The Role of Perchloric Acid in Graphene Hydrogels' Synthesis
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphene Hydrogel Synthesis Background
Graphene hydrogels have emerged as a promising class of materials in recent years, combining the exceptional properties of graphene with the versatility and functionality of hydrogels. The synthesis of these advanced materials has been a subject of intense research due to their potential applications in various fields, including energy storage, environmental remediation, and biomedical engineering.
The development of graphene hydrogels can be traced back to the discovery of graphene itself in 2004 by Andre Geim and Konstantin Novoselov. This groundbreaking work opened up new avenues for material science and led to the exploration of graphene-based three-dimensional structures. The concept of graphene hydrogels was first introduced in the late 2000s as researchers sought to harness the unique properties of graphene in a three-dimensional, porous network.
Initially, the synthesis of graphene hydrogels faced several challenges, including the difficulty in achieving uniform dispersion of graphene sheets and maintaining structural integrity. Early methods often resulted in weak mechanical properties and limited functionality. However, as research progressed, various techniques were developed to overcome these limitations, including chemical reduction, hydrothermal treatment, and self-assembly approaches.
One of the key breakthroughs in graphene hydrogel synthesis came with the introduction of chemical crosslinking agents. These agents helped to create stronger interconnections between graphene sheets, resulting in improved mechanical strength and stability. Among the various crosslinking agents explored, perchloric acid emerged as a particularly effective option due to its strong oxidizing properties and ability to form stable bonds with graphene oxide.
The role of perchloric acid in graphene hydrogel synthesis became a focal point of research in the 2010s. Its unique ability to simultaneously reduce graphene oxide and promote crosslinking led to the development of more efficient and scalable synthesis methods. This advancement significantly improved the structural integrity and functional properties of graphene hydrogels, making them more suitable for practical applications.
As the field progressed, researchers began to explore the synergistic effects of combining perchloric acid with other additives and processing techniques. This led to the development of hybrid graphene hydrogels with enhanced properties, such as improved electrical conductivity, higher surface area, and better mechanical strength. The versatility of perchloric acid in graphene hydrogel synthesis has enabled the creation of tailored materials for specific applications, ranging from supercapacitors to tissue engineering scaffolds.
The evolution of graphene hydrogel synthesis, particularly with the incorporation of perchloric acid, has set the stage for further advancements in this field. Current research aims to optimize synthesis conditions, explore new precursor materials, and develop novel functionalization strategies to expand the potential applications of these remarkable materials.
The development of graphene hydrogels can be traced back to the discovery of graphene itself in 2004 by Andre Geim and Konstantin Novoselov. This groundbreaking work opened up new avenues for material science and led to the exploration of graphene-based three-dimensional structures. The concept of graphene hydrogels was first introduced in the late 2000s as researchers sought to harness the unique properties of graphene in a three-dimensional, porous network.
Initially, the synthesis of graphene hydrogels faced several challenges, including the difficulty in achieving uniform dispersion of graphene sheets and maintaining structural integrity. Early methods often resulted in weak mechanical properties and limited functionality. However, as research progressed, various techniques were developed to overcome these limitations, including chemical reduction, hydrothermal treatment, and self-assembly approaches.
One of the key breakthroughs in graphene hydrogel synthesis came with the introduction of chemical crosslinking agents. These agents helped to create stronger interconnections between graphene sheets, resulting in improved mechanical strength and stability. Among the various crosslinking agents explored, perchloric acid emerged as a particularly effective option due to its strong oxidizing properties and ability to form stable bonds with graphene oxide.
The role of perchloric acid in graphene hydrogel synthesis became a focal point of research in the 2010s. Its unique ability to simultaneously reduce graphene oxide and promote crosslinking led to the development of more efficient and scalable synthesis methods. This advancement significantly improved the structural integrity and functional properties of graphene hydrogels, making them more suitable for practical applications.
As the field progressed, researchers began to explore the synergistic effects of combining perchloric acid with other additives and processing techniques. This led to the development of hybrid graphene hydrogels with enhanced properties, such as improved electrical conductivity, higher surface area, and better mechanical strength. The versatility of perchloric acid in graphene hydrogel synthesis has enabled the creation of tailored materials for specific applications, ranging from supercapacitors to tissue engineering scaffolds.
The evolution of graphene hydrogel synthesis, particularly with the incorporation of perchloric acid, has set the stage for further advancements in this field. Current research aims to optimize synthesis conditions, explore new precursor materials, and develop novel functionalization strategies to expand the potential applications of these remarkable materials.
