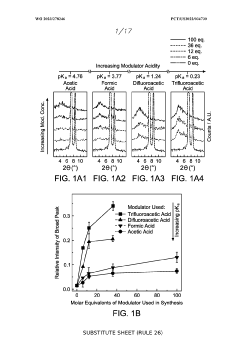
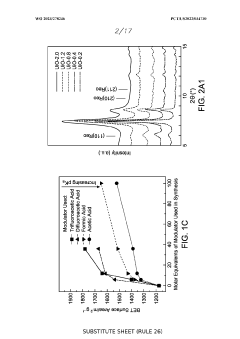
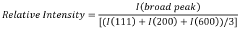The Impact of Perchloric Acid on Metal Organic Framework Synthesis
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Synthesis Background
Metal-Organic Frameworks (MOFs) have emerged as a revolutionary class of porous materials over the past few decades. These crystalline structures consist of metal ions or clusters coordinated to organic ligands, forming three-dimensional networks with exceptional porosity and surface area. The synthesis of MOFs has become a cornerstone in materials science, with applications spanning gas storage, catalysis, drug delivery, and environmental remediation.
The development of MOF synthesis techniques has evolved significantly since their initial discovery. Traditional solvothermal methods, involving high temperatures and pressures, were the primary approach for MOF production. However, these methods often resulted in low yields and limited control over crystal size and morphology. As research progressed, room-temperature synthesis methods emerged, offering more accessible and scalable production routes.
The role of acid modulators in MOF synthesis has gained considerable attention in recent years. Acids can influence the crystallization process, affecting nucleation rates, crystal growth, and the final structure of MOFs. Among various acids, perchloric acid (HClO4) has shown unique properties in MOF synthesis due to its strong oxidizing nature and ability to form stable perchlorates with metal ions.
Perchloric acid's impact on MOF synthesis is multifaceted. It can act as a pH modulator, influencing the solubility and reactivity of metal precursors and organic ligands. The presence of perchloric acid can also affect the coordination environment of metal centers, potentially leading to the formation of novel MOF structures or improved crystallinity in existing frameworks.
Furthermore, the use of perchloric acid in MOF synthesis has been associated with enhanced porosity and surface area in some cases. This effect is attributed to the acid's ability to promote the formation of defects or create additional coordination sites within the MOF structure. Such modifications can significantly alter the material's adsorption properties and catalytic performance.
However, the use of perchloric acid in MOF synthesis also presents challenges. Its strong oxidizing nature can lead to undesired side reactions or degradation of organic ligands under certain conditions. Additionally, safety considerations must be taken into account when handling perchloric acid due to its potential explosive properties when in contact with organic compounds.
As research in this field continues to advance, understanding the precise mechanisms by which perchloric acid influences MOF formation remains a key focus. This knowledge is crucial for developing more efficient and controlled synthesis methods, as well as for designing MOFs with tailored properties for specific applications.
The development of MOF synthesis techniques has evolved significantly since their initial discovery. Traditional solvothermal methods, involving high temperatures and pressures, were the primary approach for MOF production. However, these methods often resulted in low yields and limited control over crystal size and morphology. As research progressed, room-temperature synthesis methods emerged, offering more accessible and scalable production routes.
The role of acid modulators in MOF synthesis has gained considerable attention in recent years. Acids can influence the crystallization process, affecting nucleation rates, crystal growth, and the final structure of MOFs. Among various acids, perchloric acid (HClO4) has shown unique properties in MOF synthesis due to its strong oxidizing nature and ability to form stable perchlorates with metal ions.
Perchloric acid's impact on MOF synthesis is multifaceted. It can act as a pH modulator, influencing the solubility and reactivity of metal precursors and organic ligands. The presence of perchloric acid can also affect the coordination environment of metal centers, potentially leading to the formation of novel MOF structures or improved crystallinity in existing frameworks.
Furthermore, the use of perchloric acid in MOF synthesis has been associated with enhanced porosity and surface area in some cases. This effect is attributed to the acid's ability to promote the formation of defects or create additional coordination sites within the MOF structure. Such modifications can significantly alter the material's adsorption properties and catalytic performance.
However, the use of perchloric acid in MOF synthesis also presents challenges. Its strong oxidizing nature can lead to undesired side reactions or degradation of organic ligands under certain conditions. Additionally, safety considerations must be taken into account when handling perchloric acid due to its potential explosive properties when in contact with organic compounds.
