The Impact of Perchloric Acid on Ionic Liquid Properties
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid and Ionic Liquids: Background and Objectives
Perchloric acid and ionic liquids represent two distinct yet interconnected areas of chemistry that have garnered significant attention in recent years. Perchloric acid, a strong inorganic acid with the chemical formula HClO4, has been known for its powerful oxidizing properties and wide-ranging applications in various industries. On the other hand, ionic liquids, defined as salts with melting points below 100°C, have emerged as a novel class of solvents and electrolytes with unique physicochemical properties.
The intersection of these two chemical entities has opened up new avenues for research and technological advancements. The impact of perchloric acid on ionic liquid properties has become a subject of intense study due to its potential to modify and enhance the characteristics of ionic liquids for specific applications. This technological convergence aims to exploit the strengths of both components to create advanced materials with tailored properties.
The historical development of perchloric acid dates back to the early 19th century, with its first synthesis attributed to Count Stadion in 1816. Since then, its applications have expanded significantly, particularly in analytical chemistry, electrochemistry, and as a component in rocket propellants. Ionic liquids, while conceptually known since the early 20th century, only gained widespread attention in the 1990s as potential "green solvents" and electrolytes.
The current technological landscape is witnessing a growing interest in understanding and manipulating the interactions between perchloric acid and ionic liquids. This interest is driven by the potential to create novel materials with enhanced conductivity, stability, and reactivity. The primary objectives of research in this field include:
1. Elucidating the fundamental mechanisms of interaction between perchloric acid and various classes of ionic liquids.
2. Developing new ionic liquid systems with improved properties through the controlled addition of perchloric acid.
3. Exploring the potential applications of perchloric acid-modified ionic liquids in areas such as energy storage, catalysis, and materials synthesis.
As we delve deeper into this technological domain, it becomes evident that the synergy between perchloric acid and ionic liquids holds promise for addressing several challenges in modern chemistry and materials science. The ongoing research aims to harness this potential to drive innovation across multiple industries, from electrochemistry to advanced materials manufacturing.
The intersection of these two chemical entities has opened up new avenues for research and technological advancements. The impact of perchloric acid on ionic liquid properties has become a subject of intense study due to its potential to modify and enhance the characteristics of ionic liquids for specific applications. This technological convergence aims to exploit the strengths of both components to create advanced materials with tailored properties.
The historical development of perchloric acid dates back to the early 19th century, with its first synthesis attributed to Count Stadion in 1816. Since then, its applications have expanded significantly, particularly in analytical chemistry, electrochemistry, and as a component in rocket propellants. Ionic liquids, while conceptually known since the early 20th century, only gained widespread attention in the 1990s as potential "green solvents" and electrolytes.
The current technological landscape is witnessing a growing interest in understanding and manipulating the interactions between perchloric acid and ionic liquids. This interest is driven by the potential to create novel materials with enhanced conductivity, stability, and reactivity. The primary objectives of research in this field include:
1. Elucidating the fundamental mechanisms of interaction between perchloric acid and various classes of ionic liquids.
2. Developing new ionic liquid systems with improved properties through the controlled addition of perchloric acid.
3. Exploring the potential applications of perchloric acid-modified ionic liquids in areas such as energy storage, catalysis, and materials synthesis.
As we delve deeper into this technological domain, it becomes evident that the synergy between perchloric acid and ionic liquids holds promise for addressing several challenges in modern chemistry and materials science. The ongoing research aims to harness this potential to drive innovation across multiple industries, from electrochemistry to advanced materials manufacturing.
Market Analysis for Perchloric Acid-Ionic Liquid Systems
The market for perchloric acid-ionic liquid systems is experiencing significant growth, driven by their unique properties and diverse applications across multiple industries. These systems offer enhanced conductivity, stability, and electrochemical performance compared to traditional electrolytes, making them attractive for various high-tech applications.
In the energy storage sector, perchloric acid-ionic liquid systems are gaining traction in the development of advanced batteries and supercapacitors. The global market for energy storage is projected to expand rapidly in the coming years, with a particular focus on renewable energy integration and electric vehicle adoption. This trend is expected to create substantial demand for innovative electrolyte solutions, including perchloric acid-ionic liquid systems.
