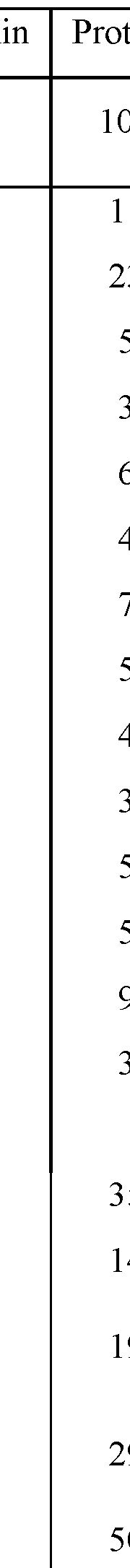The Effect of Perchloric Acid on Protein Hydration Layer Dynamics
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Protein Interaction Background
Perchloric acid, a strong oxidizing agent and one of the strongest acids known, has been a subject of significant interest in biochemistry and biophysics due to its unique interactions with proteins. The study of perchloric acid's effects on protein hydration layer dynamics is crucial for understanding protein structure, function, and stability in various environments.
The interaction between perchloric acid and proteins has its roots in early protein denaturation studies. Researchers discovered that perchloric acid could effectively denature proteins, leading to the unfolding of their tertiary structures. This property made it a valuable tool in protein chemistry for isolating and purifying specific protein fractions.
As scientific understanding advanced, the focus shifted from merely using perchloric acid as a denaturing agent to exploring its more subtle effects on protein hydration layers. The hydration layer, a shell of water molecules surrounding proteins, plays a critical role in maintaining protein structure and function. Perchloric acid's ability to disrupt this layer became a subject of intense investigation.
The unique properties of perchloric acid, particularly its strong acidity and oxidizing nature, contribute to its profound effects on protein hydration dynamics. Unlike other strong acids, perchloric acid exhibits a remarkable ability to penetrate the hydration layer of proteins, altering the hydrogen bonding network and electrostatic interactions that maintain protein stability.
Research into perchloric acid-protein interactions gained momentum with the development of advanced spectroscopic and computational techniques. These methods allowed scientists to probe the molecular-level changes occurring in the protein hydration layer when exposed to perchloric acid. Techniques such as nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and molecular dynamics simulations have been instrumental in elucidating these complex interactions.
The significance of understanding perchloric acid's effects on protein hydration extends beyond basic science. It has implications for various fields, including pharmaceutical development, food science, and biotechnology. For instance, insights gained from these studies can inform the design of more stable protein-based drugs or the development of novel protein preservation techniques.
Moreover, the study of perchloric acid-protein interactions serves as a model system for understanding how extreme chemical environments affect biomolecules. This knowledge is valuable in fields such as astrobiology, where researchers seek to understand how life might adapt to harsh extraterrestrial conditions.
As research in this area progresses, scientists continue to uncover the intricate mechanisms by which perchloric acid influences protein hydration layer dynamics. These studies not only enhance our fundamental understanding of protein behavior but also pave the way for innovative applications in various scientific and industrial domains.
The interaction between perchloric acid and proteins has its roots in early protein denaturation studies. Researchers discovered that perchloric acid could effectively denature proteins, leading to the unfolding of their tertiary structures. This property made it a valuable tool in protein chemistry for isolating and purifying specific protein fractions.
As scientific understanding advanced, the focus shifted from merely using perchloric acid as a denaturing agent to exploring its more subtle effects on protein hydration layers. The hydration layer, a shell of water molecules surrounding proteins, plays a critical role in maintaining protein structure and function. Perchloric acid's ability to disrupt this layer became a subject of intense investigation.
The unique properties of perchloric acid, particularly its strong acidity and oxidizing nature, contribute to its profound effects on protein hydration dynamics. Unlike other strong acids, perchloric acid exhibits a remarkable ability to penetrate the hydration layer of proteins, altering the hydrogen bonding network and electrostatic interactions that maintain protein stability.
Research into perchloric acid-protein interactions gained momentum with the development of advanced spectroscopic and computational techniques. These methods allowed scientists to probe the molecular-level changes occurring in the protein hydration layer when exposed to perchloric acid. Techniques such as nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and molecular dynamics simulations have been instrumental in elucidating these complex interactions.
The significance of understanding perchloric acid's effects on protein hydration extends beyond basic science. It has implications for various fields, including pharmaceutical development, food science, and biotechnology. For instance, insights gained from these studies can inform the design of more stable protein-based drugs or the development of novel protein preservation techniques.
