Tartaric Acid vs Carbonic Acid in Effervescent Reactions
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Effervescent Reaction Chemistry Background and Objectives
Effervescent reactions have been utilized for centuries, dating back to ancient civilizations where natural mineral waters containing dissolved carbon dioxide were prized for their therapeutic properties. The modern understanding of effervescent chemistry began in the 18th century with Joseph Priestley's work on "fixed air" (carbon dioxide) and its interaction with water. This foundation led to the development of commercial effervescent products in the 19th century, primarily for medicinal purposes.
The evolution of effervescent technology has been marked by significant advancements in formulation science, particularly in understanding the acid-base reactions that generate carbon dioxide gas. Tartaric acid, derived primarily from wine production byproducts, has been a traditional acidulant in effervescent formulations since the early pharmaceutical applications. Carbonic acid, formed when carbon dioxide dissolves in water, represents an alternative approach to effervescence through pressure-based carbonation rather than reactive chemistry.
The fundamental chemistry of effervescent reactions involves the interaction between acidic components (such as tartaric acid) and alkaline components (typically sodium bicarbonate) in an aqueous environment. This reaction produces carbon dioxide gas, creating the characteristic fizzing effect. The reaction kinetics, gas generation rate, and stability of the resulting solution are critical parameters that determine product performance and shelf-life.
Current technological trends in effervescent chemistry focus on enhancing stability, controlling dissolution rates, and improving sensory attributes. The pharmaceutical industry has driven much of this innovation, seeking to optimize drug delivery through effervescent dosage forms. Simultaneously, the food and beverage sector has expanded applications beyond traditional carbonated drinks to include effervescent nutritional supplements, flavor enhancers, and novel sensory experiences.
The primary technical objectives of this investigation include: quantifying the comparative efficiency of tartaric acid versus carbonic acid in generating controlled effervescence; evaluating stability profiles under various environmental conditions; assessing organoleptic properties of resulting solutions; and determining cost-effectiveness in commercial applications. Additionally, we aim to explore potential synergistic combinations that might overcome the limitations of single-acid systems.
Environmental considerations have become increasingly important in effervescent technology development, with emphasis on sustainable sourcing of acids, reduced packaging waste, and lower carbon footprints in manufacturing processes. The growing consumer preference for natural ingredients has also sparked interest in alternative acidulants derived from renewable resources, positioning tartaric acid favorably in this context compared to synthetic alternatives.
The evolution of effervescent technology has been marked by significant advancements in formulation science, particularly in understanding the acid-base reactions that generate carbon dioxide gas. Tartaric acid, derived primarily from wine production byproducts, has been a traditional acidulant in effervescent formulations since the early pharmaceutical applications. Carbonic acid, formed when carbon dioxide dissolves in water, represents an alternative approach to effervescence through pressure-based carbonation rather than reactive chemistry.
The fundamental chemistry of effervescent reactions involves the interaction between acidic components (such as tartaric acid) and alkaline components (typically sodium bicarbonate) in an aqueous environment. This reaction produces carbon dioxide gas, creating the characteristic fizzing effect. The reaction kinetics, gas generation rate, and stability of the resulting solution are critical parameters that determine product performance and shelf-life.
Current technological trends in effervescent chemistry focus on enhancing stability, controlling dissolution rates, and improving sensory attributes. The pharmaceutical industry has driven much of this innovation, seeking to optimize drug delivery through effervescent dosage forms. Simultaneously, the food and beverage sector has expanded applications beyond traditional carbonated drinks to include effervescent nutritional supplements, flavor enhancers, and novel sensory experiences.
The primary technical objectives of this investigation include: quantifying the comparative efficiency of tartaric acid versus carbonic acid in generating controlled effervescence; evaluating stability profiles under various environmental conditions; assessing organoleptic properties of resulting solutions; and determining cost-effectiveness in commercial applications. Additionally, we aim to explore potential synergistic combinations that might overcome the limitations of single-acid systems.