Market Demand Analysis
The market demand for graphene hydrogels synthesized using perchloric acid has been steadily growing, driven by their unique properties and diverse applications across multiple industries. These advanced materials exhibit exceptional mechanical strength, electrical conductivity, and high surface area, making them attractive for various high-tech applications.
In the energy storage sector, graphene hydrogels are gaining significant traction for use in supercapacitors and batteries. The global supercapacitor market is projected to reach substantial growth in the coming years, with graphene-based materials playing a crucial role in enhancing energy density and charge-discharge cycles. This trend is further fueled by the increasing demand for electric vehicles and renewable energy storage solutions.
The biomedical field represents another key market for graphene hydrogels. Their biocompatibility and tunable properties make them ideal candidates for drug delivery systems, tissue engineering scaffolds, and biosensors. The global biomaterials market, which includes advanced hydrogels, is experiencing robust growth, driven by an aging population and the need for innovative medical solutions.
Environmental applications, particularly water purification and environmental sensing, are emerging as significant market opportunities for graphene hydrogels. The global water treatment market is expanding rapidly, with a growing emphasis on advanced materials for contaminant removal and water quality monitoring. Graphene hydrogels' high adsorption capacity and selectivity position them as promising materials in this sector.
The electronics industry is also showing increased interest in graphene hydrogels for flexible electronics, wearable devices, and sensors. As the Internet of Things (IoT) continues to expand, the demand for lightweight, flexible, and highly conductive materials is expected to surge, creating new opportunities for graphene-based materials.
Despite the promising market outlook, challenges remain in scaling up production and reducing costs. The synthesis process using perchloric acid, while effective, requires optimization for large-scale manufacturing. This presents both a challenge and an opportunity for innovation in production techniques.
Overall, the market demand for graphene hydrogels synthesized with perchloric acid is poised for significant growth across multiple sectors. The unique properties offered by these materials align well with the evolving needs of various industries, from energy and healthcare to environmental protection and electronics. As research and development efforts continue to advance, addressing scalability and cost-effectiveness will be crucial in fully realizing the market potential of these innovative materials.
In the energy storage sector, graphene hydrogels are gaining significant traction for use in supercapacitors and batteries. The global supercapacitor market is projected to reach substantial growth in the coming years, with graphene-based materials playing a crucial role in enhancing energy density and charge-discharge cycles. This trend is further fueled by the increasing demand for electric vehicles and renewable energy storage solutions.
The biomedical field represents another key market for graphene hydrogels. Their biocompatibility and tunable properties make them ideal candidates for drug delivery systems, tissue engineering scaffolds, and biosensors. The global biomaterials market, which includes advanced hydrogels, is experiencing robust growth, driven by an aging population and the need for innovative medical solutions.
Environmental applications, particularly water purification and environmental sensing, are emerging as significant market opportunities for graphene hydrogels. The global water treatment market is expanding rapidly, with a growing emphasis on advanced materials for contaminant removal and water quality monitoring. Graphene hydrogels' high adsorption capacity and selectivity position them as promising materials in this sector.
The electronics industry is also showing increased interest in graphene hydrogels for flexible electronics, wearable devices, and sensors. As the Internet of Things (IoT) continues to expand, the demand for lightweight, flexible, and highly conductive materials is expected to surge, creating new opportunities for graphene-based materials.
Despite the promising market outlook, challenges remain in scaling up production and reducing costs. The synthesis process using perchloric acid, while effective, requires optimization for large-scale manufacturing. This presents both a challenge and an opportunity for innovation in production techniques.
Overall, the market demand for graphene hydrogels synthesized with perchloric acid is poised for significant growth across multiple sectors. The unique properties offered by these materials align well with the evolving needs of various industries, from energy and healthcare to environmental protection and electronics. As research and development efforts continue to advance, addressing scalability and cost-effectiveness will be crucial in fully realizing the market potential of these innovative materials.
Perchloric Acid Challenges
The synthesis of graphene hydrogels using perchloric acid presents several significant challenges that researchers and manufacturers must address. One of the primary issues is the highly corrosive and oxidizing nature of perchloric acid, which poses safety risks during handling and storage. This characteristic necessitates stringent safety protocols and specialized equipment, potentially increasing production costs and complexity.
Another challenge lies in controlling the reaction conditions precisely. The concentration of perchloric acid, temperature, and reaction time must be carefully managed to achieve the desired graphene hydrogel properties. Even slight variations in these parameters can lead to inconsistent product quality, affecting the hydrogel's mechanical strength, porosity, and electrical conductivity.
The environmental impact of using perchloric acid is also a significant concern. Its strong oxidizing properties can lead to the formation of harmful byproducts, necessitating comprehensive waste management strategies. This aspect not only adds to the production costs but also raises regulatory compliance issues in various jurisdictions.