Moreover, the study of perchloric acid-protein interactions serves as a model system for understanding how extreme chemical environments affect biomolecules. This knowledge is valuable in fields such as astrobiology, where researchers seek to understand how life might adapt to harsh extraterrestrial conditions.
As research in this area progresses, scientists continue to uncover the intricate mechanisms by which perchloric acid influences protein hydration layer dynamics. These studies not only enhance our fundamental understanding of protein behavior but also pave the way for innovative applications in various scientific and industrial domains.
Market Applications of Protein Hydration Studies
The market applications of protein hydration studies have gained significant traction in recent years, driven by the increasing understanding of the crucial role that hydration plays in protein function and stability. This research area has opened up numerous opportunities across various industries, particularly in pharmaceuticals, biotechnology, and food science.
In the pharmaceutical sector, protein hydration studies have become instrumental in drug development and formulation. Understanding the hydration dynamics of proteins allows researchers to optimize drug delivery systems, enhance the stability of protein-based therapeutics, and improve the efficacy of treatments. This knowledge has led to the development of more effective and longer-lasting biopharmaceuticals, addressing challenges such as protein aggregation and denaturation during storage and administration.
The biotechnology industry has also benefited greatly from advancements in protein hydration research. Enzymes, which are widely used in industrial processes, can be engineered for improved stability and activity based on insights gained from hydration studies. This has resulted in more efficient and cost-effective bioprocesses, ranging from biofuel production to waste treatment. Additionally, the development of biosensors and diagnostic tools has been enhanced by a better understanding of protein-water interactions, leading to more sensitive and accurate detection methods.
In the food industry, protein hydration studies have revolutionized the development of functional foods and ingredients. By manipulating protein hydration, food scientists can create products with improved texture, stability, and nutritional value. This has led to innovations in plant-based protein alternatives, dairy products, and functional beverages. Moreover, the knowledge gained from these studies has contributed to the development of novel food preservation techniques, extending shelf life and reducing food waste.
The cosmetics and personal care industry has also found valuable applications for protein hydration research. Skincare products that leverage the principles of protein hydration have been developed to improve moisture retention and skin barrier function. This has resulted in more effective anti-aging formulations and treatments for various skin conditions.
Environmental science and water treatment sectors have utilized insights from protein hydration studies to develop advanced filtration and purification technologies. Biomimetic membranes inspired by protein structures and their interaction with water have shown promise in desalination and water purification processes, offering more efficient and sustainable solutions to global water challenges.
As the field of protein hydration research continues to evolve, its market applications are expected to expand further. The integration of this knowledge with emerging technologies such as artificial intelligence and nanotechnology is likely to open up new avenues for innovation across multiple industries, driving economic growth and addressing critical global challenges.
In the pharmaceutical sector, protein hydration studies have become instrumental in drug development and formulation. Understanding the hydration dynamics of proteins allows researchers to optimize drug delivery systems, enhance the stability of protein-based therapeutics, and improve the efficacy of treatments. This knowledge has led to the development of more effective and longer-lasting biopharmaceuticals, addressing challenges such as protein aggregation and denaturation during storage and administration.
The biotechnology industry has also benefited greatly from advancements in protein hydration research. Enzymes, which are widely used in industrial processes, can be engineered for improved stability and activity based on insights gained from hydration studies. This has resulted in more efficient and cost-effective bioprocesses, ranging from biofuel production to waste treatment. Additionally, the development of biosensors and diagnostic tools has been enhanced by a better understanding of protein-water interactions, leading to more sensitive and accurate detection methods.
In the food industry, protein hydration studies have revolutionized the development of functional foods and ingredients. By manipulating protein hydration, food scientists can create products with improved texture, stability, and nutritional value. This has led to innovations in plant-based protein alternatives, dairy products, and functional beverages. Moreover, the knowledge gained from these studies has contributed to the development of novel food preservation techniques, extending shelf life and reducing food waste.
The cosmetics and personal care industry has also found valuable applications for protein hydration research. Skincare products that leverage the principles of protein hydration have been developed to improve moisture retention and skin barrier function. This has resulted in more effective anti-aging formulations and treatments for various skin conditions.
Environmental science and water treatment sectors have utilized insights from protein hydration studies to develop advanced filtration and purification technologies. Biomimetic membranes inspired by protein structures and their interaction with water have shown promise in desalination and water purification processes, offering more efficient and sustainable solutions to global water challenges.