Environmental considerations have become increasingly important in effervescent technology development, with emphasis on sustainable sourcing of acids, reduced packaging waste, and lower carbon footprints in manufacturing processes. The growing consumer preference for natural ingredients has also sparked interest in alternative acidulants derived from renewable resources, positioning tartaric acid favorably in this context compared to synthetic alternatives.
Market Analysis of Effervescent Products
The global effervescent products market has been experiencing robust growth, valued at approximately 36 billion USD in 2022 and projected to reach 57 billion USD by 2030, with a compound annual growth rate (CAGR) of around 6.8%. This growth is primarily driven by increasing consumer preference for convenient dosage forms, rising health consciousness, and the growing prevalence of digestive disorders and vitamin deficiencies worldwide.
The pharmaceutical sector dominates the effervescent products market, accounting for nearly 65% of the total market share. Within this sector, effervescent tablets for pain management, vitamin supplements, and cold medications represent the largest segments. The food and beverage industry follows as the second-largest consumer of effervescent technology, with products like effervescent energy drinks and flavored tablets gaining popularity.
Geographically, North America and Europe currently lead the market due to high healthcare expenditure, advanced pharmaceutical infrastructure, and greater consumer awareness. However, the Asia-Pacific region is emerging as the fastest-growing market, with countries like China, India, and Japan showing significant potential due to improving healthcare access, rising disposable incomes, and changing lifestyle patterns.
Consumer behavior analysis reveals a strong preference for effervescent products due to their ease of administration, rapid dissolution, pleasant taste, and improved bioavailability compared to conventional dosage forms. The COVID-19 pandemic further accelerated this trend, with a notable surge in demand for effervescent vitamin C and immune-boosting supplements.
The choice between tartaric acid and carbonic acid in effervescent formulations significantly impacts market positioning. Products utilizing tartaric acid generally command premium pricing due to better taste profiles and stability characteristics, particularly evident in the high-end supplement market. Conversely, carbonic acid-based products typically target more cost-sensitive market segments.
Distribution channels for effervescent products have evolved considerably, with online pharmacies and e-commerce platforms gaining substantial market share, especially post-pandemic. This shift has democratized access to these products and expanded their reach beyond traditional brick-and-mortar pharmacies.
Market forecasts indicate continued growth potential, particularly in emerging economies and untapped therapeutic areas. The development of sugar-free, natural ingredient-based effervescent formulations represents a significant opportunity, aligning with the global trend toward healthier consumption patterns and sustainable product development.
The pharmaceutical sector dominates the effervescent products market, accounting for nearly 65% of the total market share. Within this sector, effervescent tablets for pain management, vitamin supplements, and cold medications represent the largest segments. The food and beverage industry follows as the second-largest consumer of effervescent technology, with products like effervescent energy drinks and flavored tablets gaining popularity.
Geographically, North America and Europe currently lead the market due to high healthcare expenditure, advanced pharmaceutical infrastructure, and greater consumer awareness. However, the Asia-Pacific region is emerging as the fastest-growing market, with countries like China, India, and Japan showing significant potential due to improving healthcare access, rising disposable incomes, and changing lifestyle patterns.
Consumer behavior analysis reveals a strong preference for effervescent products due to their ease of administration, rapid dissolution, pleasant taste, and improved bioavailability compared to conventional dosage forms. The COVID-19 pandemic further accelerated this trend, with a notable surge in demand for effervescent vitamin C and immune-boosting supplements.
The choice between tartaric acid and carbonic acid in effervescent formulations significantly impacts market positioning. Products utilizing tartaric acid generally command premium pricing due to better taste profiles and stability characteristics, particularly evident in the high-end supplement market. Conversely, carbonic acid-based products typically target more cost-sensitive market segments.
Distribution channels for effervescent products have evolved considerably, with online pharmacies and e-commerce platforms gaining substantial market share, especially post-pandemic. This shift has democratized access to these products and expanded their reach beyond traditional brick-and-mortar pharmacies.