Scalability presents another hurdle in the industrial application of this synthesis method. While perchloric acid-based synthesis may be effective at laboratory scales, translating this process to large-scale production introduces additional complexities. Maintaining uniform reaction conditions across larger volumes and ensuring consistent product quality become increasingly challenging as production scales up.
The purity of the resulting graphene hydrogel is another critical issue. Residual perchloric acid or its byproducts can significantly affect the final product's properties and potential applications. Developing efficient purification techniques that do not compromise the hydrogel's structure or properties is essential but often difficult to achieve.
Lastly, the cost-effectiveness of using perchloric acid in graphene hydrogel synthesis remains a challenge. While it offers certain advantages in terms of reaction efficiency, the high cost of high-purity perchloric acid, coupled with the expenses associated with safety measures and waste management, may make this method less economically viable compared to alternative synthesis routes.
Addressing these challenges requires interdisciplinary research efforts, focusing on optimizing reaction conditions, developing safer handling protocols, improving purification techniques, and exploring more environmentally friendly alternatives. Overcoming these hurdles is crucial for realizing the full potential of perchloric acid in the large-scale production of graphene hydrogels with consistent quality and properties.
Another challenge lies in controlling the reaction conditions precisely. The concentration of perchloric acid, temperature, and reaction time must be carefully managed to achieve the desired graphene hydrogel properties. Even slight variations in these parameters can lead to inconsistent product quality, affecting the hydrogel's mechanical strength, porosity, and electrical conductivity.
The environmental impact of using perchloric acid is also a significant concern. Its strong oxidizing properties can lead to the formation of harmful byproducts, necessitating comprehensive waste management strategies. This aspect not only adds to the production costs but also raises regulatory compliance issues in various jurisdictions.
Scalability presents another hurdle in the industrial application of this synthesis method. While perchloric acid-based synthesis may be effective at laboratory scales, translating this process to large-scale production introduces additional complexities. Maintaining uniform reaction conditions across larger volumes and ensuring consistent product quality become increasingly challenging as production scales up.
The purity of the resulting graphene hydrogel is another critical issue. Residual perchloric acid or its byproducts can significantly affect the final product's properties and potential applications. Developing efficient purification techniques that do not compromise the hydrogel's structure or properties is essential but often difficult to achieve.
Lastly, the cost-effectiveness of using perchloric acid in graphene hydrogel synthesis remains a challenge. While it offers certain advantages in terms of reaction efficiency, the high cost of high-purity perchloric acid, coupled with the expenses associated with safety measures and waste management, may make this method less economically viable compared to alternative synthesis routes.
Addressing these challenges requires interdisciplinary research efforts, focusing on optimizing reaction conditions, developing safer handling protocols, improving purification techniques, and exploring more environmentally friendly alternatives. Overcoming these hurdles is crucial for realizing the full potential of perchloric acid in the large-scale production of graphene hydrogels with consistent quality and properties.
Current Synthesis Methods
01 Synthesis and preparation methods of graphene hydrogels
Various methods for synthesizing and preparing graphene hydrogels are described, including chemical reduction, self-assembly, and hydrothermal processes. These techniques aim to create three-dimensional porous structures with high surface area and excellent mechanical properties.- Synthesis and preparation methods of graphene hydrogels: Various methods for synthesizing and preparing graphene hydrogels are described, including chemical reduction, self-assembly, and hydrothermal processes. These methods aim to create three-dimensional porous structures with high surface area and excellent mechanical properties.
- Applications of graphene hydrogels in energy storage: Graphene hydrogels show promising applications in energy storage devices such as supercapacitors and batteries. Their high conductivity, large surface area, and porous structure contribute to improved energy storage capacity and performance.
- Functionalization of graphene hydrogels: Techniques for functionalizing graphene hydrogels with various materials or molecules to enhance their properties or tailor them for specific applications. This includes doping, surface modification, and incorporation of nanoparticles or polymers.
- Graphene hydrogels for environmental applications: The use of graphene hydrogels in environmental applications such as water purification, pollutant removal, and environmental sensing. Their high adsorption capacity and large surface area make them effective for removing contaminants from water and air.
- Mechanical and structural properties of graphene hydrogels: Studies on the mechanical and structural properties of graphene hydrogels, including their compressibility, elasticity, and self-healing abilities. These properties are crucial for applications in flexible electronics, sensors, and actuators.
