The Interaction of Perchloric Acid with Semiconducting Materials
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid-Semiconductor Interaction Background
The interaction between perchloric acid and semiconducting materials has been a subject of significant interest in the field of materials science and electronics for several decades. Perchloric acid, a strong oxidizing agent, has unique properties that make it both useful and challenging when interacting with semiconductors. This interaction has implications for various applications, including etching processes, surface modification, and the development of novel electronic devices.
Historically, the study of perchloric acid-semiconductor interactions began in the mid-20th century as the semiconductor industry started to grow rapidly. Early research focused primarily on the etching capabilities of perchloric acid on silicon and germanium, the two most common semiconductor materials at the time. As the field progressed, researchers expanded their investigations to include compound semiconductors such as gallium arsenide and indium phosphide.
The interaction between perchloric acid and semiconductors is primarily characterized by its oxidizing nature. When perchloric acid comes into contact with a semiconductor surface, it can initiate a series of redox reactions. These reactions can lead to the formation of oxide layers, the dissolution of the semiconductor material, or the modification of surface properties. The specific outcomes depend on factors such as the concentration of the acid, the temperature, and the crystallographic orientation of the semiconductor.
One of the key aspects of this interaction is the formation of surface oxides. In the case of silicon, for example, perchloric acid can promote the growth of a thin layer of silicon dioxide. This oxide layer can significantly alter the electrical and chemical properties of the semiconductor surface, which has implications for device fabrication and performance. The controlled growth of these oxide layers has been exploited in various applications, including the development of insulating barriers in electronic devices.
The etching capabilities of perchloric acid on semiconductors have been extensively studied and utilized in the manufacturing of electronic components. The acid's ability to selectively etch certain crystallographic planes of semiconductors has made it valuable in creating specific surface structures and patterns. This property has been particularly useful in the fabrication of microelectromechanical systems (MEMS) and in the preparation of samples for electron microscopy.
As research in this area has progressed, scientists have discovered that the interaction between perchloric acid and semiconductors can be modulated by various factors. The presence of light, for instance, can significantly affect the reaction kinetics and outcomes. This photochemical aspect has opened up new avenues for controlled surface modification and has been explored for potential applications in photovoltaics and optoelectronics.
Historically, the study of perchloric acid-semiconductor interactions began in the mid-20th century as the semiconductor industry started to grow rapidly. Early research focused primarily on the etching capabilities of perchloric acid on silicon and germanium, the two most common semiconductor materials at the time. As the field progressed, researchers expanded their investigations to include compound semiconductors such as gallium arsenide and indium phosphide.
The interaction between perchloric acid and semiconductors is primarily characterized by its oxidizing nature. When perchloric acid comes into contact with a semiconductor surface, it can initiate a series of redox reactions. These reactions can lead to the formation of oxide layers, the dissolution of the semiconductor material, or the modification of surface properties. The specific outcomes depend on factors such as the concentration of the acid, the temperature, and the crystallographic orientation of the semiconductor.
One of the key aspects of this interaction is the formation of surface oxides. In the case of silicon, for example, perchloric acid can promote the growth of a thin layer of silicon dioxide. This oxide layer can significantly alter the electrical and chemical properties of the semiconductor surface, which has implications for device fabrication and performance. The controlled growth of these oxide layers has been exploited in various applications, including the development of insulating barriers in electronic devices.
The etching capabilities of perchloric acid on semiconductors have been extensively studied and utilized in the manufacturing of electronic components. The acid's ability to selectively etch certain crystallographic planes of semiconductors has made it valuable in creating specific surface structures and patterns. This property has been particularly useful in the fabrication of microelectromechanical systems (MEMS) and in the preparation of samples for electron microscopy.
As research in this area has progressed, scientists have discovered that the interaction between perchloric acid and semiconductors can be modulated by various factors. The presence of light, for instance, can significantly affect the reaction kinetics and outcomes. This photochemical aspect has opened up new avenues for controlled surface modification and has been explored for potential applications in photovoltaics and optoelectronics.