Market forecasts indicate continued growth potential, particularly in emerging economies and untapped therapeutic areas. The development of sugar-free, natural ingredient-based effervescent formulations represents a significant opportunity, aligning with the global trend toward healthier consumption patterns and sustainable product development.
Current Challenges in Acid Selection for Effervescent Formulations
The selection of appropriate acids for effervescent formulations presents significant challenges for pharmaceutical and food industry researchers. Currently, tartaric acid and citric acid remain the predominant choices for commercial effervescent products, while carbonic acid serves primarily as the reaction product rather than an ingredient. This established preference stems from stability, solubility, and reaction kinetics considerations that continue to shape formulation decisions.
A primary challenge in acid selection involves balancing reaction rate with shelf stability. Tartaric acid offers superior stability in humid conditions compared to alternatives, but its reaction kinetics with carbonates can be difficult to control precisely. Manufacturers must carefully calibrate acid-base ratios to achieve desired dissolution times without compromising product integrity during storage.
Moisture sensitivity represents another critical obstacle, as premature reactions during manufacturing or storage can significantly reduce product efficacy. Tartaric acid demonstrates relatively lower hygroscopicity than citric acid, yet still requires specialized packaging and processing environments to maintain stability. The development of cost-effective moisture barriers remains an ongoing industry challenge.
Taste profile management presents additional complexity in acid selection. Tartaric acid imparts a distinctly tart flavor that may require masking agents in certain applications, while the carbonic acid produced during effervescence contributes a characteristic tingling sensation that must be balanced with other formulation components to achieve consumer acceptance.
Manufacturing scalability introduces further complications, as acid selection impacts production equipment requirements, processing parameters, and quality control measures. Tartaric acid's physical properties necessitate specific handling protocols that may limit production efficiency or require specialized equipment investments.
Regulatory considerations also influence acid selection decisions. While both tartaric and citric acids hold GRAS (Generally Recognized As Safe) status, their application in specific product categories may trigger different regulatory requirements across global markets. Manufacturers must navigate these varying standards while maintaining consistent product performance.
Cost fluctuations in raw material markets add another dimension to the acid selection challenge. Tartaric acid, derived primarily from wine industry byproducts, experiences price volatility tied to grape harvests and wine production volumes. This economic uncertainty complicates long-term formulation planning and may drive interest in alternative acid sources.
Environmental sustainability concerns are increasingly influencing acid selection decisions. The carbon footprint associated with tartaric acid production and transportation must be weighed against performance benefits, particularly as consumers and regulatory bodies place greater emphasis on sustainable manufacturing practices.
A primary challenge in acid selection involves balancing reaction rate with shelf stability. Tartaric acid offers superior stability in humid conditions compared to alternatives, but its reaction kinetics with carbonates can be difficult to control precisely. Manufacturers must carefully calibrate acid-base ratios to achieve desired dissolution times without compromising product integrity during storage.
Moisture sensitivity represents another critical obstacle, as premature reactions during manufacturing or storage can significantly reduce product efficacy. Tartaric acid demonstrates relatively lower hygroscopicity than citric acid, yet still requires specialized packaging and processing environments to maintain stability. The development of cost-effective moisture barriers remains an ongoing industry challenge.
Taste profile management presents additional complexity in acid selection. Tartaric acid imparts a distinctly tart flavor that may require masking agents in certain applications, while the carbonic acid produced during effervescence contributes a characteristic tingling sensation that must be balanced with other formulation components to achieve consumer acceptance.
Manufacturing scalability introduces further complications, as acid selection impacts production equipment requirements, processing parameters, and quality control measures. Tartaric acid's physical properties necessitate specific handling protocols that may limit production efficiency or require specialized equipment investments.
Regulatory considerations also influence acid selection decisions. While both tartaric and citric acids hold GRAS (Generally Recognized As Safe) status, their application in specific product categories may trigger different regulatory requirements across global markets. Manufacturers must navigate these varying standards while maintaining consistent product performance.