02 Applications of graphene hydrogels in energy storage
Graphene hydrogels show promising applications in energy storage devices such as supercapacitors and batteries. Their high conductivity, large surface area, and porous structure contribute to improved energy storage capacity and performance.Expand Specific Solutions03 Functionalization of graphene hydrogels
Various methods for functionalizing graphene hydrogels are explored, including doping with heteroatoms, incorporating metal nanoparticles, and surface modification. These techniques enhance the hydrogels' properties for specific applications such as sensing, catalysis, and environmental remediation.Expand Specific Solutions04 Graphene hydrogels for biomedical applications
The use of graphene hydrogels in biomedical applications is investigated, including drug delivery systems, tissue engineering scaffolds, and biosensors. Their biocompatibility, mechanical strength, and ability to incorporate various biomolecules make them suitable for these applications.Expand Specific Solutions05 Characterization and property analysis of graphene hydrogels
Various techniques for characterizing and analyzing the properties of graphene hydrogels are described. These include methods for determining their mechanical strength, electrical conductivity, porosity, and surface area, which are crucial for optimizing their performance in different applications.Expand Specific Solutions
Key Industry Players
The development of graphene hydrogels using perchloric acid is in an early stage, with significant potential for growth. The market size is expanding as research progresses, but commercialization remains limited. Technologically, it's still evolving, with academic institutions like the University of New Hampshire, Zhejiang University, and Gwangju Institute of Science & Technology leading research efforts. Companies such as ExxonMobil Chemical Patents and DuPont de Nemours are also involved, indicating growing industrial interest. However, the technology's maturity varies across different applications, with some areas more advanced than others. The competitive landscape is diverse, featuring both academic and corporate players, suggesting a dynamic and collaborative environment for innovation in this field.
Zhejiang University
Technical Solution: Zhejiang University has developed an innovative approach to graphene hydrogel synthesis using perchloric acid. Their method involves a one-step hydrothermal reduction process, where graphene oxide (GO) is treated with perchloric acid at elevated temperatures. This process results in the simultaneous reduction of GO and the formation of a 3D porous network structure. The perchloric acid acts as both a reducing agent and a structure-directing agent, facilitating the formation of a highly interconnected graphene network[1]. The resulting hydrogels exhibit enhanced electrical conductivity and mechanical strength compared to traditional graphene hydrogels[2]. Additionally, the university has explored the incorporation of metal nanoparticles during the synthesis process, further expanding the potential applications of these materials in areas such as energy storage and catalysis[3].
Strengths: High electrical conductivity, improved mechanical properties, and potential for functionalization with metal nanoparticles. Weaknesses: Potential safety concerns due to the use of perchloric acid, which is a strong oxidizer.
Shanghai Institute of Applied Physics, Chinese Academy of Sci
Technical Solution: The Shanghai Institute of Applied Physics has developed a novel approach to graphene hydrogel synthesis utilizing perchloric acid as a key component. Their method involves a controlled oxidation-reduction process, where graphene oxide is first treated with perchloric acid to create oxygen-containing functional groups on the graphene sheets. This is followed by a reduction step using a mild reducing agent, resulting in a highly porous and interconnected 3D graphene network[1]. The institute has also investigated the role of perchloric acid concentration on the final hydrogel properties, demonstrating that higher concentrations lead to increased porosity and surface area[2]. Furthermore, they have explored the incorporation of metal ions during the synthesis process, which has shown promise for enhancing the hydrogel's catalytic and electrochemical properties[3].
Strengths: High degree of control over hydrogel properties, potential for tailored functionalization, and improved surface area. Weaknesses: Multi-step process may be more complex and time-consuming compared to one-pot synthesis methods.
