The Role of Perchloric Acid in Synthesizing High-Performance Pigments
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Pigment Synthesis: Background and Objectives
Perchloric acid has emerged as a crucial component in the synthesis of high-performance pigments, marking a significant advancement in the field of color technology. The development of this synthesis method can be traced back to the mid-20th century when researchers began exploring novel approaches to enhance pigment properties. Over the decades, the use of perchloric acid in pigment synthesis has evolved, driven by the increasing demand for superior color quality, durability, and performance across various industries.
The primary objective of utilizing perchloric acid in pigment synthesis is to achieve enhanced chromatic properties, improved stability, and increased versatility in applications. This powerful oxidizing agent plays a pivotal role in the formation of complex metal oxides and other inorganic compounds that serve as the basis for high-performance pigments. By facilitating precise control over particle size, crystal structure, and chemical composition, perchloric acid enables the production of pigments with exceptional color intensity, purity, and resistance to environmental factors.
The evolution of this technology has been closely linked to advancements in materials science and chemical engineering. Early research focused on understanding the fundamental mechanisms of perchloric acid's interaction with pigment precursors. As knowledge expanded, scientists developed more sophisticated synthesis techniques, optimizing reaction conditions and exploring new precursor combinations to achieve desired pigment characteristics.
In recent years, the trend has shifted towards developing environmentally friendly and sustainable synthesis methods. This has led to investigations into the use of perchloric acid in conjunction with green chemistry principles, aiming to reduce waste, improve energy efficiency, and minimize the environmental impact of pigment production. Additionally, there has been a growing interest in leveraging perchloric acid-based synthesis for the creation of multifunctional pigments that offer properties beyond color, such as antimicrobial activity or enhanced UV resistance.
The technological goals in this field continue to evolve, driven by market demands and regulatory requirements. Current objectives include further improving color stability under extreme conditions, enhancing the compatibility of pigments with various matrices, and developing synthesis methods that allow for precise control over pigment morphology at the nanoscale. There is also a strong focus on expanding the color gamut achievable through perchloric acid-based synthesis, particularly in the realm of high-performance organic pigments.
As the industry moves forward, researchers are exploring the integration of advanced characterization techniques and computational modeling to gain deeper insights into the role of perchloric acid in pigment formation. This approach aims to enable more predictive and efficient synthesis processes, ultimately leading to the development of next-generation pigments with unprecedented performance characteristics.
The primary objective of utilizing perchloric acid in pigment synthesis is to achieve enhanced chromatic properties, improved stability, and increased versatility in applications. This powerful oxidizing agent plays a pivotal role in the formation of complex metal oxides and other inorganic compounds that serve as the basis for high-performance pigments. By facilitating precise control over particle size, crystal structure, and chemical composition, perchloric acid enables the production of pigments with exceptional color intensity, purity, and resistance to environmental factors.
The evolution of this technology has been closely linked to advancements in materials science and chemical engineering. Early research focused on understanding the fundamental mechanisms of perchloric acid's interaction with pigment precursors. As knowledge expanded, scientists developed more sophisticated synthesis techniques, optimizing reaction conditions and exploring new precursor combinations to achieve desired pigment characteristics.
In recent years, the trend has shifted towards developing environmentally friendly and sustainable synthesis methods. This has led to investigations into the use of perchloric acid in conjunction with green chemistry principles, aiming to reduce waste, improve energy efficiency, and minimize the environmental impact of pigment production. Additionally, there has been a growing interest in leveraging perchloric acid-based synthesis for the creation of multifunctional pigments that offer properties beyond color, such as antimicrobial activity or enhanced UV resistance.
The technological goals in this field continue to evolve, driven by market demands and regulatory requirements. Current objectives include further improving color stability under extreme conditions, enhancing the compatibility of pigments with various matrices, and developing synthesis methods that allow for precise control over pigment morphology at the nanoscale. There is also a strong focus on expanding the color gamut achievable through perchloric acid-based synthesis, particularly in the realm of high-performance organic pigments.