Cost fluctuations in raw material markets add another dimension to the acid selection challenge. Tartaric acid, derived primarily from wine industry byproducts, experiences price volatility tied to grape harvests and wine production volumes. This economic uncertainty complicates long-term formulation planning and may drive interest in alternative acid sources.
Environmental sustainability concerns are increasingly influencing acid selection decisions. The carbon footprint associated with tartaric acid production and transportation must be weighed against performance benefits, particularly as consumers and regulatory bodies place greater emphasis on sustainable manufacturing practices.
Comparative Analysis of Tartaric vs Carbonic Acid Solutions
01 Basic effervescent reaction mechanisms
The fundamental chemical reaction between tartaric acid and carbonic acid sources (typically sodium bicarbonate) produces carbon dioxide gas, creating the effervescent effect. This reaction occurs when the acids and carbonates come into contact with water, resulting in rapid gas formation. The stoichiometry and reaction kinetics can be controlled by adjusting the ratio of acid to carbonate, which affects the speed and duration of the effervescence.- Basic effervescent reaction mechanisms: The fundamental chemical reaction between tartaric acid and carbonic acid sources (typically sodium bicarbonate) produces carbon dioxide gas, creating the effervescent effect. This reaction occurs when the acids and carbonates come into contact with water, resulting in rapid gas formation. The stoichiometry and reaction kinetics can be controlled through precise formulation of the acid and carbonate components to achieve desired dissolution rates and gas evolution profiles.
- Formulation techniques for effervescent products: Various formulation techniques are employed to create stable effervescent products using tartaric acid and carbonic acid sources. These include granulation methods, particle coating, and the use of binding agents to prevent premature reactions. Controlling moisture content during manufacturing is critical, as is the selection of appropriate excipients that enhance stability without interfering with the effervescent reaction. Specialized drying techniques and packaging solutions help maintain product integrity until use.
- Applications in pharmaceutical and nutraceutical products: Tartaric acid and carbonic acid effervescent systems are widely used in pharmaceutical and nutraceutical formulations to enhance dissolution, improve taste, and increase bioavailability of active ingredients. These systems can mask bitter flavors of medications, improve patient compliance, and provide rapid delivery of therapeutic agents. The effervescent reaction creates a pleasant mouthfeel and can help transport active ingredients across biological membranes more effectively than non-effervescent formulations.
- Innovations in food and beverage applications: Effervescent reactions between tartaric acid and carbonic acid sources are utilized in various food and beverage applications to create novel sensory experiences and functional benefits. These include effervescent candies, powdered drink mixes, and instant carbonation systems. Recent innovations focus on controlling the timing and intensity of effervescence, incorporating natural flavors that complement the acidic taste profile, and developing systems that maintain stability under various environmental conditions.
- Sustainable and eco-friendly effervescent formulations: Recent developments in effervescent technology focus on creating more sustainable and eco-friendly formulations using tartaric acid and carbonic acid sources. These include the use of naturally derived acids, biodegradable packaging materials, and manufacturing processes with reduced environmental impact. Some innovations incorporate organic tartaric acid from wine industry byproducts, water-soluble packaging that dissolves completely without residue, and effervescent systems that require less energy to produce while maintaining efficacy and stability.
