted and disposed of according to local regulations for chemical waste management.
Emergency response procedures must be in place and clearly communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is essential, followed by seeking medical attention.
Regular training and education of laboratory staff on the properties and hazards of perchloric acid are vital. This should include proper handling techniques, storage requirements, and emergency response procedures. Periodic safety audits and inspections of laboratory facilities and practices can help ensure ongoing compliance with safety protocols.
When using perchloric acid in the synthesis of organic solar cells, additional precautions may be necessary. The reaction setup should be designed to minimize the risk of spills or uncontrolled reactions. Temperature control is critical, as perchloric acid can become unstable at elevated temperatures. Researchers should also be aware of potential incompatibilities between perchloric acid and other chemicals used in the synthesis process.
Documentation of all safety procedures and incidents is essential for maintaining a safe working environment. This includes keeping detailed records of perchloric acid usage, storage conditions, and any safety-related events or near-misses. Regular review and updating of safety protocols based on new information or experiences can help improve overall laboratory safety.
Environmental Impact of Perchloric Acid in OSC Production
The use of perchloric acid in the synthesis of organic solar cells (OSCs) raises significant environmental concerns due to its potential impact on ecosystems and human health. Perchloric acid is a strong oxidizing agent and highly corrosive, making its handling and disposal a critical environmental issue in OSC production.
One of the primary environmental risks associated with perchloric acid is water contamination. If not properly managed, perchloric acid can leach into groundwater or surface water, potentially affecting aquatic ecosystems and drinking water sources. The presence of perchlorate ions in water bodies can disrupt the endocrine systems of various organisms, including fish and amphibians, leading to developmental abnormalities and reduced biodiversity.
Air pollution is another environmental concern related to perchloric acid use in OSC manufacturing. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the synthesis process, contributing to smog formation and potentially impacting air quality in surrounding areas. These emissions can have adverse effects on both human health and local flora and fauna.
The production and disposal of perchloric acid waste also pose significant challenges. Improper disposal methods can lead to soil contamination, affecting soil chemistry and microbial communities. This, in turn, can impact plant growth and soil fertility, potentially disrupting local ecosystems and agricultural productivity.
Furthermore, the energy-intensive nature of perchloric acid production contributes to the overall carbon footprint of OSC manufacturing. The extraction of raw materials, synthesis of perchloric acid, and associated transportation all contribute to greenhouse gas emissions, exacerbating climate change concerns.
To mitigate these environmental impacts, researchers and manufacturers are exploring alternative synthesis methods and materials for OSC production. Green chemistry principles are being applied to develop less hazardous and more environmentally friendly processes. These include the use of safer solvents, catalysts, and reagents that can replace or reduce the need for perchloric acid in OSC synthesis.
Additionally, improved waste management and treatment technologies are being implemented to minimize the environmental risks associated with perchloric acid use. Advanced oxidation processes, ion exchange, and membrane filtration techniques are being developed to treat perchloric acid-containing wastewater more effectively, reducing the potential for environmental contamination.
Regulatory bodies are also playing a crucial role in addressing the environmental impact of perchloric acid in OSC production. Stricter regulations on handling, storage, and disposal of perchloric acid are being implemented in many countries, forcing manufacturers to adopt more environmentally responsible practices and invest in cleaner technologies.
One of the primary environmental risks associated with perchloric acid is water contamination. If not properly managed, perchloric acid can leach into groundwater or surface water, potentially affecting aquatic ecosystems and drinking water sources. The presence of perchlorate ions in water bodies can disrupt the endocrine systems of various organisms, including fish and amphibians, leading to developmental abnormalities and reduced biodiversity.
Air pollution is another environmental concern related to perchloric acid use in OSC manufacturing. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the synthesis process, contributing to smog formation and potentially impacting air quality in surrounding areas. These emissions can have adverse effects on both human health and local flora and fauna.
The production and disposal of perchloric acid waste also pose significant challenges. Improper disposal methods can lead to soil contamination, affecting soil chemistry and microbial communities. This, in turn, can impact plant growth and soil fertility, potentially disrupting local ecosystems and agricultural productivity.
Furthermore, the energy-intensive nature of perchloric acid production contributes to the overall carbon footprint of OSC manufacturing. The extraction of raw materials, synthesis of perchloric acid, and associated transportation all contribute to greenhouse gas emissions, exacerbating climate change concerns.
To mitigate these environmental impacts, researchers and manufacturers are exploring alternative synthesis methods and materials for OSC production. Green chemistry principles are being applied to develop less hazardous and more environmentally friendly processes. These include the use of safer solvents, catalysts, and reagents that can replace or reduce the need for perchloric acid in OSC synthesis.
Additionally, improved waste management and treatment technologies are being implemented to minimize the environmental risks associated with perchloric acid use. Advanced oxidation processes, ion exchange, and membrane filtration techniques are being developed to treat perchloric acid-containing wastewater more effectively, reducing the potential for environmental contamination.
Regulatory bodies are also playing a crucial role in addressing the environmental impact of perchloric acid in OSC production. Stricter regulations on handling, storage, and disposal of perchloric acid are being implemented in many countries, forcing manufacturers to adopt more environmentally responsible practices and invest in cleaner technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!