As research in this field continues to advance, understanding the precise mechanisms by which perchloric acid influences MOF formation remains a key focus. This knowledge is crucial for developing more efficient and controlled synthesis methods, as well as for designing MOFs with tailored properties for specific applications.
Perchloric Acid Market
The perchloric acid market has witnessed significant growth in recent years, driven by its diverse applications across various industries. As a strong oxidizing agent, perchloric acid plays a crucial role in metal organic framework (MOF) synthesis, which has contributed to its increasing demand. The market is characterized by a complex interplay of factors, including technological advancements, regulatory frameworks, and evolving end-user requirements.
In terms of market size, the global perchloric acid market has been expanding steadily, with a notable uptick in demand from the chemical and pharmaceutical sectors. This growth is primarily attributed to the rising adoption of MOFs in gas storage, catalysis, and drug delivery applications. The market's geographical distribution shows a concentration in regions with advanced chemical manufacturing capabilities, such as North America, Europe, and Asia-Pacific.
The demand dynamics of perchloric acid are closely tied to the research and development activities in MOF synthesis. As MOFs gain traction in emerging applications like carbon capture and water purification, the market for perchloric acid is expected to experience a corresponding surge. However, the market faces challenges related to the hazardous nature of perchloric acid, necessitating stringent handling and storage protocols.
Key market players in the perchloric acid industry include established chemical manufacturers and specialized suppliers. These companies are investing in research and development to enhance the purity and efficiency of perchloric acid production, particularly for high-end applications like MOF synthesis. The competitive landscape is characterized by a focus on product quality, supply chain optimization, and strategic partnerships with end-users in the MOF research community.
Pricing trends in the perchloric acid market reflect the balance between production costs, regulatory compliance expenses, and demand fluctuations. The market has seen a gradual increase in prices, driven by the growing sophistication of MOF synthesis techniques and the need for higher purity grades. This trend is likely to continue as the applications of MOFs expand into new domains, potentially creating niche markets for specialized perchloric acid formulations.
Looking ahead, the perchloric acid market is poised for continued growth, with the MOF synthesis segment acting as a key driver. The market is expected to benefit from ongoing research into novel MOF structures and their applications, which could lead to increased demand for high-quality perchloric acid. However, market participants must navigate challenges related to environmental regulations and safety concerns, which may influence future market dynamics and innovation trajectories in perchloric acid production and utilization.
In terms of market size, the global perchloric acid market has been expanding steadily, with a notable uptick in demand from the chemical and pharmaceutical sectors. This growth is primarily attributed to the rising adoption of MOFs in gas storage, catalysis, and drug delivery applications. The market's geographical distribution shows a concentration in regions with advanced chemical manufacturing capabilities, such as North America, Europe, and Asia-Pacific.
The demand dynamics of perchloric acid are closely tied to the research and development activities in MOF synthesis. As MOFs gain traction in emerging applications like carbon capture and water purification, the market for perchloric acid is expected to experience a corresponding surge. However, the market faces challenges related to the hazardous nature of perchloric acid, necessitating stringent handling and storage protocols.
Key market players in the perchloric acid industry include established chemical manufacturers and specialized suppliers. These companies are investing in research and development to enhance the purity and efficiency of perchloric acid production, particularly for high-end applications like MOF synthesis. The competitive landscape is characterized by a focus on product quality, supply chain optimization, and strategic partnerships with end-users in the MOF research community.
Pricing trends in the perchloric acid market reflect the balance between production costs, regulatory compliance expenses, and demand fluctuations. The market has seen a gradual increase in prices, driven by the growing sophistication of MOF synthesis techniques and the need for higher purity grades. This trend is likely to continue as the applications of MOFs expand into new domains, potentially creating niche markets for specialized perchloric acid formulations.
Looking ahead, the perchloric acid market is poised for continued growth, with the MOF synthesis segment acting as a key driver. The market is expected to benefit from ongoing research into novel MOF structures and their applications, which could lead to increased demand for high-quality perchloric acid. However, market participants must navigate challenges related to environmental regulations and safety concerns, which may influence future market dynamics and innovation trajectories in perchloric acid production and utilization.
Challenges in MOF Synthesis
Metal Organic Frameworks (MOFs) synthesis presents several significant challenges that researchers and industry professionals must overcome to advance this field. One of the primary obstacles is achieving precise control over the synthesis process. The formation of MOFs involves complex interactions between metal ions and organic ligands, which can be highly sensitive to reaction conditions.