02 Formulation stability and shelf life enhancement
Specialized formulation techniques are employed to prevent premature reaction between tartaric acid and carbonate components during storage. These include physical separation of reactants, use of moisture-resistant coatings, addition of desiccants, and controlled humidity manufacturing environments. Stabilizers and binding agents can be incorporated to maintain product integrity while ensuring rapid dissolution when intended for use.Expand Specific Solutions03 Applications in pharmaceutical and nutraceutical products
Tartaric acid and carbonic acid effervescent systems are widely used in pharmaceutical formulations to enhance drug dissolution, improve palatability, and increase bioavailability of active ingredients. These systems can mask unpleasant tastes, facilitate rapid disintegration of tablets, and provide a convenient delivery method for medications. The effervescent reaction can also be tailored to create controlled release profiles for therapeutic compounds.Expand Specific Solutions04 Food and beverage applications
Effervescent reactions between tartaric acid and carbonic acid sources are utilized in food and beverage products to create fizzing sensations, enhance flavor release, and improve consumer experience. These systems can be formulated with various flavoring agents, sweeteners, and colorants to create instant beverages, confectionery items, and specialty food products. The reaction parameters can be adjusted to control dissolution time and gas release intensity.Expand Specific Solutions05 Advanced manufacturing techniques
Innovative manufacturing processes have been developed to optimize effervescent formulations containing tartaric acid and carbonic acid sources. These include specialized granulation methods, controlled compression techniques, and precise particle size distribution control. Low-humidity production environments, specialized packaging systems, and novel coating technologies help maintain product stability. Recent advancements include continuous manufacturing processes and precision dosing systems for consistent effervescent performance.Expand Specific Solutions
Leading Manufacturers in Effervescent Product Industry
The effervescent reaction market involving tartaric acid versus carbonic acid is in a growth phase, with an estimated global market size of $7-9 billion and expanding at 5-6% annually. The technology is mature but evolving, with companies pursuing differentiated formulations. Pharmaceutical leaders like Novartis, AbbVie, and Takeda dominate the medical applications, while consumer goods companies such as Colgate-Palmolive and Cosmetic Warriors focus on personal care products. Chemical specialists including Lonza, Lubrizol, and Eastman Chemical provide raw materials and technical expertise. Recent innovations focus on improved stability, faster dissolution rates, and environmentally sustainable formulations, with increasing patent activity suggesting continued competitive development.
Sandoz AG
Technical Solution: Sandoz has developed proprietary effervescent tablet formulations utilizing tartaric acid as the primary acidifying agent in combination with sodium bicarbonate. Their technology employs a specialized granulation process that creates a stable matrix where tartaric acid and bicarbonate components remain separated until dissolution. This prevents premature reaction during manufacturing and storage while ensuring rapid effervescence when exposed to water. Their formulations typically contain 15-25% tartaric acid with precise particle size distribution (75-150 μm) to optimize dissolution kinetics. Sandoz has also pioneered humidity-resistant coating technologies that extend shelf life to 24-36 months without compromising effervescent performance.
Strengths: Superior stability in various climate conditions; faster dissolution rates compared to citric acid alternatives; reduced hygroscopicity leading to longer shelf life. Weaknesses: Higher production costs due to tartaric acid pricing; slightly more acidic taste profile requiring additional flavor masking agents.
Novartis AG
Technical Solution: Novartis has developed an advanced effervescent delivery system that strategically utilizes both tartaric and carbonic acid components in pharmaceutical formulations. Their patented "Dual-Acid Matrix Technology" incorporates tartaric acid (12-18% w/w) as the primary acidulant with controlled-release carbonic acid precursors to create a biphasic effervescent reaction. This approach delivers an initial rapid effervescence followed by a sustained release phase, enhancing drug dissolution and bioavailability. The technology employs specialized microencapsulation techniques to protect moisture-sensitive components and incorporates proprietary desiccant-integrated packaging systems that maintain stability even in challenging environmental conditions.
Strengths: Enhanced drug absorption profiles through optimized effervescent reaction kinetics; superior taste masking capabilities; extended shelf stability (3+ years). Weaknesses: Complex manufacturing process requiring specialized equipment; higher production costs compared to conventional effervescent systems.
Key Patents in Effervescent Acid Technology
Brewable beverage in a cold liquid with controlled effervescence
PatentPendingUS20230148631A1
Innovation
- Development of brewable infusions using selected botanical and fruit species with quinine, naringin, and steviol glycosides for bitterness and sweetness, combined with specific effervescent ingredients like sodium bicarbonate and tartaric acid, optimized for cold water infusion and stability, to create a gas-free, sugar-free alternative with controlled effervescence.