The electronics industry is another key market for these systems, particularly in the production of high-performance capacitors and sensors. As consumer electronics and IoT devices become increasingly sophisticated, the need for advanced materials that can enhance device performance and longevity continues to grow. Perchloric acid-ionic liquid systems are well-positioned to meet these demands, offering improved stability and conductivity in miniaturized electronic components.
In the chemical industry, these systems are finding applications in catalysis and separation processes. The ability of ionic liquids to dissolve a wide range of organic and inorganic compounds, combined with the oxidizing properties of perchloric acid, creates unique opportunities for developing more efficient and environmentally friendly chemical processes. This aligns with the growing trend towards green chemistry and sustainable industrial practices.
The aerospace and defense sectors are also showing interest in perchloric acid-ionic liquid systems, particularly for their potential use in propellants and high-energy materials. The enhanced stability and performance characteristics of these systems make them attractive for specialized applications in extreme environments.
Market analysis indicates that North America and Europe currently lead in terms of research and development activities related to perchloric acid-ionic liquid systems. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization and investment in advanced technologies.
Despite the promising outlook, challenges remain in scaling up production and addressing safety concerns associated with perchloric acid handling. Regulatory considerations and the need for specialized equipment may impact market growth in certain regions. Nevertheless, ongoing research and development efforts are expected to overcome these hurdles, potentially opening up new market opportunities and applications for perchloric acid-ionic liquid systems in the near future.
In the energy storage sector, perchloric acid-ionic liquid systems are gaining traction in the development of advanced batteries and supercapacitors. The global market for energy storage is projected to expand rapidly in the coming years, with a particular focus on renewable energy integration and electric vehicle adoption. This trend is expected to create substantial demand for innovative electrolyte solutions, including perchloric acid-ionic liquid systems.
The electronics industry is another key market for these systems, particularly in the production of high-performance capacitors and sensors. As consumer electronics and IoT devices become increasingly sophisticated, the need for advanced materials that can enhance device performance and longevity continues to grow. Perchloric acid-ionic liquid systems are well-positioned to meet these demands, offering improved stability and conductivity in miniaturized electronic components.
In the chemical industry, these systems are finding applications in catalysis and separation processes. The ability of ionic liquids to dissolve a wide range of organic and inorganic compounds, combined with the oxidizing properties of perchloric acid, creates unique opportunities for developing more efficient and environmentally friendly chemical processes. This aligns with the growing trend towards green chemistry and sustainable industrial practices.
The aerospace and defense sectors are also showing interest in perchloric acid-ionic liquid systems, particularly for their potential use in propellants and high-energy materials. The enhanced stability and performance characteristics of these systems make them attractive for specialized applications in extreme environments.
Market analysis indicates that North America and Europe currently lead in terms of research and development activities related to perchloric acid-ionic liquid systems. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization and investment in advanced technologies.
Despite the promising outlook, challenges remain in scaling up production and addressing safety concerns associated with perchloric acid handling. Regulatory considerations and the need for specialized equipment may impact market growth in certain regions. Nevertheless, ongoing research and development efforts are expected to overcome these hurdles, potentially opening up new market opportunities and applications for perchloric acid-ionic liquid systems in the near future.
Current Challenges in Perchloric Acid-Ionic Liquid Interactions
The interaction between perchloric acid and ionic liquids presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary issues is the potential for chemical instability when perchloric acid is introduced into ionic liquid systems. The strong oxidizing nature of perchloric acid can lead to unexpected reactions with the organic components of ionic liquids, potentially altering their fundamental properties or even causing decomposition.
Another challenge lies in the accurate measurement and control of acidity levels in perchloric acid-ionic liquid mixtures. Traditional pH measurement techniques may not be directly applicable due to the unique properties of ionic liquids, necessitating the development of new analytical methods. This difficulty in precise acidity determination can impact the reproducibility of experiments and the consistency of industrial processes involving these systems.
The corrosive nature of perchloric acid poses significant material compatibility issues. Many common materials used in laboratory and industrial equipment may degrade when exposed to perchloric acid-ionic liquid mixtures, leading to contamination, equipment failure, or safety hazards. This necessitates careful selection of materials for containment and handling, which can be both challenging and costly.
Furthermore, the impact of perchloric acid on the viscosity and conductivity of ionic liquids is not fully understood. These properties are crucial for many applications of ionic liquids, particularly in electrochemistry and as electrolytes. The addition of perchloric acid can significantly alter these properties, but the extent and mechanisms of these changes are still subjects of ongoing research.