Market Analysis for Semiconductor Etching Solutions
The semiconductor etching solutions market has experienced significant growth in recent years, driven by the increasing demand for advanced semiconductor devices across various industries. The global market for semiconductor etching solutions is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the industry's robust expansion.
The demand for semiconductor etching solutions is primarily fueled by the rapid advancement of technologies such as 5G, artificial intelligence, Internet of Things (IoT), and autonomous vehicles. These technologies require increasingly sophisticated and miniaturized semiconductor components, which in turn necessitate more advanced etching processes. The trend towards smaller node sizes and 3D chip architectures has further intensified the need for precise and efficient etching solutions.
Perchloric acid, as a key component in certain semiconductor etching processes, plays a crucial role in this market. Its unique properties make it particularly effective for etching specific semiconductor materials, especially in applications requiring high selectivity and precision. The interaction of perchloric acid with semiconducting materials has become a focal point for research and development in the industry, as manufacturers seek to optimize etching processes for next-generation devices.
Geographically, the semiconductor etching solutions market is dominated by the Asia-Pacific region, with countries like Taiwan, South Korea, and China leading in semiconductor manufacturing. North America and Europe also hold significant market shares, driven by their advanced research capabilities and presence of major semiconductor companies.
The market is characterized by intense competition among key players, including major chemical companies and specialized semiconductor material suppliers. These companies are investing heavily in research and development to create innovative etching solutions that can meet the evolving requirements of semiconductor manufacturers.
Environmental and safety concerns associated with the use of aggressive chemicals like perchloric acid are influencing market dynamics. There is a growing trend towards the development of more environmentally friendly etching solutions, which could potentially impact the market share of traditional acid-based etchants.
Looking ahead, the semiconductor etching solutions market is expected to continue its growth trajectory, driven by ongoing technological advancements in the semiconductor industry. The increasing complexity of semiconductor devices and the push towards more efficient manufacturing processes will likely sustain the demand for advanced etching solutions, including those involving perchloric acid and other specialized chemicals.
The demand for semiconductor etching solutions is primarily fueled by the rapid advancement of technologies such as 5G, artificial intelligence, Internet of Things (IoT), and autonomous vehicles. These technologies require increasingly sophisticated and miniaturized semiconductor components, which in turn necessitate more advanced etching processes. The trend towards smaller node sizes and 3D chip architectures has further intensified the need for precise and efficient etching solutions.
Perchloric acid, as a key component in certain semiconductor etching processes, plays a crucial role in this market. Its unique properties make it particularly effective for etching specific semiconductor materials, especially in applications requiring high selectivity and precision. The interaction of perchloric acid with semiconducting materials has become a focal point for research and development in the industry, as manufacturers seek to optimize etching processes for next-generation devices.
Geographically, the semiconductor etching solutions market is dominated by the Asia-Pacific region, with countries like Taiwan, South Korea, and China leading in semiconductor manufacturing. North America and Europe also hold significant market shares, driven by their advanced research capabilities and presence of major semiconductor companies.
The market is characterized by intense competition among key players, including major chemical companies and specialized semiconductor material suppliers. These companies are investing heavily in research and development to create innovative etching solutions that can meet the evolving requirements of semiconductor manufacturers.
Environmental and safety concerns associated with the use of aggressive chemicals like perchloric acid are influencing market dynamics. There is a growing trend towards the development of more environmentally friendly etching solutions, which could potentially impact the market share of traditional acid-based etchants.
Looking ahead, the semiconductor etching solutions market is expected to continue its growth trajectory, driven by ongoing technological advancements in the semiconductor industry. The increasing complexity of semiconductor devices and the push towards more efficient manufacturing processes will likely sustain the demand for advanced etching solutions, including those involving perchloric acid and other specialized chemicals.