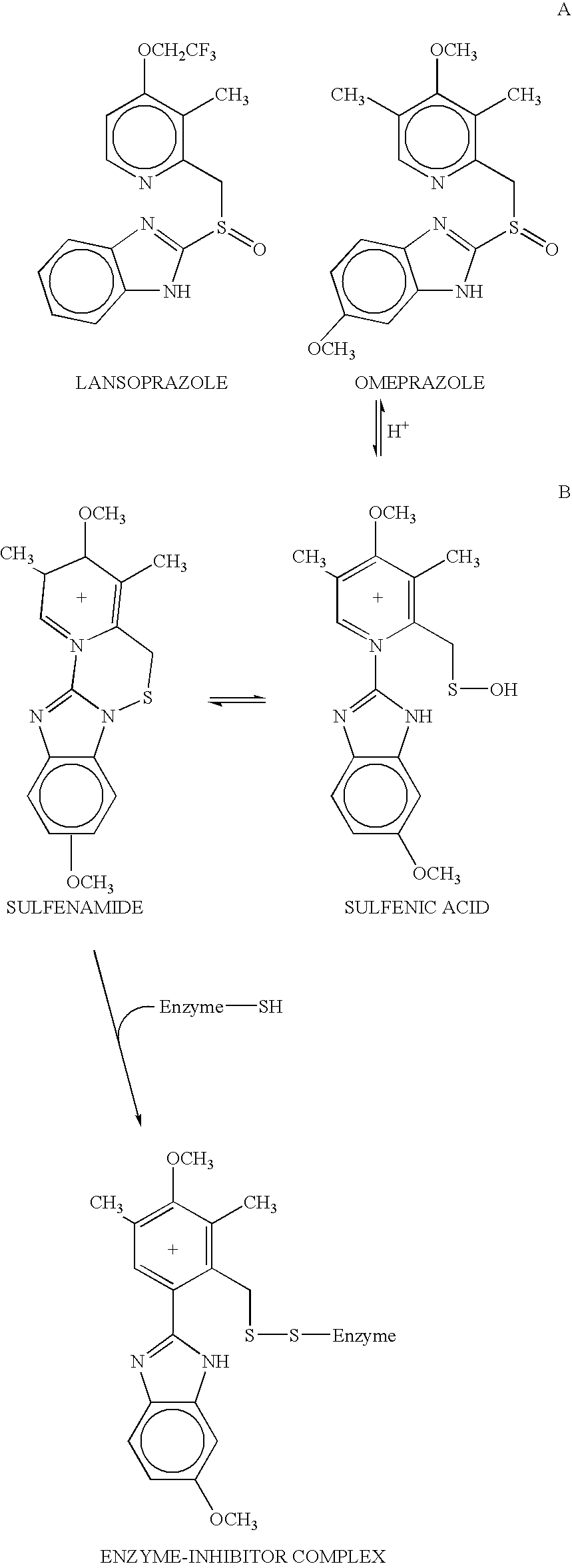
Novel substituted benzimidazole dosage forms and method of using same
PatentInactiveUS20040048896A1
Innovation
- A pharmaceutical composition comprising a proton pump inhibitor and a buffering agent, without enteric coating, that can be formulated as a solution, suspension, or solid form, including parietal cell activators to enhance pharmacological activity, allowing for easy administration and stability at room temperature, reducing the need for additional bicarbonate and simplifying dosing protocols.
Stability and Shelf-life Considerations
The stability and shelf-life of effervescent products represent critical factors in their commercial viability and consumer acceptance. When comparing tartaric acid and carbonic acid systems, significant differences emerge in their long-term stability profiles under various storage conditions.
Tartaric acid-based effervescent formulations typically demonstrate superior stability characteristics compared to carbonic acid systems. This advantage stems primarily from tartaric acid's crystalline structure, which remains relatively inert until activated by moisture. Studies indicate that properly formulated tartaric acid effervescent products can maintain over 95% of their initial reactivity after 24 months of storage under controlled conditions (25°C, 40% relative humidity).