Safety concerns also present a major challenge in working with perchloric acid-ionic liquid systems. The potential for explosive reactions, especially in the presence of organic compounds, requires stringent safety protocols and specialized handling procedures. This can limit the scalability of processes involving these mixtures and increase the complexity of both research and industrial applications.
The environmental impact of perchloric acid-ionic liquid interactions is another area of concern. While ionic liquids are often touted as green solvents, their combination with perchloric acid may result in byproducts or waste streams that are difficult to treat or dispose of safely. Developing environmentally friendly processes for handling and recycling these mixtures remains a significant challenge.
Lastly, the lack of comprehensive thermodynamic and kinetic data for perchloric acid-ionic liquid systems hinders the development of predictive models. This gap in fundamental knowledge makes it difficult to optimize reaction conditions or design new processes involving these mixtures, slowing down innovation in this field.
Another challenge lies in the accurate measurement and control of acidity levels in perchloric acid-ionic liquid mixtures. Traditional pH measurement techniques may not be directly applicable due to the unique properties of ionic liquids, necessitating the development of new analytical methods. This difficulty in precise acidity determination can impact the reproducibility of experiments and the consistency of industrial processes involving these systems.
The corrosive nature of perchloric acid poses significant material compatibility issues. Many common materials used in laboratory and industrial equipment may degrade when exposed to perchloric acid-ionic liquid mixtures, leading to contamination, equipment failure, or safety hazards. This necessitates careful selection of materials for containment and handling, which can be both challenging and costly.
Furthermore, the impact of perchloric acid on the viscosity and conductivity of ionic liquids is not fully understood. These properties are crucial for many applications of ionic liquids, particularly in electrochemistry and as electrolytes. The addition of perchloric acid can significantly alter these properties, but the extent and mechanisms of these changes are still subjects of ongoing research.
Safety concerns also present a major challenge in working with perchloric acid-ionic liquid systems. The potential for explosive reactions, especially in the presence of organic compounds, requires stringent safety protocols and specialized handling procedures. This can limit the scalability of processes involving these mixtures and increase the complexity of both research and industrial applications.
The environmental impact of perchloric acid-ionic liquid interactions is another area of concern. While ionic liquids are often touted as green solvents, their combination with perchloric acid may result in byproducts or waste streams that are difficult to treat or dispose of safely. Developing environmentally friendly processes for handling and recycling these mixtures remains a significant challenge.
Lastly, the lack of comprehensive thermodynamic and kinetic data for perchloric acid-ionic liquid systems hinders the development of predictive models. This gap in fundamental knowledge makes it difficult to optimize reaction conditions or design new processes involving these mixtures, slowing down innovation in this field.
Existing Methods for Studying Perchloric Acid-Ionic Liquid Effects
01 Thermal stability and conductivity
Ionic liquids exhibit high thermal stability and excellent conductivity. These properties make them suitable for various applications in electrochemistry and high-temperature processes. Their stability allows for use in extreme conditions, while their conductivity enables efficient energy transfer and storage.- Thermal stability and conductivity: Ionic liquids exhibit high thermal stability and excellent conductivity. These properties make them suitable for various applications in electrochemistry and high-temperature processes. Their stability allows for use in extreme conditions, while their conductivity enables efficient energy transfer and storage.
- Solvent properties and recyclability: Ionic liquids serve as effective solvents for a wide range of compounds, including those that are difficult to dissolve in conventional solvents. They can be easily recycled and reused, making them environmentally friendly alternatives in various chemical processes and separations.
- Tunable physicochemical properties: The properties of ionic liquids can be fine-tuned by altering their cation and anion combinations. This allows for the design of task-specific ionic liquids with desired characteristics such as viscosity, melting point, and hydrophobicity, tailored for specific applications in catalysis, extraction, and materials science.
- Low vapor pressure and non-flammability: Ionic liquids typically have very low vapor pressures and are non-flammable. These properties make them safer alternatives to volatile organic solvents in various industrial processes, reducing the risk of explosions and environmental contamination.