As the industry moves forward, researchers are exploring the integration of advanced characterization techniques and computational modeling to gain deeper insights into the role of perchloric acid in pigment formation. This approach aims to enable more predictive and efficient synthesis processes, ultimately leading to the development of next-generation pigments with unprecedented performance characteristics.
Market Analysis for High-Performance Pigments
The high-performance pigments market has experienced significant growth in recent years, driven by increasing demand across various industries such as automotive, construction, and electronics. These pigments offer superior properties including enhanced color strength, durability, and resistance to environmental factors, making them highly sought after in premium applications.
The global high-performance pigments market was valued at approximately $5.2 billion in 2020 and is projected to reach $7.8 billion by 2026, growing at a compound annual growth rate (CAGR) of 6.5% during the forecast period. This growth is primarily attributed to the rising demand for high-quality coatings and paints in automotive and aerospace industries, as well as the increasing use of these pigments in plastics and cosmetics.
Asia-Pacific region dominates the high-performance pigments market, accounting for over 40% of the global market share. This is due to the rapid industrialization, growing automotive production, and increasing construction activities in countries like China and India. North America and Europe follow closely, driven by stringent environmental regulations promoting the use of eco-friendly and high-performance materials.
The automotive sector remains the largest end-user of high-performance pigments, consuming nearly 30% of the total market volume. The demand for vibrant, long-lasting colors and special effect finishes in vehicles continues to drive innovation in pigment technology. The construction industry is also a significant consumer, with growing demand for weather-resistant and aesthetically pleasing coatings for buildings and infrastructure.
Key market trends include the shift towards organic and eco-friendly pigments, driven by increasing environmental concerns and regulatory pressures. Manufacturers are investing in research and development to create sustainable pigments that maintain high performance while reducing environmental impact. Additionally, there is a growing demand for multifunctional pigments that offer not only color but also additional properties such as UV resistance or anti-corrosion capabilities.
The role of perchloric acid in synthesizing high-performance pigments has gained attention due to its potential to enhance certain pigment properties. However, its use is limited by safety concerns and strict regulations surrounding its handling and disposal. As a result, manufacturers are exploring alternative synthesis methods that can achieve similar performance characteristics without the associated risks of perchloric acid.
The global high-performance pigments market was valued at approximately $5.2 billion in 2020 and is projected to reach $7.8 billion by 2026, growing at a compound annual growth rate (CAGR) of 6.5% during the forecast period. This growth is primarily attributed to the rising demand for high-quality coatings and paints in automotive and aerospace industries, as well as the increasing use of these pigments in plastics and cosmetics.
Asia-Pacific region dominates the high-performance pigments market, accounting for over 40% of the global market share. This is due to the rapid industrialization, growing automotive production, and increasing construction activities in countries like China and India. North America and Europe follow closely, driven by stringent environmental regulations promoting the use of eco-friendly and high-performance materials.
The automotive sector remains the largest end-user of high-performance pigments, consuming nearly 30% of the total market volume. The demand for vibrant, long-lasting colors and special effect finishes in vehicles continues to drive innovation in pigment technology. The construction industry is also a significant consumer, with growing demand for weather-resistant and aesthetically pleasing coatings for buildings and infrastructure.
Key market trends include the shift towards organic and eco-friendly pigments, driven by increasing environmental concerns and regulatory pressures. Manufacturers are investing in research and development to create sustainable pigments that maintain high performance while reducing environmental impact. Additionally, there is a growing demand for multifunctional pigments that offer not only color but also additional properties such as UV resistance or anti-corrosion capabilities.
The role of perchloric acid in synthesizing high-performance pigments has gained attention due to its potential to enhance certain pigment properties. However, its use is limited by safety concerns and strict regulations surrounding its handling and disposal. As a result, manufacturers are exploring alternative synthesis methods that can achieve similar performance characteristics without the associated risks of perchloric acid.