Perchloric Acid Innovations
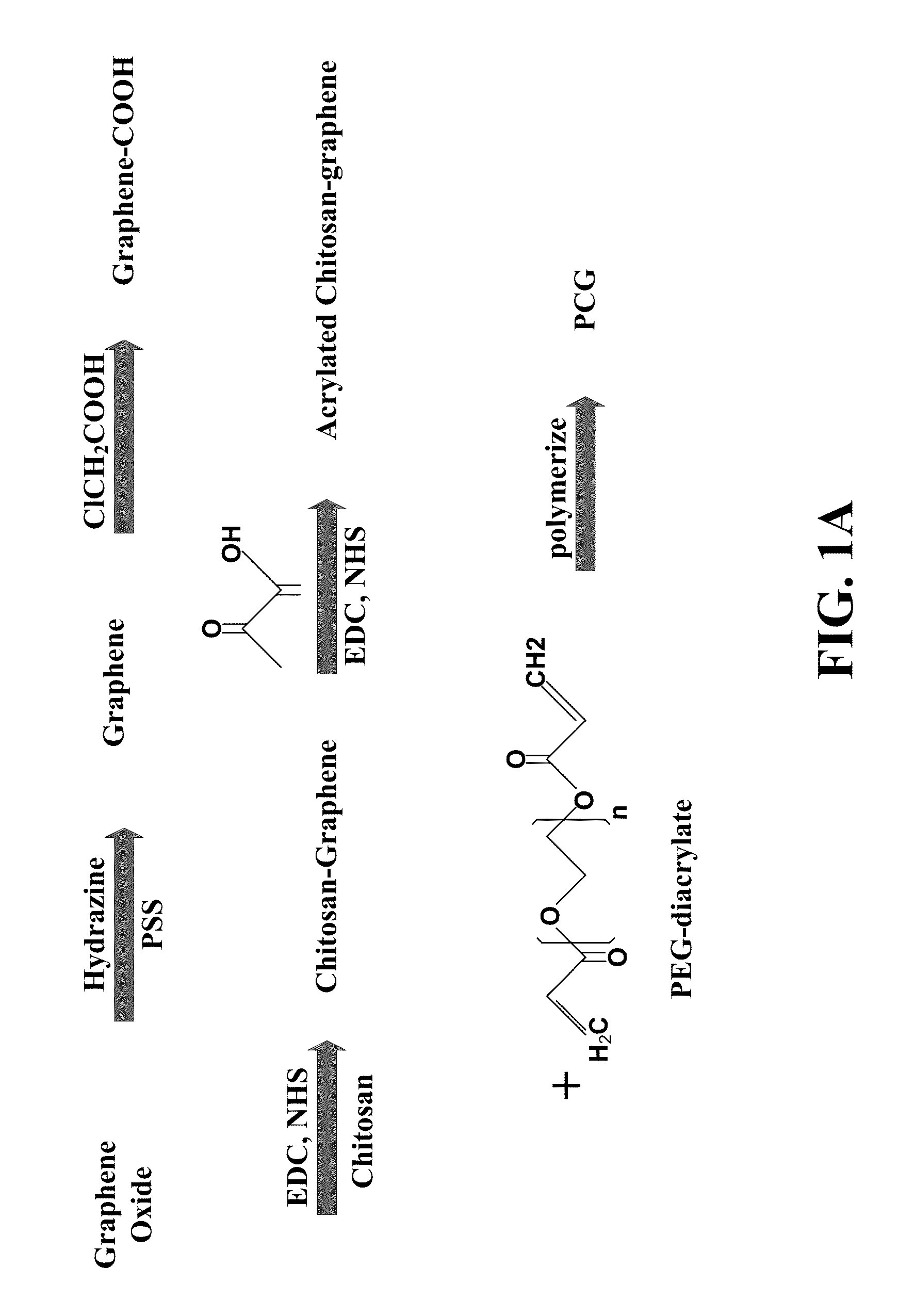
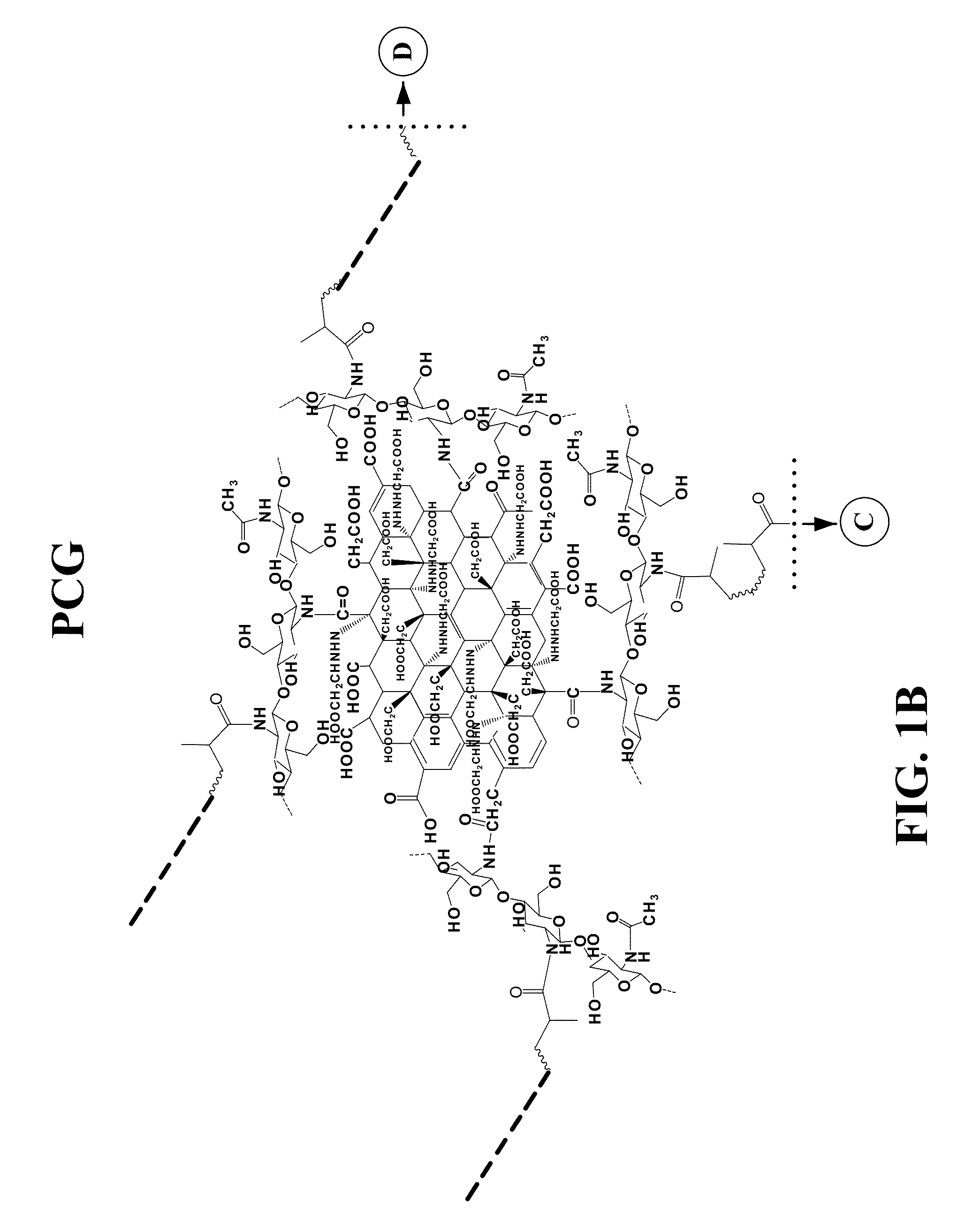
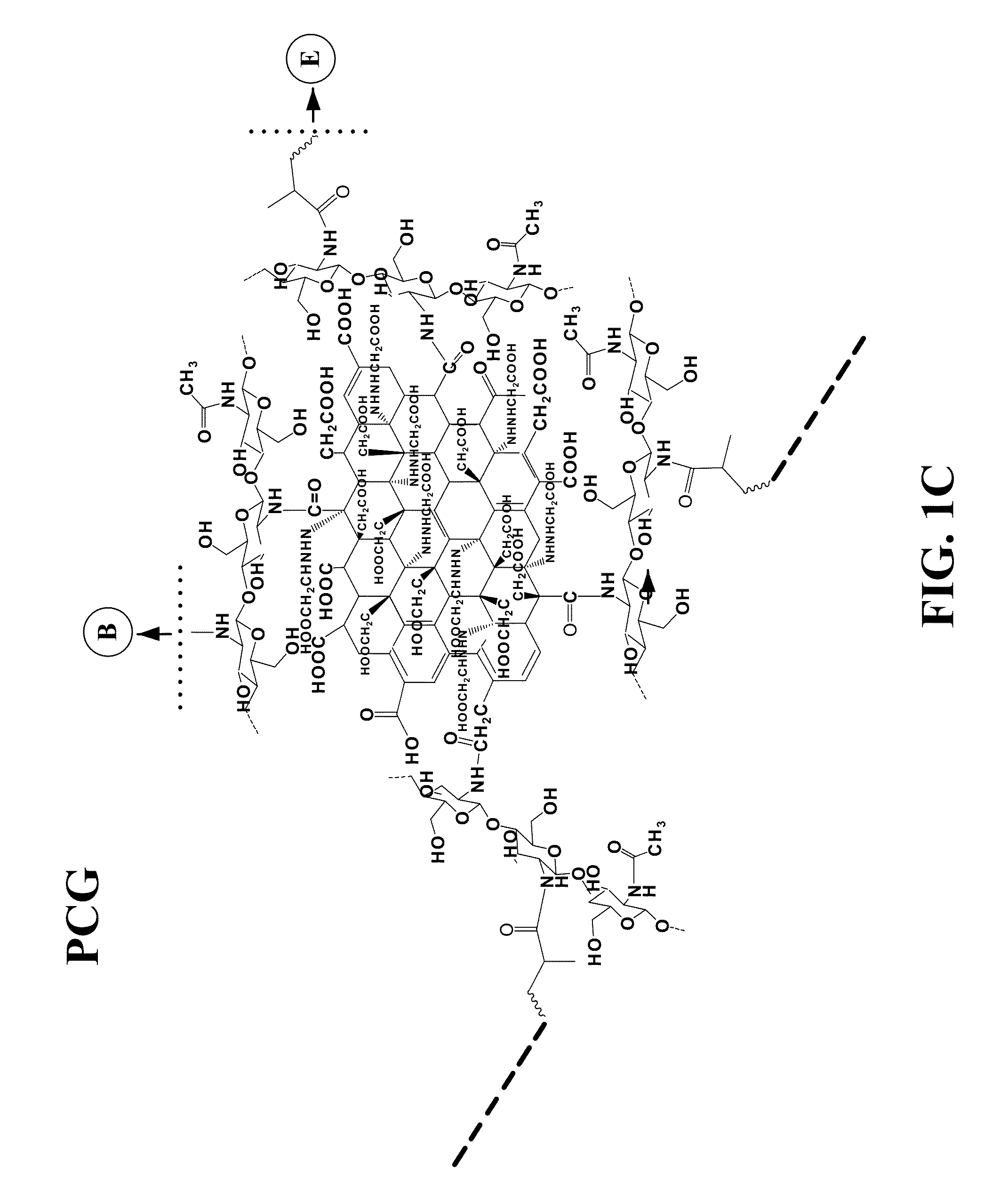
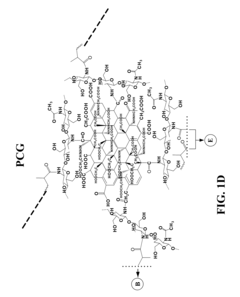
Graphene hydrogel and method for using the same
PatentInactiveUS20130230496A1
Innovation
- A hydrogel composition comprising graphene, chitosan, and polyethylene glycol diacrylate (PEGDA) is developed, which supports the differentiation of mesenchymal stem cells into adipocytes, chondrocytes, and osteocytes, and a thermally inducible hydrogel (TPCG) is created by incorporating N-isopropylacrylamide (NIPAM), enabling stable dispersion of graphene and high drug loading capacity with temperature-responsive drug release.
High surface area, high purity and high conductance mono-layered reduced graphene oxide
PatentActiveIN201711043028A
Innovation
- A method involving the preparation of graphene oxide using a mixture of flaky graphite powder and potassium permanganate, followed by a mixed acid solution with sulphuric and phosphoric acid, and subsequent reduction using formic acid and microwave treatment to produce high-purity reduced graphene oxide.
Safety and Handling Protocols
The use of perchloric acid in the synthesis of graphene hydrogels necessitates stringent safety and handling protocols due to its highly oxidizing and potentially explosive nature. Proper training and adherence to safety guidelines are paramount