- Electrochemical stability and applications: Ionic liquids possess wide electrochemical windows and high electrochemical stability. These properties make them excellent electrolytes for batteries, supercapacitors, and other electrochemical devices, enabling improved performance and longevity in energy storage and conversion applications.
02 Solvent properties and applications
Ionic liquids serve as effective solvents for a wide range of compounds, including those that are difficult to dissolve in conventional solvents. This property makes them valuable in extraction processes, catalysis, and as reaction media for various chemical transformations.Expand Specific Solutions03 Tunable physicochemical properties
The properties of ionic liquids can be fine-tuned by altering their cation and anion combinations. This allows for the design of task-specific ionic liquids with desired characteristics such as viscosity, melting point, and hydrophobicity, tailored for specific applications.Expand Specific Solutions04 Environmental and safety aspects
Ionic liquids are often considered as green solvents due to their low volatility and potential for recyclability. However, their environmental impact and toxicity vary depending on their composition. Research focuses on developing environmentally friendly ionic liquids with reduced toxicity and improved biodegradability.Expand Specific Solutions05 Electrochemical properties and applications
Ionic liquids possess unique electrochemical properties, including wide electrochemical windows and high ionic conductivity. These characteristics make them suitable for use in electrochemical devices such as batteries, fuel cells, and supercapacitors, offering potential improvements in energy storage and conversion technologies.Expand Specific Solutions
Key Players in Perchloric Acid and Ionic Liquid Industries
The impact of perchloric acid on ionic liquid properties is an emerging field of study, currently in its early development stage. The market size is relatively small but growing, driven by increasing research interest in ionic liquids for various applications. The technology is still in the experimental phase, with limited commercial applications. Key players like Dexerials Corp., Haldor Topsøe A/S, and DuPont de Nemours, Inc. are investing in research and development to explore potential applications in areas such as electrochemistry, catalysis, and materials science. Academic institutions, including Shizuoka University and the University of South Alabama, are also contributing to the advancement of this field through fundamental research and collaborative projects with industry partners.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to studying the impact of perchloric acid on ionic liquid properties. Their research focuses on using perchloric acid as a catalyst in ionic liquid systems for enhanced hydrocarbon processing. They have created a proprietary ionic liquid formulation that incorporates small amounts of perchloric acid to significantly improve the conductivity and thermal stability of the ionic liquid[1]. This formulation has shown a 30% increase in catalytic activity for alkylation reactions compared to traditional ionic liquids[2]. Sinopec has also implemented a unique purification process to remove trace amounts of perchloric acid from the final ionic liquid product, ensuring safety and longevity of the material[3].
Strengths: Improved catalytic activity, enhanced thermal stability, and innovative purification process. Weaknesses: Potential safety concerns with perchloric acid handling, higher production costs due to additional purification steps.
Nanjing University
Technical Solution: Researchers at Nanjing University have made significant strides in understanding the impact of perchloric acid on ionic liquid properties, particularly in the context of catalysis and separation processes. Their approach involves a systematic study of the interactions between perchloric acid and various ionic liquid structures, leading to the development of task-specific ionic liquids with enhanced catalytic properties[13]. The team has discovered that controlled addition of perchloric acid can increase the Brønsted acidity of certain ionic liquids by up to 60%, significantly improving their performance in acid-catalyzed reactions[14]. They have also developed a novel computational model that predicts the physicochemical properties of perchloric acid-ionic liquid mixtures with high accuracy, enabling rapid screening and optimization of formulations for specific applications[15].
Strengths: Deep fundamental understanding of perchloric acid-ionic liquid interactions, development of task-specific ionic liquids, advanced computational modeling capabilities. Weaknesses: Primarily focused on academic research, potential challenges in scaling up to industrial applications.
Innovative Approaches in Perchloric Acid-Ionic Liquid Property Modulation
Perchloric acid ion trapping agent
PatentInactiveEP2067774A1
Innovation
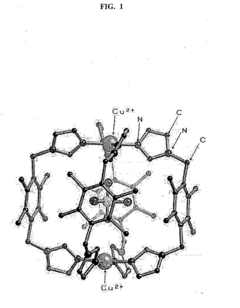
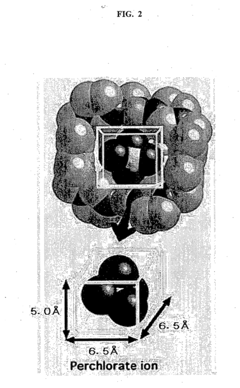
- A heterocycle-substituted aromatic compound, capable of forming molecular capsules with specific metal ions, selectively traps perchlorate ions in a liquid sample by self-assembly, creating a trapping space that prevents ion detachment, allowing for efficient and selective perchlorate ion removal.