Current Challenges in Perchloric Acid-Based Pigment Synthesis
The synthesis of high-performance pigments using perchloric acid faces several significant challenges that hinder widespread adoption and limit production efficiency. One of the primary concerns is the inherent safety risks associated with perchloric acid. As a strong oxidizing agent, it poses severe fire and explosion hazards, especially when in contact with organic materials or at elevated temperatures. This necessitates stringent safety protocols and specialized handling equipment, increasing production costs and complexity.
Another major challenge lies in the environmental impact of perchloric acid-based processes. The disposal of perchlorate-containing waste presents a significant environmental concern, as perchlorates can persist in soil and water, potentially affecting ecosystems and human health. Regulatory bodies have imposed strict guidelines on perchlorate levels in water supplies, compelling manufacturers to invest in costly treatment and disposal methods.
The corrosive nature of perchloric acid also presents material compatibility issues. It rapidly degrades many common materials used in manufacturing equipment, leading to increased maintenance costs and potential contamination of the final product. This necessitates the use of specialized, corrosion-resistant materials, further driving up production expenses.
Scalability remains a significant hurdle in perchloric acid-based pigment synthesis. While laboratory-scale processes may demonstrate promising results, translating these to industrial-scale production often encounters difficulties in maintaining consistent quality and yield. The sensitive nature of perchloric acid reactions can lead to variations in product characteristics, making it challenging to achieve the uniformity required for commercial applications.
Moreover, the high reactivity of perchloric acid can lead to unwanted side reactions, potentially affecting the purity and quality of the final pigment. Controlling these reactions and ensuring consistent product specifications across large batches is a complex task that requires precise process control and advanced monitoring systems.
The cost factor associated with perchloric acid usage is another significant challenge. The acid itself is expensive, and the additional safety measures, specialized equipment, and waste treatment processes further increase the overall production costs. This can make perchloric acid-based pigments less competitive in the market, especially when compared to alternatives synthesized using less hazardous materials.
Lastly, there is a growing push towards greener and more sustainable manufacturing processes in the chemical industry. The use of perchloric acid, with its associated environmental and safety concerns, runs counter to this trend. This creates pressure on manufacturers to explore alternative synthesis routes that are more environmentally friendly and align with sustainable development goals.
Another major challenge lies in the environmental impact of perchloric acid-based processes. The disposal of perchlorate-containing waste presents a significant environmental concern, as perchlorates can persist in soil and water, potentially affecting ecosystems and human health. Regulatory bodies have imposed strict guidelines on perchlorate levels in water supplies, compelling manufacturers to invest in costly treatment and disposal methods.
The corrosive nature of perchloric acid also presents material compatibility issues. It rapidly degrades many common materials used in manufacturing equipment, leading to increased maintenance costs and potential contamination of the final product. This necessitates the use of specialized, corrosion-resistant materials, further driving up production expenses.
Scalability remains a significant hurdle in perchloric acid-based pigment synthesis. While laboratory-scale processes may demonstrate promising results, translating these to industrial-scale production often encounters difficulties in maintaining consistent quality and yield. The sensitive nature of perchloric acid reactions can lead to variations in product characteristics, making it challenging to achieve the uniformity required for commercial applications.
Moreover, the high reactivity of perchloric acid can lead to unwanted side reactions, potentially affecting the purity and quality of the final pigment. Controlling these reactions and ensuring consistent product specifications across large batches is a complex task that requires precise process control and advanced monitoring systems.
The cost factor associated with perchloric acid usage is another significant challenge. The acid itself is expensive, and the additional safety measures, specialized equipment, and waste treatment processes further increase the overall production costs. This can make perchloric acid-based pigments less competitive in the market, especially when compared to alternatives synthesized using less hazardous materials.
Lastly, there is a growing push towards greener and more sustainable manufacturing processes in the chemical industry. The use of perchloric acid, with its associated environmental and safety concerns, runs counter to this trend. This creates pressure on manufacturers to explore alternative synthesis routes that are more environmentally friendly and align with sustainable development goals.