Current Challenges in Perchloric Acid Semiconductor Processing
The semiconductor industry faces several critical challenges in the processing of materials using perchloric acid. One of the primary issues is the highly corrosive nature of perchloric acid, which can lead to rapid degradation of equipment and potential safety hazards. This corrosiveness necessitates the use of specialized materials and protective measures, significantly increasing production costs and complexity.
Another major challenge is the precise control of etching rates and uniformity when using perchloric acid in semiconductor processing. The acid's strong oxidizing properties can result in non-uniform etching across wafer surfaces, leading to inconsistencies in device performance and yield issues. Achieving the required level of precision and repeatability in etching processes remains a significant hurdle for manufacturers.
The disposal and environmental impact of perchloric acid waste present additional challenges. Strict regulations govern the handling and disposal of perchloric acid due to its potential environmental hazards. Developing effective and economical waste treatment methods that comply with increasingly stringent environmental standards is an ongoing concern for the industry.
Furthermore, the interaction between perchloric acid and various semiconductor materials can lead to unexpected chemical reactions or the formation of unwanted byproducts. These interactions may alter the electrical properties of the semiconducting materials, potentially compromising device performance. Understanding and mitigating these complex chemical interactions require extensive research and process optimization.
The volatility of perchloric acid at elevated temperatures poses significant safety risks in semiconductor processing environments. Maintaining stable and safe operating conditions while achieving the desired etching or cleaning results demands sophisticated process control systems and robust safety protocols.
Lastly, the semiconductor industry faces challenges in scaling perchloric acid-based processes for larger wafer sizes and more complex device architectures. As the industry moves towards advanced nodes and three-dimensional structures, adapting perchloric acid processes to meet these new requirements without compromising efficiency or yield becomes increasingly difficult.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, chemical engineering, and process control technologies. Ongoing research efforts focus on developing alternative etching chemistries, improving equipment design, and enhancing process monitoring and control systems to overcome the limitations associated with perchloric acid in semiconductor processing.
Another major challenge is the precise control of etching rates and uniformity when using perchloric acid in semiconductor processing. The acid's strong oxidizing properties can result in non-uniform etching across wafer surfaces, leading to inconsistencies in device performance and yield issues. Achieving the required level of precision and repeatability in etching processes remains a significant hurdle for manufacturers.
The disposal and environmental impact of perchloric acid waste present additional challenges. Strict regulations govern the handling and disposal of perchloric acid due to its potential environmental hazards. Developing effective and economical waste treatment methods that comply with increasingly stringent environmental standards is an ongoing concern for the industry.
Furthermore, the interaction between perchloric acid and various semiconductor materials can lead to unexpected chemical reactions or the formation of unwanted byproducts. These interactions may alter the electrical properties of the semiconducting materials, potentially compromising device performance. Understanding and mitigating these complex chemical interactions require extensive research and process optimization.
The volatility of perchloric acid at elevated temperatures poses significant safety risks in semiconductor processing environments. Maintaining stable and safe operating conditions while achieving the desired etching or cleaning results demands sophisticated process control systems and robust safety protocols.
Lastly, the semiconductor industry faces challenges in scaling perchloric acid-based processes for larger wafer sizes and more complex device architectures. As the industry moves towards advanced nodes and three-dimensional structures, adapting perchloric acid processes to meet these new requirements without compromising efficiency or yield becomes increasingly difficult.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, chemical engineering, and process control technologies. Ongoing research efforts focus on developing alternative etching chemistries, improving equipment design, and enhancing process monitoring and control systems to overcome the limitations associated with perchloric acid in semiconductor processing.
Existing Perchloric Acid Etching Methodologies
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in chemical reactions, as well as its role in sample preparation and digestion for analytical chemistry applications.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.
- Perchloric acid in battery technology: The use of perchloric acid in the development and improvement of battery technologies. This may involve its application in electrolyte formulations, electrode materials, or other components to enhance battery performance, capacity, or longevity.