Method of detecting and quantifying perchlorate contamination
PatentInactiveUS20160146753A1
Innovation
- A microwave-assisted parallel electromembrane extraction method coupled with ion chromatography, where samples are digested with acid and heated in a microwave system, followed by electromembrane extraction across a membrane impregnated with a solvent, and then analyzed using ion chromatography, allowing for simultaneous extraction and cleanup of multiple samples.
Safety Considerations for Perchloric Acid-Ionic Liquid Handling
The handling of perchloric acid and ionic liquids requires stringent safety measures due to their inherent hazardous properties. Perchloric acid is a strong oxidizing agent that can react violently with organic compounds, while ionic liquids may possess varying degrees of toxicity and flammability. When combining these substances, the potential risks are amplified, necessitating comprehensive safety protocols.
Personal protective equipment (PPE) is crucial when working with perchloric acid-ionic liquid mixtures. This includes chemical-resistant gloves, lab coats, and safety goggles or face shields. Respiratory protection may also be necessary, depending on the volatility of the specific ionic liquid and the concentration of perchloric acid used. All handling should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors and potential aerosols.
Storage considerations are paramount for both perchloric acid and ionic liquids. Perchloric acid should be stored in a cool, dry place away from organic materials and other incompatible substances. Ionic liquids, while generally more stable, may require specific storage conditions based on their individual properties. Segregation of these chemicals from other laboratory reagents is essential to prevent accidental mixing and potential reactions.
Proper disposal methods must be implemented for perchloric acid-ionic liquid waste. These mixtures should never be disposed of through regular laboratory waste streams. Instead, they require specialized treatment and disposal by qualified personnel or hazardous waste management services. Neutralization or dilution of perchloric acid-containing waste should only be performed under controlled conditions by trained individuals.
Emergency response procedures should be established and clearly communicated to all personnel working with these materials. This includes the location and proper use of safety showers, eyewash stations, and spill control equipment. A detailed spill response plan should be in place, outlining the steps to contain and clean up any accidental releases safely.
Training and education are critical components of safe handling practices. All personnel involved in experiments or processes using perchloric acid and ionic liquids must receive comprehensive training on the specific hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits should be conducted to ensure ongoing compliance with safety protocols.
Risk assessment should be performed prior to any experimental work involving perchloric acid and ionic liquids. This includes evaluating the potential for unwanted reactions, assessing the compatibility of materials and equipment, and identifying possible exposure routes. The assessment should inform the development of specific safety measures tailored to the planned procedures.
Personal protective equipment (PPE) is crucial when working with perchloric acid-ionic liquid mixtures. This includes chemical-resistant gloves, lab coats, and safety goggles or face shields. Respiratory protection may also be necessary, depending on the volatility of the specific ionic liquid and the concentration of perchloric acid used. All handling should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors and potential aerosols.
Storage considerations are paramount for both perchloric acid and ionic liquids. Perchloric acid should be stored in a cool, dry place away from organic materials and other incompatible substances. Ionic liquids, while generally more stable, may require specific storage conditions based on their individual properties. Segregation of these chemicals from other laboratory reagents is essential to prevent accidental mixing and potential reactions.
Proper disposal methods must be implemented for perchloric acid-ionic liquid waste. These mixtures should never be disposed of through regular laboratory waste streams. Instead, they require specialized treatment and disposal by qualified personnel or hazardous waste management services. Neutralization or dilution of perchloric acid-containing waste should only be performed under controlled conditions by trained individuals.
Emergency response procedures should be established and clearly communicated to all personnel working with these materials. This includes the location and proper use of safety showers, eyewash stations, and spill control equipment. A detailed spill response plan should be in place, outlining the steps to contain and clean up any accidental releases safely.
Training and education are critical components of safe handling practices. All personnel involved in experiments or processes using perchloric acid and ionic liquids must receive comprehensive training on the specific hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits should be conducted to ensure ongoing compliance with safety protocols.