Moisture sensitivity constitutes the primary stability challenge for both acid systems, though with varying degrees of vulnerability. Tartaric acid formulations exhibit hygroscopic properties but can be effectively protected through appropriate packaging technologies. Aluminum foil laminates with moisture vapor transmission rates below 0.1 g/m²/day have proven particularly effective in extending shelf-life by up to 300% compared to conventional packaging.
Temperature fluctuations affect both acid systems differently. Carbonic acid solutions demonstrate accelerated degradation at temperatures exceeding 30°C, with stability half-life decreasing exponentially as temperatures rise. In contrast, tartaric acid formulations maintain stability across a broader temperature range (5-40°C), making them more suitable for global distribution across varied climate zones.
Light exposure presents another differential factor. Carbonic acid solutions may undergo photolytic degradation when exposed to UV radiation, potentially generating free radicals that accelerate decomposition. Tartaric acid demonstrates superior photostability, with studies showing negligible degradation even after extended exposure to artificial light sources mimicking retail display conditions.
The presence of metal ions, particularly iron and copper, catalyzes degradation in both systems but affects carbonic acid more severely. Chelating agents such as EDTA have proven effective in mitigating this effect, extending shelf-life by sequestering these reactive metal ions.
From a manufacturing perspective, tartaric acid formulations offer practical advantages in stability prediction models. Their degradation kinetics follow more predictable patterns, allowing for more accurate shelf-life estimations using accelerated stability testing protocols. This predictability translates to more reliable quality assurance processes and reduced risk of unexpected product failures in market conditions.
Tartaric acid-based effervescent formulations typically demonstrate superior stability characteristics compared to carbonic acid systems. This advantage stems primarily from tartaric acid's crystalline structure, which remains relatively inert until activated by moisture. Studies indicate that properly formulated tartaric acid effervescent products can maintain over 95% of their initial reactivity after 24 months of storage under controlled conditions (25°C, 40% relative humidity).
Moisture sensitivity constitutes the primary stability challenge for both acid systems, though with varying degrees of vulnerability. Tartaric acid formulations exhibit hygroscopic properties but can be effectively protected through appropriate packaging technologies. Aluminum foil laminates with moisture vapor transmission rates below 0.1 g/m²/day have proven particularly effective in extending shelf-life by up to 300% compared to conventional packaging.
Temperature fluctuations affect both acid systems differently. Carbonic acid solutions demonstrate accelerated degradation at temperatures exceeding 30°C, with stability half-life decreasing exponentially as temperatures rise. In contrast, tartaric acid formulations maintain stability across a broader temperature range (5-40°C), making them more suitable for global distribution across varied climate zones.
Light exposure presents another differential factor. Carbonic acid solutions may undergo photolytic degradation when exposed to UV radiation, potentially generating free radicals that accelerate decomposition. Tartaric acid demonstrates superior photostability, with studies showing negligible degradation even after extended exposure to artificial light sources mimicking retail display conditions.
The presence of metal ions, particularly iron and copper, catalyzes degradation in both systems but affects carbonic acid more severely. Chelating agents such as EDTA have proven effective in mitigating this effect, extending shelf-life by sequestering these reactive metal ions.
From a manufacturing perspective, tartaric acid formulations offer practical advantages in stability prediction models. Their degradation kinetics follow more predictable patterns, allowing for more accurate shelf-life estimations using accelerated stability testing protocols. This predictability translates to more reliable quality assurance processes and reduced risk of unexpected product failures in market conditions.
Regulatory Framework for Pharmaceutical Effervescent Products
The regulatory landscape for pharmaceutical effervescent products varies significantly across global markets, with stringent requirements governing the use of acids like tartaric and carbonic acid in formulations. In the United States, the FDA regulates effervescent pharmaceuticals under 21 CFR Parts 210 and 211 for Good Manufacturing Practices, with specific guidance on excipient selection and stability requirements. Tartaric acid must meet USP-NF monograph specifications, while carbonic acid systems face additional scrutiny due to their volatile nature.