The use of perchloric acid in MOF synthesis introduces additional complexities. While perchloric acid can serve as a powerful oxidizing agent and proton source, its incorporation often leads to unpredictable outcomes. The high reactivity of perchloric acid can result in uncontrolled reactions, potentially causing structural defects or even complete collapse of the MOF framework.
Another major challenge is maintaining the stability of MOFs during and after synthesis. The presence of perchloric acid can significantly affect the pH of the reaction medium, potentially leading to the degradation of organic ligands or the dissolution of metal nodes. This instability can compromise the structural integrity and porosity of the resulting MOFs, limiting their practical applications.
Reproducibility is a persistent issue in MOF synthesis, particularly when using strong acids like perchloric acid. Small variations in reaction conditions, such as temperature, concentration, or mixing rates, can lead to substantial differences in the final product. This lack of consistency poses significant hurdles for large-scale production and standardization of MOF materials.
The safety concerns associated with perchloric acid usage present another challenge. Its strong oxidizing properties and potential for forming explosive compounds necessitate stringent safety protocols, specialized equipment, and highly trained personnel. These requirements can limit the widespread adoption of perchloric acid-based synthesis methods in both research and industrial settings.
Furthermore, the environmental impact of using perchloric acid in MOF synthesis is a growing concern. The disposal of perchlorate-containing waste and the potential for environmental contamination require careful consideration and management, adding complexity to the overall synthesis process.
Lastly, the characterization of MOFs synthesized using perchloric acid can be challenging. The presence of perchlorate ions or residual acid can interfere with common analytical techniques, making it difficult to accurately assess the structure, composition, and properties of the resulting materials. This complication can hinder the development and optimization of new MOF structures and applications.
The use of perchloric acid in MOF synthesis introduces additional complexities. While perchloric acid can serve as a powerful oxidizing agent and proton source, its incorporation often leads to unpredictable outcomes. The high reactivity of perchloric acid can result in uncontrolled reactions, potentially causing structural defects or even complete collapse of the MOF framework.
Another major challenge is maintaining the stability of MOFs during and after synthesis. The presence of perchloric acid can significantly affect the pH of the reaction medium, potentially leading to the degradation of organic ligands or the dissolution of metal nodes. This instability can compromise the structural integrity and porosity of the resulting MOFs, limiting their practical applications.
Reproducibility is a persistent issue in MOF synthesis, particularly when using strong acids like perchloric acid. Small variations in reaction conditions, such as temperature, concentration, or mixing rates, can lead to substantial differences in the final product. This lack of consistency poses significant hurdles for large-scale production and standardization of MOF materials.
The safety concerns associated with perchloric acid usage present another challenge. Its strong oxidizing properties and potential for forming explosive compounds necessitate stringent safety protocols, specialized equipment, and highly trained personnel. These requirements can limit the widespread adoption of perchloric acid-based synthesis methods in both research and industrial settings.
Furthermore, the environmental impact of using perchloric acid in MOF synthesis is a growing concern. The disposal of perchlorate-containing waste and the potential for environmental contamination require careful consideration and management, adding complexity to the overall synthesis process.
Lastly, the characterization of MOFs synthesized using perchloric acid can be challenging. The presence of perchlorate ions or residual acid can interfere with common analytical techniques, making it difficult to accurately assess the structure, composition, and properties of the resulting materials. This complication can hinder the development and optimization of new MOF structures and applications.
Current Perchloric Acid Use
01 Synthesis and structure of Metal Organic Frameworks
Metal Organic Frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands. The synthesis of MOFs involves various methods such as solvothermal, microwave-assisted, and mechanochemical techniques. The structure of MOFs can be tailored by selecting different metal nodes and organic linkers, resulting in diverse pore sizes and functionalities.- Synthesis and structure of Metal Organic Frameworks: This category focuses on the methods and techniques for synthesizing Metal Organic Frameworks (MOFs), including the selection of metal ions and organic ligands, as well as the control of reaction conditions to achieve desired structures and properties. It also covers the characterization of MOF structures using various analytical techniques.
- Applications of Metal Organic Frameworks in gas storage and separation: MOFs are utilized for gas storage and separation applications due to their high surface area and tunable pore sizes. This includes the use of MOFs for hydrogen storage, carbon dioxide capture, and the separation of gas mixtures. The development of MOFs with enhanced selectivity and capacity for specific gases is a key focus in this area.