- Perchloric acid in material processing: Applications of perchloric acid in various material processing techniques, such as etching, surface treatment, or purification of materials. This includes its use in semiconductor manufacturing, metal processing, and other industrial applications where its strong oxidizing properties are beneficial.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in chemical reactions, as well as its role in sample preparation and digestion for analytical chemistry applications.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.Expand Specific Solutions04 Perchloric acid in battery technology
Use of perchloric acid in the development and improvement of battery technologies. This may involve its application in electrolyte formulations, electrode materials, or other components to enhance battery performance, capacity, or longevity.Expand Specific Solutions05 Purification and concentration of perchloric acid
Techniques and processes for purifying and concentrating perchloric acid to meet specific industrial or laboratory requirements. This may include distillation methods, membrane separation, or other advanced purification technologies to achieve high-purity perchloric acid.Expand Specific Solutions
Key Players in Semiconductor Chemical Industry
The interaction of perchloric acid with semiconducting materials represents a niche but critical area in the semiconductor industry. The market is currently in a growth phase, driven by increasing demand for advanced electronic devices. While the global semiconductor market is valued at hundreds of billions of dollars, this specific application likely constitutes a smaller, specialized segment. Companies like Renesas Electronics, Samsung Electronics, and SMIC are at the forefront of semiconductor technology, potentially leading research and development in this area. The technology's maturity varies, with established players having more advanced capabilities, while newer entrants are still developing their expertise. As the industry evolves, collaboration between academic institutions like Zhejiang University and companies may accelerate technological advancements in this field.
Semiconductor Manufacturing International (Shanghai) Corp.
Technical Solution: SMIC has developed a proprietary etching process using perchloric acid for advanced semiconductor manufacturing. Their approach focuses on optimizing the etching uniformity and selectivity for various materials used in their fabrication processes[4]. SMIC has implemented a controlled dilution system to maintain precise perchloric acid concentrations throughout the etching process, ensuring consistent results across wafers[5]. The company has also invested in advanced waste treatment facilities to handle the byproducts of perchloric acid etching, aligning with environmental regulations[6].
Strengths: In-house expertise in integrating perchloric acid etching into semiconductor manufacturing processes, and advanced waste treatment capabilities. Weaknesses: Potential limitations in scaling the process for larger wafer sizes and adapting to new materials.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a novel approach to using perchloric acid in semiconductor processing, focusing on its application in advanced memory technologies. Their method involves a carefully controlled etching process that utilizes perchloric acid in combination with other chemicals to achieve high-precision patterning for 3D NAND and DRAM structures[7]. Samsung has also explored the use of perchloric acid in cleaning processes to remove metallic contaminants from semiconductor surfaces without damaging the underlying materials[8]. Additionally, the company has invested in advanced automation and monitoring systems to ensure safe handling and precise control of perchloric acid throughout their manufacturing facilities[9].
Strengths: Cutting-edge research in applying perchloric acid to advanced memory technologies, strong focus on safety and automation. Weaknesses: Potential challenges in adapting the process to non-memory semiconductor applications.
Core Innovations in Perchloric Acid-Semiconductor Interactions
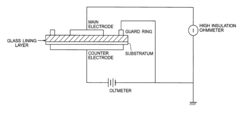
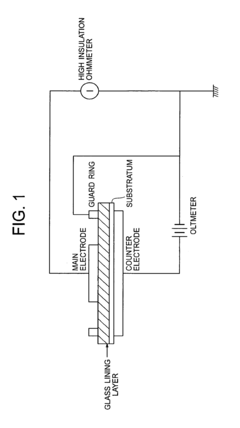
Cover coating composition for glass lining
PatentActiveUS20120118202A1
Innovation
- A cover coating composition for glass linings is developed, comprising 65-75 mol% SiO2, 2-8 mol% ZrO2, 10-22 mol% R2O (where R is Li, K, or Cs), and 2-12 mol% R'O (where R' is Mg, Ca, or Ba), without Na2O, along with optional additives like TiO2, Al2O3, and metal fibers, to create a durable and sodium-free enamel layer.