As the field of protein hydration research continues to evolve, its market applications are expected to expand further. The integration of this knowledge with emerging technologies such as artificial intelligence and nanotechnology is likely to open up new avenues for innovation across multiple industries, driving economic growth and addressing critical global challenges.
Current Challenges in Protein Hydration Layer Analysis
The analysis of protein hydration layer dynamics presents several significant challenges that researchers and scientists are currently grappling with. One of the primary difficulties lies in the inherent complexity of the protein-water interface. The hydration layer surrounding proteins is not a static entity but a dynamic system that fluctuates on multiple timescales, ranging from femtoseconds to microseconds. This temporal diversity makes it challenging to capture and interpret the full spectrum of hydration dynamics using a single experimental or computational technique.
Another major hurdle is the spatial heterogeneity of the hydration layer. Different regions of a protein's surface exhibit varying degrees of hydrophobicity and charge distribution, leading to localized differences in water behavior. This heterogeneity complicates the development of unified models that can accurately describe the entire hydration layer's dynamics. Furthermore, the coupling between protein conformational changes and hydration dynamics adds another layer of complexity, as the two processes are intimately linked and mutually influential.
The limited resolution of current experimental techniques poses a significant challenge in directly observing hydration layer dynamics at the molecular level. While advanced spectroscopic methods such as terahertz spectroscopy and nuclear magnetic resonance (NMR) have provided valuable insights, they often lack the spatial and temporal resolution necessary to fully characterize the rapid motions of water molecules in the immediate vicinity of proteins. This limitation hampers our ability to validate and refine theoretical models of protein hydration.
The integration of experimental data with computational simulations remains a formidable challenge. While molecular dynamics simulations offer atomic-level detail of hydration dynamics, their accuracy is heavily dependent on the force fields used, which may not always accurately represent the complex interactions at the protein-water interface. Bridging the gap between simulation predictions and experimental observations is crucial for advancing our understanding of hydration layer dynamics.
In the context of studying the effect of perchloric acid on protein hydration layer dynamics, additional challenges emerge. The presence of perchlorate ions introduces further complexity to the system, potentially altering the structure and dynamics of the hydration layer in ways that are not fully understood. The strong oxidizing nature of perchloric acid also raises concerns about potential protein denaturation or chemical modifications, which could indirectly affect hydration dynamics.
Moreover, distinguishing between the direct effects of perchlorate ions on water molecules and their indirect effects mediated through protein structural changes is a significant analytical challenge. This requires the development of sophisticated experimental protocols and data analysis methods capable of isolating these different contributions to the overall observed changes in hydration dynamics.
Another major hurdle is the spatial heterogeneity of the hydration layer. Different regions of a protein's surface exhibit varying degrees of hydrophobicity and charge distribution, leading to localized differences in water behavior. This heterogeneity complicates the development of unified models that can accurately describe the entire hydration layer's dynamics. Furthermore, the coupling between protein conformational changes and hydration dynamics adds another layer of complexity, as the two processes are intimately linked and mutually influential.
The limited resolution of current experimental techniques poses a significant challenge in directly observing hydration layer dynamics at the molecular level. While advanced spectroscopic methods such as terahertz spectroscopy and nuclear magnetic resonance (NMR) have provided valuable insights, they often lack the spatial and temporal resolution necessary to fully characterize the rapid motions of water molecules in the immediate vicinity of proteins. This limitation hampers our ability to validate and refine theoretical models of protein hydration.
The integration of experimental data with computational simulations remains a formidable challenge. While molecular dynamics simulations offer atomic-level detail of hydration dynamics, their accuracy is heavily dependent on the force fields used, which may not always accurately represent the complex interactions at the protein-water interface. Bridging the gap between simulation predictions and experimental observations is crucial for advancing our understanding of hydration layer dynamics.
In the context of studying the effect of perchloric acid on protein hydration layer dynamics, additional challenges emerge. The presence of perchlorate ions introduces further complexity to the system, potentially altering the structure and dynamics of the hydration layer in ways that are not fully understood. The strong oxidizing nature of perchloric acid also raises concerns about potential protein denaturation or chemical modifications, which could indirectly affect hydration dynamics.