- Metal Organic Frameworks for catalysis: MOFs are employed as catalysts or catalyst supports in various chemical reactions. Their large surface area, well-defined pore structure, and the ability to incorporate catalytically active sites make them attractive for heterogeneous catalysis. This includes their use in organic transformations, electrochemical reactions, and photocatalysis.
- Functionalization and modification of Metal Organic Frameworks: This category covers methods for modifying MOFs to enhance their properties or introduce new functionalities. This includes post-synthetic modification, incorporation of functional groups into organic linkers, and the development of composite materials combining MOFs with other materials such as polymers or nanoparticles.
- Metal Organic Frameworks for sensing and detection: MOFs are utilized in sensing and detection applications due to their ability to selectively interact with target molecules. This includes the development of MOF-based sensors for detecting gases, ions, and biomolecules. The focus is on improving sensitivity, selectivity, and response time of these sensors.
02 Applications of Metal Organic Frameworks
MOFs have a wide range of applications due to their high surface area and tunable properties. They are used in gas storage and separation, catalysis, drug delivery, and environmental remediation. MOFs can also be employed in sensors, energy storage devices, and as components in membranes for various separation processes.Expand Specific Solutions03 Functionalization and modification of Metal Organic Frameworks
MOFs can be functionalized or modified to enhance their properties and expand their applications. This can be achieved through post-synthetic modification, incorporation of functional groups in the organic linkers, or by creating composite materials. Such modifications can improve stability, selectivity, and performance in specific applications.Expand Specific Solutions04 Characterization techniques for Metal Organic Frameworks
Various analytical techniques are used to characterize MOFs, including X-ray diffraction, gas adsorption analysis, spectroscopic methods, and microscopy. These techniques help determine the crystal structure, surface area, pore size distribution, and chemical composition of MOFs. Advanced characterization methods can provide insights into the dynamic behavior and in-situ performance of MOFs.Expand Specific Solutions05 Scale-up and industrial production of Metal Organic Frameworks
The scale-up and industrial production of MOFs present challenges in maintaining consistent quality, reducing production costs, and optimizing synthesis conditions. Continuous flow synthesis, spray-drying, and other innovative manufacturing techniques are being developed to address these challenges and enable large-scale production of MOFs for commercial applications.Expand Specific Solutions
Key MOF Industry Players
The impact of perchloric acid on metal organic framework synthesis represents an emerging field in materials science, with the competitive landscape still in its early stages. The market size is relatively small but growing, driven by increasing research interest and potential applications in gas storage, catalysis, and sensing. The technology is in the developmental phase, with varying levels of maturity across different research groups and companies. Leading academic institutions like the University of California, Texas A&M University, and National University of Singapore are at the forefront of research, while industrial players such as BASF Corp. and ExxonMobil Technology & Engineering Co. are exploring commercial applications. The field is characterized by collaborative efforts between academia and industry, with a focus on optimizing synthesis methods and exploring novel MOF structures.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to metal-organic framework (MOF) synthesis using perchloric acid as a modulator. Their method involves the controlled addition of perchloric acid during the solvothermal synthesis process, which significantly enhances the crystallinity and porosity of the resulting MOFs [1]. This technique allows for the fine-tuning of pore size and surface area, leading to MOFs with improved gas storage and separation properties. BASF's research has shown that perchloric acid can accelerate the formation of metal-ligand bonds, resulting in more uniform and defect-free MOF structures [3]. Additionally, they have optimized the concentration of perchloric acid to achieve a balance between reaction rate and structural integrity, enabling the production of high-quality MOFs at industrial scales [5].
Strengths: Enhanced crystallinity and porosity, improved gas storage and separation properties, scalable production. Weaknesses: Potential safety concerns due to perchloric acid's oxidizing nature, increased production costs.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed a novel approach to MOF synthesis utilizing perchloric acid as a structure-directing agent. Their method involves the careful introduction of perchloric acid during the initial stages of MOF formation, which promotes the growth of specific crystal facets and enhances overall structural stability [2]. This technique has been particularly effective in creating MOFs with improved thermal and chemical resistance, making them suitable for applications in harsh industrial environments. ExxonMobil's research has demonstrated that perchloric acid can facilitate the formation of stronger metal-ligand coordination bonds, resulting in MOFs with enhanced catalytic properties [4]. Furthermore, they have optimized the synthesis conditions to achieve a balance between reaction kinetics and product quality, enabling the production of high-performance MOFs for various applications, including gas separation and heterogeneous catalysis [6].