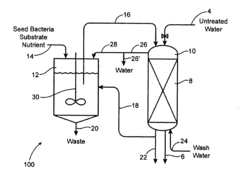
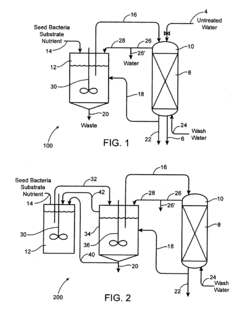
Bio degradation of oxyanions such as perchlorate on ion exchange resins
PatentInactiveUS20090092584A1
Innovation
- Contacting perchlorate-loaded ion exchange resins with a perchlorate-destroying microorganism fluid product under anaerobic conditions to convert perchlorate to nonperchlorate species, such as chlorate, chlorite, hypochlorite, and chloride, reducing the resin's perchlorate load and allowing for safe reuse or disposal.
Safety Protocols for Handling Perchloric Acid
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature, especially when interacting with semiconducting materials. Proper personal protective equipment (PPE) is essential, including chemical-resistant gloves, safety goggles, and a lab coat. A face shield should be worn when handling large quantities. All work with perchloric acid must be conducted in a designated fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Before beginning any procedure, a thorough risk assessment should be conducted, and all personnel involved must be trained in the specific hazards and emergency procedures associated with perchloric acid. The work area should be free from organic materials, as perchloric acid can form explosive compounds when in contact with these substances. Dedicated glassware and equipment should be used exclusively for perchloric acid work and must be thoroughly cleaned after each use.
Storage of perchloric acid requires special consideration. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, and secondary containment should be used to prevent spills. Regular inspections of storage areas are necessary to detect any signs of degradation or leakage.
When working with semiconducting materials, additional precautions are necessary. The interaction between perchloric acid and semiconductors can produce volatile compounds or generate heat. Therefore, the reaction should be carried out in small quantities and closely monitored for temperature changes. A spill response kit specifically designed for perchloric acid should be readily available in the laboratory.
Waste disposal is a critical aspect of perchloric acid safety protocols. Neutralization should only be performed by trained personnel using appropriate methods. Dilution with water is generally the first step, followed by neutralization with a suitable base. The resulting waste must be collected separately and disposed of through a certified chemical waste management service.
Emergency procedures must be clearly defined and communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and fire extinguishers. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is crucial. Any exposure incident should be reported and medical attention sought promptly.
Regular safety audits and refresher training sessions should be conducted to ensure compliance with safety protocols and to address any new hazards or procedures. Documentation of all safety measures, including standard operating procedures (SOPs), safety data sheets (SDS), and incident reports, must be maintained and readily accessible to all laboratory personnel.
Before beginning any procedure, a thorough risk assessment should be conducted, and all personnel involved must be trained in the specific hazards and emergency procedures associated with perchloric acid. The work area should be free from organic materials, as perchloric acid can form explosive compounds when in contact with these substances. Dedicated glassware and equipment should be used exclusively for perchloric acid work and must be thoroughly cleaned after each use.
Storage of perchloric acid requires special consideration. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, and secondary containment should be used to prevent spills. Regular inspections of storage areas are necessary to detect any signs of degradation or leakage.
When working with semiconducting materials, additional precautions are necessary. The interaction between perchloric acid and semiconductors can produce volatile compounds or generate heat. Therefore, the reaction should be carried out in small quantities and closely monitored for temperature changes. A spill response kit specifically designed for perchloric acid should be readily available in the laboratory.
Waste disposal is a critical aspect of perchloric acid safety protocols. Neutralization should only be performed by trained personnel using appropriate methods. Dilution with water is generally the first step, followed by neutralization with a suitable base. The resulting waste must be collected separately and disposed of through a certified chemical waste management service.