for all personnel involved in the synthesis process. Personal protective equipment (PPE) is essential, including chemical-resistant gloves, safety goggles, and a lab coat. A face shield should be worn when handling larger quantities of perchloric acid.
Perchloric acid must be stored in a cool, dry, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are suitable for storage, but metal containers should be avoided due to the risk of corrosion. Regular inspections of storage areas and containers are necessary to detect any signs of leakage or degradation.
When working with perchloric acid, it is crucial to use a properly designed fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. The work area should be free from organic materials, and all equipment must be thoroughly cleaned after use to remove any residual acid.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent dangerous heat generation and potential splashing. Accurate measurement and careful handling are essential to maintain the precise concentrations required for graphene hydrogel synthesis.
Emergency response procedures must be established and clearly communicated. This includes the availability of appropriate fire extinguishing agents, such as water or foam, and eyewash stations and safety showers in close proximity to the work area. A spill response kit specifically designed for perchloric acid should be readily accessible.
Waste management is a critical aspect of perchloric acid handling. Neutralization and proper disposal methods must be followed, and perchloric acid waste should never be mixed with organic solvents or other incompatible materials. All waste containers must be clearly labeled and stored separately from other chemical waste.
Regular safety audits and refresher training sessions should be conducted to ensure ongoing compliance with safety protocols. Documentation of all safety procedures, incident reports, and training records is essential for maintaining a safe working environment and meeting regulatory requirements.
Perchloric acid must be stored in a cool, dry, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are suitable for storage, but metal containers should be avoided due to the risk of corrosion. Regular inspections of storage areas and containers are necessary to detect any signs of leakage or degradation.
When working with perchloric acid, it is crucial to use a properly designed fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. The work area should be free from organic materials, and all equipment must be thoroughly cleaned after use to remove any residual acid.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent dangerous heat generation and potential splashing. Accurate measurement and careful handling are essential to maintain the precise concentrations required for graphene hydrogel synthesis.