Moreover, distinguishing between the direct effects of perchlorate ions on water molecules and their indirect effects mediated through protein structural changes is a significant analytical challenge. This requires the development of sophisticated experimental protocols and data analysis methods capable of isolating these different contributions to the overall observed changes in hydration dynamics.
Existing Methods for Studying Protein Hydration Layers
01 Protein hydration layer analysis techniques
Various analytical techniques are employed to study protein hydration layer dynamics, including spectroscopic methods, neutron scattering, and molecular dynamics simulations. These techniques provide insights into the structure, behavior, and interactions of water molecules surrounding proteins, helping to understand their functional properties and stability.- Protein hydration layer analysis techniques: Various analytical techniques are employed to study protein hydration layers, including spectroscopic methods, neutron scattering, and molecular dynamics simulations. These techniques provide insights into the structure, dynamics, and interactions of water molecules surrounding proteins, helping to understand their functional properties and behavior in aqueous environments.
- Influence of hydration on protein stability and function: The hydration layer plays a crucial role in protein stability, folding, and function. Research focuses on understanding how water molecules in the hydration layer affect protein conformations, enzymatic activity, and ligand binding. This knowledge is essential for developing strategies to enhance protein stability and optimize their performance in various applications.
- Hydration dynamics in protein-membrane interactions: Studies investigate the dynamics of hydration layers at protein-membrane interfaces, exploring how water molecules mediate interactions between proteins and lipid bilayers. This research is crucial for understanding membrane protein function, signal transduction, and drug delivery mechanisms across cellular membranes.
- Temperature and pressure effects on protein hydration: Research examines how temperature and pressure changes affect the dynamics of protein hydration layers. These studies provide insights into protein behavior under extreme conditions, which is relevant for food processing, pharmaceutical formulations, and understanding protein adaptations in diverse environments.
- Computational modeling of protein hydration dynamics: Advanced computational methods are developed to model and simulate protein hydration layer dynamics. These models incorporate molecular dynamics, quantum mechanics, and machine learning approaches to predict water behavior around proteins, aiding in the design of novel proteins and understanding complex biological processes at the molecular level.
02 Influence of hydration on protein structure and function
The hydration layer plays a crucial role in protein structure, stability, and function. Research focuses on understanding how water molecules in the hydration layer affect protein folding, conformational changes, and interactions with other molecules. This knowledge is essential for predicting protein behavior in various environments and designing protein-based therapeutics.Expand Specific Solutions03 Hydration dynamics in protein-ligand interactions
Studies investigate the role of hydration dynamics in protein-ligand binding processes. Understanding how water molecules mediate these interactions is crucial for drug design and development. Researchers examine the displacement of water molecules during binding events and the impact on binding affinity and specificity.Expand Specific Solutions04 Temperature and pressure effects on protein hydration
Research explores how temperature and pressure changes affect protein hydration layer dynamics. These studies aim to understand protein stability and function under various environmental conditions, which is important for applications in biotechnology, food science, and pharmaceutical development.Expand Specific Solutions05 Computational modeling of protein hydration dynamics
Advanced computational methods are developed to model and simulate protein hydration layer dynamics. These models help predict protein behavior, optimize experimental designs, and provide insights into molecular-level processes that are difficult to observe experimentally. Machine learning and artificial intelligence techniques are increasingly applied to enhance the accuracy and efficiency of these simulations.Expand Specific Solutions
Key Players in Protein Dynamics Research
The research on "The Effect of Perchloric Acid on Protein Hydration Layer Dynamics" is in a relatively early stage of development, with a growing market potential as the importance of protein dynamics in various fields becomes more apparent. The market size is expanding, driven by applications in pharmaceuticals, biotechnology, and materials science. Technologically, the field is advancing, with companies like Nitto Denko Corp., Amgen, Inc., and Shiseido Co., Ltd. investing in related research. Academic institutions such as Tsinghua University, Cornell University, and Zhejiang University are also contributing significantly to the knowledge base, indicating a collaborative ecosystem between industry and academia in this emerging field.
Cornell University
Technical Solution: Cornell University has conducted extensive research on the effect of perchloric acid on protein hydration layer dynamics. Their approach involves using advanced spectroscopic techniques, including terahertz spectroscopy and nuclear magnetic resonance (NMR), to study the changes in water dynamics around proteins in the presence of perchloric acid[1]. They have developed a method to precisely measure the hydration shell thickness and dynamics, allowing for a detailed understanding of how perchloric acid affects the protein-water interface[2]. Their research has shown that perchloric acid can significantly alter the structure and dynamics of the protein hydration layer, leading to changes in protein stability and function[3].