Emergency procedures must be clearly defined and communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and fire extinguishers. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is crucial. Any exposure incident should be reported and medical attention sought promptly.
Regular safety audits and refresher training sessions should be conducted to ensure compliance with safety protocols and to address any new hazards or procedures. Documentation of all safety measures, including standard operating procedures (SOPs), safety data sheets (SDS), and incident reports, must be maintained and readily accessible to all laboratory personnel.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in semiconductor manufacturing processes raises significant environmental concerns due to its potential impact on ecosystems and human health. When released into the environment, perchloric acid can contaminate soil and water sources, leading to long-term ecological damage. The high oxidizing power of perchloric acid can disrupt natural chemical balances in aquatic systems, affecting pH levels and potentially harming aquatic life.
One of the primary environmental risks associated with perchloric acid is its persistence in groundwater. Unlike many other contaminants, perchlorate ions (derived from perchloric acid) are highly soluble and mobile in water, making them difficult to remove once they enter the water table. This persistence can lead to long-term contamination of drinking water sources, posing potential health risks to human populations.
In areas where semiconductor manufacturing facilities are located, there is an increased risk of perchlorate contamination in surrounding soil and water bodies. Studies have shown that perchlorate can accumulate in plants, potentially entering the food chain and affecting both wildlife and human consumers. This bioaccumulation effect amplifies the environmental impact, as even small releases of perchloric acid can lead to significant concentrations in biological systems over time.
The atmospheric release of perchloric acid vapors during semiconductor processing can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Additionally, the deposition of these airborne compounds can lead to soil and surface water contamination far from the original source.
To mitigate these environmental risks, stringent regulations and waste management practices are essential in the semiconductor industry. Advanced treatment technologies, such as ion exchange and biological reduction processes, are being developed and implemented to remove perchlorate from wastewater and contaminated groundwater. However, the effectiveness of these treatments varies, and complete removal of perchlorate remains challenging.
The semiconductor industry is increasingly focusing on developing alternative processes that reduce or eliminate the use of perchloric acid. This includes exploring less hazardous oxidizing agents and implementing closed-loop systems to minimize waste generation. Additionally, improved containment and handling procedures are being adopted to prevent accidental releases and reduce the overall environmental footprint of semiconductor manufacturing processes.
One of the primary environmental risks associated with perchloric acid is its persistence in groundwater. Unlike many other contaminants, perchlorate ions (derived from perchloric acid) are highly soluble and mobile in water, making them difficult to remove once they enter the water table. This persistence can lead to long-term contamination of drinking water sources, posing potential health risks to human populations.
In areas where semiconductor manufacturing facilities are located, there is an increased risk of perchlorate contamination in surrounding soil and water bodies. Studies have shown that perchlorate can accumulate in plants, potentially entering the food chain and affecting both wildlife and human consumers. This bioaccumulation effect amplifies the environmental impact, as even small releases of perchloric acid can lead to significant concentrations in biological systems over time.
The atmospheric release of perchloric acid vapors during semiconductor processing can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Additionally, the deposition of these airborne compounds can lead to soil and surface water contamination far from the original source.
To mitigate these environmental risks, stringent regulations and waste management practices are essential in the semiconductor industry. Advanced treatment technologies, such as ion exchange and biological reduction processes, are being developed and implemented to remove perchlorate from wastewater and contaminated groundwater. However, the effectiveness of these treatments varies, and complete removal of perchlorate remains challenging.
The semiconductor industry is increasingly focusing on developing alternative processes that reduce or eliminate the use of perchloric acid. This includes exploring less hazardous oxidizing agents and implementing closed-loop systems to minimize waste generation. Additionally, improved containment and handling procedures are being adopted to prevent accidental releases and reduce the overall environmental footprint of semiconductor manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!