European regulations through the European Medicines Agency (EMA) impose more comprehensive documentation requirements, particularly under Directive 2001/83/EC. The EMA mandates detailed stability studies for effervescent products, with specific attention to moisture control and packaging integrity. Notably, the European Pharmacopoeia includes specific monographs for effervescent tablets (EP 0478) that outline disintegration time limits and acid content parameters.
Japanese regulatory authorities through the PMDA have established unique requirements for effervescent formulations, particularly regarding dissolution profiles and stability under humid conditions. Their guidelines specifically address the concentration limits of acidifying agents, with tartaric acid generally permitted at higher concentrations than carbonic acid systems.
International Conference on Harmonization (ICH) guidelines, particularly ICH Q3C for residual solvents and Q1A(R2) for stability testing, provide a harmonized framework that manufacturers must consider when selecting between tartaric and carbonic acid systems. These guidelines emphasize the importance of demonstrating consistent effervescent reaction profiles throughout the product's shelf life.
Labeling requirements also differ substantially between regulatory jurisdictions. The FDA requires specific storage warnings for moisture-sensitive effervescent products, while the EMA mandates comprehensive patient information leaflets detailing proper administration techniques. Products utilizing carbonic acid systems typically require more extensive storage warnings due to their greater sensitivity to environmental conditions.
Recent regulatory trends indicate a growing focus on environmental impact assessments for pharmaceutical excipients. The European Union's REACH regulation increasingly scrutinizes the environmental footprint of acids used in pharmaceutical formulations, with tartaric acid generally receiving more favorable environmental classifications than carbonic acid production processes.
Manufacturers must also navigate country-specific regulations in emerging markets, where requirements for effervescent products can vary dramatically. China's NMPA and India's CDSCO have established unique specifications for acid content in effervescent formulations, often requiring additional stability studies under tropical conditions that significantly impact the choice between tartaric and carbonic acid systems.
European regulations through the European Medicines Agency (EMA) impose more comprehensive documentation requirements, particularly under Directive 2001/83/EC. The EMA mandates detailed stability studies for effervescent products, with specific attention to moisture control and packaging integrity. Notably, the European Pharmacopoeia includes specific monographs for effervescent tablets (EP 0478) that outline disintegration time limits and acid content parameters.
Japanese regulatory authorities through the PMDA have established unique requirements for effervescent formulations, particularly regarding dissolution profiles and stability under humid conditions. Their guidelines specifically address the concentration limits of acidifying agents, with tartaric acid generally permitted at higher concentrations than carbonic acid systems.
International Conference on Harmonization (ICH) guidelines, particularly ICH Q3C for residual solvents and Q1A(R2) for stability testing, provide a harmonized framework that manufacturers must consider when selecting between tartaric and carbonic acid systems. These guidelines emphasize the importance of demonstrating consistent effervescent reaction profiles throughout the product's shelf life.
Labeling requirements also differ substantially between regulatory jurisdictions. The FDA requires specific storage warnings for moisture-sensitive effervescent products, while the EMA mandates comprehensive patient information leaflets detailing proper administration techniques. Products utilizing carbonic acid systems typically require more extensive storage warnings due to their greater sensitivity to environmental conditions.
Recent regulatory trends indicate a growing focus on environmental impact assessments for pharmaceutical excipients. The European Union's REACH regulation increasingly scrutinizes the environmental footprint of acids used in pharmaceutical formulations, with tartaric acid generally receiving more favorable environmental classifications than carbonic acid production processes.
Manufacturers must also navigate country-specific regulations in emerging markets, where requirements for effervescent products can vary dramatically. China's NMPA and India's CDSCO have established unique specifications for acid content in effervescent formulations, often requiring additional stability studies under tropical conditions that significantly impact the choice between tartaric and carbonic acid systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!