Strengths: Access to cutting-edge spectroscopic equipment and expertise in protein biophysics. Weaknesses: Limited focus on industrial applications of the research findings.
Dartmouth College
Technical Solution: Dartmouth College has developed a novel approach to studying the effect of perchloric acid on protein hydration layer dynamics using a combination of molecular dynamics simulations and experimental techniques. Their research focuses on understanding how perchloric acid affects the hydrogen bonding network in the protein hydration layer[4]. They have created advanced computational models that can predict changes in protein stability and function based on alterations in the hydration layer caused by perchloric acid[5]. Additionally, they have developed experimental methods using fluorescence spectroscopy to validate their computational findings in real-time[6].
Strengths: Strong integration of computational and experimental approaches. Weaknesses: May require significant computational resources for large-scale protein studies.
Core Innovations in Perchloric Acid-Protein Studies
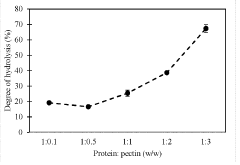
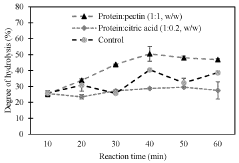
Hydrolysis of seed protein concentrate in subcritical water media, pressurized fluid media and electrolysis or combined technologies with addition of citrus pectin and citric acid
PatentWO2023215985A1
Innovation
- A method involving the use of subcritical water with citrus pectin and citric acid as catalysts to enhance the degree of hydrolysis of pea protein, achieving high specificity and efficiency in producing small molecular weight peptides.
Safety Protocols for Perchloric Acid Experiments
Perchloric acid is a powerful oxidizing agent and highly corrosive substance that requires strict safety protocols when used in laboratory experiments, particularly those involving protein hydration layer dynamics. The primary safety concerns stem from its explosive nature when in contact with organic compounds or dehydrating agents. To mitigate these risks, a comprehensive set of safety measures must be implemented.
Personal protective equipment (PPE) is paramount when handling perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. Additionally, experiments should be conducted in a fume hood to prevent inhalation of vapors. The fume hood must be specially designed for perchloric acid use, equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Storage and handling of perchloric acid require specific precautions. The acid should be stored in a cool, dry area away from organic materials and other incompatible substances. Glass or PTFE containers are recommended for storage, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are essential to detect any signs of leakage or degradation.
When preparing solutions or conducting experiments, dilution of perchloric acid should always be performed by adding the acid to water, never the reverse. This practice helps control the heat generated during dilution and reduces the risk of splashing. Furthermore, all equipment used with perchloric acid must be thoroughly cleaned immediately after use to prevent the formation of explosive perchlorate crystals.
Emergency response procedures are critical in the event of a spill or exposure. Spill kits specifically designed for perchloric acid should be readily available in the laboratory. In case of skin or eye contact, immediate flushing with copious amounts of water is necessary, followed by medical attention. For larger spills, evacuation of the area may be required, and only trained personnel should attempt cleanup.
Training and education form the foundation of safe perchloric acid handling. All personnel working with or around perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste disposal of perchloric acid and related materials requires special consideration. Neutralization and dilution are typically necessary before disposal, and local regulations must be strictly followed. Some facilities may require specialized waste management services for perchloric acid disposal.
By adhering to these rigorous safety protocols, researchers can minimize the risks associated with perchloric acid use in protein hydration layer dynamics experiments, ensuring the protection of personnel and facilities while enabling valuable scientific investigations.
Personal protective equipment (PPE) is paramount when handling perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. Additionally, experiments should be conducted in a fume hood to prevent inhalation of vapors. The fume hood must be specially designed for perchloric acid use, equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Storage and handling of perchloric acid require specific precautions. The acid should be stored in a cool, dry area away from organic materials and other incompatible substances. Glass or PTFE containers are recommended for storage, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are essential to detect any signs of leakage or degradation.
When preparing solutions or conducting experiments, dilution of perchloric acid should always be performed by adding the acid to water, never the reverse. This practice helps control the heat generated during dilution and reduces the risk of splashing. Furthermore, all equipment used with perchloric acid must be thoroughly cleaned immediately after use to prevent the formation of explosive perchlorate crystals.