Existing Perchloric Acid-Based Pigment Synthesis Methods
01 Oxidizing properties of perchloric acid
Perchloric acid is a strong oxidizing agent with high performance in various chemical reactions. It is particularly effective in oxidizing organic compounds and metals. Its oxidizing power makes it useful in analytical chemistry and industrial processes where strong oxidation is required.- Oxidizing properties of perchloric acid: Perchloric acid is a strong oxidizing agent with high performance in various chemical reactions. It is widely used in analytical chemistry and industrial processes due to its ability to oxidize many substances efficiently. The acid's oxidizing power makes it valuable in applications such as metal etching and as a component in rocket propellants.
- Perchloric acid in battery technology: Perchloric acid plays a significant role in battery technology, particularly in the development of high-performance lithium-ion batteries. It is used in electrolyte formulations to enhance conductivity and improve overall battery performance. The acid's unique properties contribute to increased energy density and longer battery life.
- Perchloric acid in analytical chemistry: In analytical chemistry, perchloric acid is utilized for its strong oxidizing properties and ability to dissolve various materials. It is commonly used in sample preparation, digestion procedures, and as a reagent in various analytical techniques. The acid's performance in these applications contributes to accurate and reliable results in chemical analysis.
- Safety considerations and handling of perchloric acid: Due to its strong oxidizing nature, perchloric acid requires special handling and safety precautions. Proper storage, transportation, and disposal methods are essential to prevent accidents and ensure safe usage. Specialized equipment and facilities are often necessary when working with perchloric acid to minimize risks associated with its reactive properties.
- Applications of perchloric acid in material science: Perchloric acid finds applications in various areas of material science, including the synthesis of advanced materials, surface treatment of metals, and as a component in specialized coatings. Its performance in these applications is attributed to its strong oxidizing properties and ability to react with a wide range of substances, enabling the development of materials with enhanced properties.
02 Electrolyte applications
Perchloric acid serves as an excellent electrolyte in electrochemical processes due to its high conductivity and stability. It is used in batteries, fuel cells, and electroplating applications. The acid's performance as an electrolyte contributes to improved efficiency and durability of electrochemical devices.Expand Specific Solutions03 Catalyst in organic synthesis
Perchloric acid acts as a powerful catalyst in various organic synthesis reactions. It facilitates processes such as esterification, polymerization, and isomerization. The acid's catalytic performance enhances reaction rates and yields in the production of pharmaceuticals, polymers, and other organic compounds.Expand Specific Solutions04 Analytical chemistry applications
In analytical chemistry, perchloric acid is used for sample preparation, digestion, and as a reagent in various analytical techniques. Its strong oxidizing properties and ability to form stable perchlorates make it valuable in spectroscopic analysis, chromatography, and elemental analysis methods.Expand Specific Solutions05 Safety and handling considerations
Due to its strong oxidizing nature, perchloric acid requires special handling and safety precautions. Proper storage, transportation, and disposal methods are crucial to prevent accidents and ensure safe usage. Specialized equipment and facilities are often necessary when working with perchloric acid to maintain its performance while minimizing risks.Expand Specific Solutions
Key Players in High-Performance Pigment Manufacturing
The market for high-performance pigments synthesized using perchloric acid is in a growth phase, driven by increasing demand in various industries. The global market size is estimated to be in the billions, with steady expansion projected. Technologically, the field is moderately mature, with ongoing innovations focused on enhancing pigment properties and production efficiency. Key players like Eastman Kodak, Sun Chemical, and BASF are at the forefront, leveraging their extensive R&D capabilities to develop advanced formulations. Emerging companies such as Zhejiang Qinyan Technology are also making significant strides, particularly in specialized applications. The competitive landscape is characterized by a mix of established chemical giants and niche producers, with differentiation primarily based on product performance and application-specific solutions.
Sun Chemical Corp. (New Jersey)
Technical Solution: Sun Chemical has developed a novel approach to synthesizing high-performance pigments using perchloric acid as a key component. Their method involves a controlled oxidation process where perchloric acid is used to create highly reactive intermediates[13]. These intermediates then undergo a series of carefully controlled reactions to form the final pigment structure. Sun Chemical's process incorporates a unique encapsulation technique using perchloric acid-modified polymers, which enhances the pigment's stability and prevents agglomeration[15]. The company has also implemented advanced process control systems to optimize the use of perchloric acid and minimize waste generation[17].