Strengths: Improved thermal and chemical stability, enhanced catalytic properties, versatile applications. Weaknesses: Potential environmental concerns, higher production costs due to specialized handling of perchloric acid.
Perchloric Acid Innovations
Metal organic frameworks having node defects and methods of making the same
PatentWO2023278246A1
Innovation
- The synthesis of metal-organic frameworks with tetravalent cations and terephthalate linkers in a primitive cubic lattice, using a process involving a first metal source, a polytopic organic carboxylic acid, and a second metal source, along with monocarboxylic acids, to create a reaction solution that is heated, resulting in frameworks with controlled node defects and enhanced porosity and surface area.
Light activatable polypyrrolic metal organic framework composites and applications thereof
PatentActiveIN202011028994A
Innovation
- Development of polypyrrole-based metal-organic framework (MOF) composites through conjugation or encapsulation of porphyrins and BODIPYs onto MOFs like UIO-66-NH2, MIL-101-NH2, and MIL-53-NH2, utilizing O/W/O emulsion templating for scalable synthesis of microspheres and nanostructures, enhancing recyclability and wavelength-specific activation.
Safety Regulations
The use of perchloric acid in Metal Organic Framework (MOF) synthesis necessitates stringent safety regulations due to its highly reactive and potentially explosive nature. Laboratories and industrial facilities working with perchloric acid must adhere to strict guidelines to ensure the safety of personnel and prevent accidents.
Personal protective equipment (PPE) is paramount when handling perchloric acid. Workers must wear chemical-resistant gloves, safety goggles, face shields, and acid-resistant aprons or suits. Respiratory protection may also be required, depending on the concentration and volume of perchloric acid being used.
Storage and handling regulations for perchloric acid are particularly crucial. The acid must be stored in dedicated, well-ventilated areas away from organic materials, reducing agents, and other incompatible substances. Containers should be made of appropriate materials, such as glass or certain plastics, and must be properly labeled with hazard warnings.
Workplace design and engineering controls play a significant role in safety regulations. Fume hoods used for perchloric acid work must be specially designed with wash-down systems to prevent the accumulation of explosive perchlorates. These hoods should be constructed of acid-resistant materials and equipped with dedicated exhaust systems.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment equipment. Personnel must be trained in proper spill cleanup techniques and the use of neutralizing agents specific to perchloric acid.
Waste disposal regulations for perchloric acid and its byproducts are stringent. Neutralization and dilution procedures must be followed carefully, and disposal should only be carried out by qualified personnel or specialized waste management services.
Regular safety audits and inspections are mandated to ensure compliance with regulations. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training and education form a critical component of safety regulations. All personnel working with perchloric acid must undergo comprehensive training on its properties, hazards, safe handling procedures, and emergency response protocols. Refresher courses should be conducted periodically to maintain awareness and update knowledge on any new safety guidelines.
Documentation and record-keeping are essential aspects of regulatory compliance. Detailed logs of perchloric acid usage, storage conditions, and any incidents or near-misses must be maintained. These records are crucial for regulatory inspections and for continuously improving safety protocols.
Personal protective equipment (PPE) is paramount when handling perchloric acid. Workers must wear chemical-resistant gloves, safety goggles, face shields, and acid-resistant aprons or suits. Respiratory protection may also be required, depending on the concentration and volume of perchloric acid being used.
Storage and handling regulations for perchloric acid are particularly crucial. The acid must be stored in dedicated, well-ventilated areas away from organic materials, reducing agents, and other incompatible substances. Containers should be made of appropriate materials, such as glass or certain plastics, and must be properly labeled with hazard warnings.
Workplace design and engineering controls play a significant role in safety regulations. Fume hoods used for perchloric acid work must be specially designed with wash-down systems to prevent the accumulation of explosive perchlorates. These hoods should be constructed of acid-resistant materials and equipped with dedicated exhaust systems.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment equipment. Personnel must be trained in proper spill cleanup techniques and the use of neutralizing agents specific to perchloric acid.
Waste disposal regulations for perchloric acid and its byproducts are stringent. Neutralization and dilution procedures must be followed carefully, and disposal should only be carried out by qualified personnel or specialized waste management services.
Regular safety audits and inspections are mandated to ensure compliance with regulations. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
Training and education form a critical component of safety regulations. All personnel working with perchloric acid must undergo comprehensive training on its properties, hazards, safe handling procedures, and emergency response protocols. Refresher courses should be conducted periodically to maintain awareness and update knowledge on any new safety guidelines.