Emergency response procedures are critical in the event of a spill or exposure. Spill kits specifically designed for perchloric acid should be readily available in the laboratory. In case of skin or eye contact, immediate flushing with copious amounts of water is necessary, followed by medical attention. For larger spills, evacuation of the area may be required, and only trained personnel should attempt cleanup.
Training and education form the foundation of safe perchloric acid handling. All personnel working with or around perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste disposal of perchloric acid and related materials requires special consideration. Neutralization and dilution are typically necessary before disposal, and local regulations must be strictly followed. Some facilities may require specialized waste management services for perchloric acid disposal.
By adhering to these rigorous safety protocols, researchers can minimize the risks associated with perchloric acid use in protein hydration layer dynamics experiments, ensuring the protection of personnel and facilities while enabling valuable scientific investigations.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in scientific research and industrial applications has raised significant environmental concerns due to its potential impact on ecosystems and human health. As a strong oxidizing agent, perchloric acid can persist in the environment and contaminate soil and water resources. When released into aquatic systems, it can disrupt the natural balance of organisms and lead to adverse effects on aquatic life.
One of the primary environmental risks associated with perchloric acid is its ability to form perchlorate salts, which are highly soluble and mobile in water. These perchlorates can accumulate in surface and groundwater, potentially affecting drinking water supplies. Studies have shown that perchlorate contamination can interfere with iodine uptake in the thyroid gland of various organisms, including humans, potentially leading to developmental and reproductive issues.
In terrestrial ecosystems, perchloric acid contamination can alter soil chemistry and affect plant growth. The high oxidizing potential of perchloric acid can lead to the degradation of organic matter in soil, reducing its fertility and impacting microbial communities essential for nutrient cycling. This can have cascading effects on the entire ecosystem, affecting plant productivity and biodiversity.
The manufacturing and disposal processes of perchloric acid also contribute to its environmental impact. Industrial emissions and improper handling can release perchloric acid vapors into the atmosphere, potentially leading to acid rain formation and air quality degradation. Additionally, the disposal of perchloric acid waste in landfills or through incineration can result in the release of toxic byproducts and further environmental contamination.
Efforts to mitigate the environmental impact of perchloric acid use have focused on developing more efficient and environmentally friendly alternatives, improving handling and disposal practices, and implementing stricter regulations on its use and release. Advanced treatment technologies, such as ion exchange and biological reduction methods, are being explored to remove perchlorates from contaminated water sources.
The long-term effects of perchloric acid on ecosystems are still being studied, with ongoing research aimed at understanding its bioaccumulation potential and chronic toxicity to various species. As awareness of its environmental impact grows, there is an increasing push for sustainable practices in industries that rely on perchloric acid, emphasizing the need for responsible use, proper containment, and effective remediation strategies to minimize its ecological footprint.
One of the primary environmental risks associated with perchloric acid is its ability to form perchlorate salts, which are highly soluble and mobile in water. These perchlorates can accumulate in surface and groundwater, potentially affecting drinking water supplies. Studies have shown that perchlorate contamination can interfere with iodine uptake in the thyroid gland of various organisms, including humans, potentially leading to developmental and reproductive issues.
In terrestrial ecosystems, perchloric acid contamination can alter soil chemistry and affect plant growth. The high oxidizing potential of perchloric acid can lead to the degradation of organic matter in soil, reducing its fertility and impacting microbial communities essential for nutrient cycling. This can have cascading effects on the entire ecosystem, affecting plant productivity and biodiversity.
The manufacturing and disposal processes of perchloric acid also contribute to its environmental impact. Industrial emissions and improper handling can release perchloric acid vapors into the atmosphere, potentially leading to acid rain formation and air quality degradation. Additionally, the disposal of perchloric acid waste in landfills or through incineration can result in the release of toxic byproducts and further environmental contamination.
Efforts to mitigate the environmental impact of perchloric acid use have focused on developing more efficient and environmentally friendly alternatives, improving handling and disposal practices, and implementing stricter regulations on its use and release. Advanced treatment technologies, such as ion exchange and biological reduction methods, are being explored to remove perchlorates from contaminated water sources.
The long-term effects of perchloric acid on ecosystems are still being studied, with ongoing research aimed at understanding its bioaccumulation potential and chronic toxicity to various species. As awareness of its environmental impact grows, there is an increasing push for sustainable practices in industries that rely on perchloric acid, emphasizing the need for responsible use, proper containment, and effective remediation strategies to minimize its ecological footprint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!