Emergency response procedures must be established and clearly communicated. This includes the availability of appropriate fire extinguishing agents, such as water or foam, and eyewash stations and safety showers in close proximity to the work area. A spill response kit specifically designed for perchloric acid should be readily accessible.
Waste management is a critical aspect of perchloric acid handling. Neutralization and proper disposal methods must be followed, and perchloric acid waste should never be mixed with organic solvents or other incompatible materials. All waste containers must be clearly labeled and stored separately from other chemical waste.
Regular safety audits and refresher training sessions should be conducted to ensure ongoing compliance with safety protocols. Documentation of all safety procedures, incident reports, and training records is essential for maintaining a safe working environment and meeting regulatory requirements.
Environmental Impact Assessment
The synthesis of graphene hydrogels using perchloric acid requires careful consideration of its environmental impact. Perchloric acid, a strong oxidizing agent, poses significant risks to both human health and the environment if not properly managed during the production process.
In aquatic ecosystems, the release of perchlorate ions from improperly treated waste can disrupt the endocrine systems of various organisms, particularly affecting thyroid function in fish and amphibians. This can lead to developmental abnormalities and population decline in affected species. Furthermore, perchlorate contamination in water sources can persist for extended periods due to its high solubility and stability, potentially impacting drinking water quality for both wildlife and human populations.
Atmospheric emissions during the synthesis process may contribute to the formation of secondary pollutants. When perchloric acid vapors react with atmospheric moisture, they can form aerosols that may lead to localized acid rain events. This can result in soil acidification, damage to vegetation, and corrosion of infrastructure in affected areas.
Soil contamination is another concern, as perchlorate salts can accumulate in soil layers, affecting soil microbial communities and plant growth. This may lead to reduced agricultural productivity in contaminated areas and potential bioaccumulation of perchlorate in food chains.
To mitigate these environmental risks, stringent waste management protocols must be implemented. This includes the use of specialized treatment systems to neutralize and remove perchlorate ions from wastewater before discharge. Closed-loop production systems and vapor recovery technologies can significantly reduce atmospheric emissions and minimize the risk of accidental releases.
Additionally, the development of alternative synthesis methods that reduce or eliminate the use of perchloric acid should be prioritized. Green chemistry approaches, such as using less hazardous oxidizing agents or exploring electrochemical synthesis routes, could potentially offer more environmentally friendly alternatives for graphene hydrogel production.
Continuous environmental monitoring around production facilities is crucial to detect and address any potential contamination quickly. This should include regular testing of soil, water, and air quality to ensure compliance with environmental regulations and to protect local ecosystems.
In conclusion, while the use of perchloric acid in graphene hydrogel synthesis offers certain technical advantages, its potential environmental impacts necessitate careful management and ongoing research into more sustainable production methods. Balancing technological progress with environmental stewardship remains a key challenge in this field.
In aquatic ecosystems, the release of perchlorate ions from improperly treated waste can disrupt the endocrine systems of various organisms, particularly affecting thyroid function in fish and amphibians. This can lead to developmental abnormalities and population decline in affected species. Furthermore, perchlorate contamination in water sources can persist for extended periods due to its high solubility and stability, potentially impacting drinking water quality for both wildlife and human populations.
Atmospheric emissions during the synthesis process may contribute to the formation of secondary pollutants. When perchloric acid vapors react with atmospheric moisture, they can form aerosols that may lead to localized acid rain events. This can result in soil acidification, damage to vegetation, and corrosion of infrastructure in affected areas.
Soil contamination is another concern, as perchlorate salts can accumulate in soil layers, affecting soil microbial communities and plant growth. This may lead to reduced agricultural productivity in contaminated areas and potential bioaccumulation of perchlorate in food chains.
To mitigate these environmental risks, stringent waste management protocols must be implemented. This includes the use of specialized treatment systems to neutralize and remove perchlorate ions from wastewater before discharge. Closed-loop production systems and vapor recovery technologies can significantly reduce atmospheric emissions and minimize the risk of accidental releases.
Additionally, the development of alternative synthesis methods that reduce or eliminate the use of perchloric acid should be prioritized. Green chemistry approaches, such as using less hazardous oxidizing agents or exploring electrochemical synthesis routes, could potentially offer more environmentally friendly alternatives for graphene hydrogel production.
Continuous environmental monitoring around production facilities is crucial to detect and address any potential contamination quickly. This should include regular testing of soil, water, and air quality to ensure compliance with environmental regulations and to protect local ecosystems.
In conclusion, while the use of perchloric acid in graphene hydrogel synthesis offers certain technical advantages, its potential environmental impacts necessitate careful management and ongoing research into more sustainable production methods. Balancing technological progress with environmental stewardship remains a key challenge in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!