Risk assessment should be performed prior to any experimental work involving perchloric acid and ionic liquids. This includes evaluating the potential for unwanted reactions, assessing the compatibility of materials and equipment, and identifying possible exposure routes. The assessment should inform the development of specific safety measures tailored to the planned procedures.
Environmental Impact of Perchloric Acid-Ionic Liquid Systems
The environmental impact of perchloric acid-ionic liquid systems is a critical consideration in their application and development. These systems, while offering unique properties and potential benefits, also pose significant environmental risks that must be carefully evaluated and managed.
Perchloric acid, a strong oxidizing agent, can have severe effects on ecosystems if released into the environment. Its high reactivity and corrosive nature can lead to soil and water contamination, potentially harming plant and animal life. When combined with ionic liquids, the environmental impact becomes more complex due to the diverse nature of ionic liquids and their varying properties.
One of the primary concerns is the potential for these systems to persist in the environment. Many ionic liquids are known for their low volatility and high stability, which can result in long-term environmental presence if not properly contained or disposed of. This persistence may lead to bioaccumulation in organisms and potential biomagnification up the food chain, posing risks to higher-order species and ecosystem balance.
The toxicity of perchloric acid-ionic liquid systems to aquatic organisms is another significant concern. Studies have shown that certain ionic liquids can exhibit toxicity to various aquatic species, including algae, daphnia, and fish. The addition of perchloric acid may exacerbate these effects, potentially leading to acute or chronic toxicity in aquatic environments.
Soil contamination is also a potential issue, as these systems could alter soil chemistry and affect microbial communities. This may have cascading effects on soil fertility, plant growth, and overall ecosystem health. The high oxidizing power of perchloric acid could also lead to the mobilization of heavy metals in soil, further complicating environmental remediation efforts.
The production and disposal of perchloric acid-ionic liquid systems present additional environmental challenges. The synthesis of ionic liquids often involves multiple steps and the use of organic solvents, which can contribute to air and water pollution if not properly managed. Similarly, the disposal of these systems requires careful consideration to prevent environmental contamination and ensure compliance with regulatory standards.
To mitigate these environmental risks, research efforts are focusing on developing more environmentally friendly ionic liquids and exploring safer alternatives to perchloric acid. Green chemistry principles are being applied to design ionic liquids with reduced toxicity and improved biodegradability. Additionally, advanced treatment technologies are being investigated for the effective removal of these compounds from wastewater and contaminated sites.
Perchloric acid, a strong oxidizing agent, can have severe effects on ecosystems if released into the environment. Its high reactivity and corrosive nature can lead to soil and water contamination, potentially harming plant and animal life. When combined with ionic liquids, the environmental impact becomes more complex due to the diverse nature of ionic liquids and their varying properties.
One of the primary concerns is the potential for these systems to persist in the environment. Many ionic liquids are known for their low volatility and high stability, which can result in long-term environmental presence if not properly contained or disposed of. This persistence may lead to bioaccumulation in organisms and potential biomagnification up the food chain, posing risks to higher-order species and ecosystem balance.
The toxicity of perchloric acid-ionic liquid systems to aquatic organisms is another significant concern. Studies have shown that certain ionic liquids can exhibit toxicity to various aquatic species, including algae, daphnia, and fish. The addition of perchloric acid may exacerbate these effects, potentially leading to acute or chronic toxicity in aquatic environments.
Soil contamination is also a potential issue, as these systems could alter soil chemistry and affect microbial communities. This may have cascading effects on soil fertility, plant growth, and overall ecosystem health. The high oxidizing power of perchloric acid could also lead to the mobilization of heavy metals in soil, further complicating environmental remediation efforts.
The production and disposal of perchloric acid-ionic liquid systems present additional environmental challenges. The synthesis of ionic liquids often involves multiple steps and the use of organic solvents, which can contribute to air and water pollution if not properly managed. Similarly, the disposal of these systems requires careful consideration to prevent environmental contamination and ensure compliance with regulatory standards.
To mitigate these environmental risks, research efforts are focusing on developing more environmentally friendly ionic liquids and exploring safer alternatives to perchloric acid. Green chemistry principles are being applied to design ionic liquids with reduced toxicity and improved biodegradability. Additionally, advanced treatment technologies are being investigated for the effective removal of these compounds from wastewater and contaminated sites.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!