Documentation and record-keeping are essential aspects of regulatory compliance. Detailed logs of perchloric acid usage, storage conditions, and any incidents or near-misses must be maintained. These records are crucial for regulatory inspections and for continuously improving safety protocols.
Environmental Impact
The use of perchloric acid in metal organic framework (MOF) synthesis raises significant environmental concerns that warrant careful consideration. The production, handling, and disposal of perchloric acid can have substantial impacts on ecosystems and human health if not managed properly.
Perchloric acid is a strong oxidizing agent and can react violently with organic compounds, posing risks of fire and explosion. Its production process often involves the use of chlorine and other hazardous chemicals, which can lead to air and water pollution if not adequately controlled. Emissions from perchloric acid manufacturing facilities may contribute to acid rain and smog formation, affecting local air quality and potentially harming vegetation and aquatic life.
In the context of MOF synthesis, the use of perchloric acid generates acidic waste streams that require specialized treatment before disposal. If not properly neutralized, these wastes can alter the pH of aquatic environments, leading to detrimental effects on fish, plants, and microorganisms. Additionally, perchlorate ions, which are byproducts of perchloric acid use, are known to persist in the environment and can contaminate groundwater sources.
The potential for perchlorate contamination is particularly concerning due to its ability to interfere with iodine uptake in the thyroid gland, potentially affecting hormonal balance in humans and wildlife. Studies have shown that even low levels of perchlorate exposure can impact the development of aquatic organisms and terrestrial animals, particularly during early life stages.
From a lifecycle perspective, the environmental footprint of perchloric acid extends beyond its immediate use in MOF synthesis. The energy-intensive production process contributes to greenhouse gas emissions, while transportation and storage of this hazardous material pose risks of accidental releases during handling and distribution.
To mitigate these environmental impacts, researchers and industries are exploring alternative synthesis methods that reduce or eliminate the need for perchloric acid in MOF production. Green chemistry approaches, such as using less hazardous solvents or developing solvent-free synthesis techniques, are gaining traction. Additionally, improved waste treatment technologies and stricter regulations on perchloric acid handling and disposal are being implemented to minimize environmental risks.
As the field of MOF synthesis continues to evolve, it is crucial to balance the pursuit of novel materials with environmental stewardship. Ongoing research into sustainable synthesis methods and life cycle assessments of MOF production processes will be essential in developing more environmentally friendly approaches to these promising materials.
Perchloric acid is a strong oxidizing agent and can react violently with organic compounds, posing risks of fire and explosion. Its production process often involves the use of chlorine and other hazardous chemicals, which can lead to air and water pollution if not adequately controlled. Emissions from perchloric acid manufacturing facilities may contribute to acid rain and smog formation, affecting local air quality and potentially harming vegetation and aquatic life.
In the context of MOF synthesis, the use of perchloric acid generates acidic waste streams that require specialized treatment before disposal. If not properly neutralized, these wastes can alter the pH of aquatic environments, leading to detrimental effects on fish, plants, and microorganisms. Additionally, perchlorate ions, which are byproducts of perchloric acid use, are known to persist in the environment and can contaminate groundwater sources.
The potential for perchlorate contamination is particularly concerning due to its ability to interfere with iodine uptake in the thyroid gland, potentially affecting hormonal balance in humans and wildlife. Studies have shown that even low levels of perchlorate exposure can impact the development of aquatic organisms and terrestrial animals, particularly during early life stages.
From a lifecycle perspective, the environmental footprint of perchloric acid extends beyond its immediate use in MOF synthesis. The energy-intensive production process contributes to greenhouse gas emissions, while transportation and storage of this hazardous material pose risks of accidental releases during handling and distribution.
To mitigate these environmental impacts, researchers and industries are exploring alternative synthesis methods that reduce or eliminate the need for perchloric acid in MOF production. Green chemistry approaches, such as using less hazardous solvents or developing solvent-free synthesis techniques, are gaining traction. Additionally, improved waste treatment technologies and stricter regulations on perchloric acid handling and disposal are being implemented to minimize environmental risks.
As the field of MOF synthesis continues to evolve, it is crucial to balance the pursuit of novel materials with environmental stewardship. Ongoing research into sustainable synthesis methods and life cycle assessments of MOF production processes will be essential in developing more environmentally friendly approaches to these promising materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!