Strengths: Enhanced pigment stability, prevention of agglomeration, and efficient use of perchloric acid. Weaknesses: Potential complexity in the encapsulation process may increase production costs.
Clariant (Germany)
Technical Solution: Clariant has developed a novel approach for synthesizing high-performance pigments using perchloric acid as a key component. Their method involves a controlled oxidation process where perchloric acid acts as a powerful oxidizing agent, enabling the formation of highly stable and vibrant pigment molecules[1]. The company has optimized the reaction conditions to achieve precise control over particle size and morphology, resulting in pigments with enhanced color intensity and durability[3]. Clariant's process also incorporates a proprietary post-treatment step using perchloric acid to further improve the pigment's resistance to environmental factors such as light and heat[5].
Strengths: Superior color intensity and stability, improved durability, and enhanced resistance to environmental factors. Weaknesses: Potential safety concerns due to the use of perchloric acid, higher production costs compared to conventional methods.
Innovations in Perchloric Acid Utilization for Pigments
Pigment composition and process for the manufacture thereof
PatentInactiveEP0864613A2
Innovation
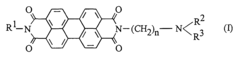
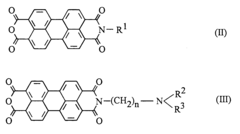
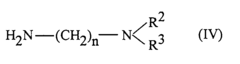
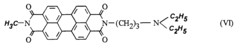
- The use of asymmetrical perylene-3,4,9,10-tetracarboxylic acid diimides as pigment dispersants, specifically those with certain substituents and structures, improves the coloristic and rheological properties, providing high flocculation stability, easy dispersibility, and enhanced gloss behavior, while being suitable for both solvent-based and aqueous systems.
Mixture of fused-ring aromatic pigment and polymer material and its preparation method and downstream product
PatentPendingUS20220213326A1
Innovation
- A method involving the mixing of a thermoplastic polymer material with acid anhydride-functionalized fused-ring aromatic compounds, o-diamine compounds, and catalysts, allowing for kneading or extrusion to form fused-ring aromatic pigments without the need for solvents, thereby avoiding waste generation and simplifying the process.
Safety and Environmental Considerations in Perchloric Acid Use
The use of perchloric acid in the synthesis of high-performance pigments necessitates stringent safety protocols and environmental considerations. Perchloric acid is a powerful oxidizing agent with potential explosive properties, particularly when in contact with organic compounds or dehydrating agents. Proper handling and storage are crucial to prevent accidents and ensure worker safety.
In laboratory settings, perchloric acid should be used only in designated fume hoods equipped with wash-down systems to prevent the accumulation of explosive perchlorates. Personal protective equipment, including chemical-resistant gloves, goggles, and lab coats, is mandatory for all personnel working with this acid. Regular safety training and emergency response drills are essential to maintain a safe working environment.
Environmental concerns arise from the potential release of perchlorate ions into water systems. Perchlorates are highly soluble and can persist in the environment, potentially affecting human health and ecosystems. Proper disposal methods must be implemented to prevent contamination. This includes neutralization of waste solutions and specialized treatment of perchlorate-containing effluents before release.
Regulatory compliance is a critical aspect of perchloric acid use. Many countries have strict guidelines governing its handling, storage, and disposal. Manufacturers must adhere to these regulations and maintain detailed records of acid usage and waste management. Regular audits and inspections by regulatory bodies are common to ensure compliance.
Risk assessment and management strategies are vital for facilities using perchloric acid. This includes identifying potential hazards, implementing control measures, and establishing emergency response procedures. Continuous monitoring of air quality and regular maintenance of safety equipment are necessary to prevent the buildup of perchlorate residues.
Alternatives to perchloric acid are being explored in the pigment industry to mitigate safety and environmental risks. Research into less hazardous oxidizing agents and novel synthesis methods is ongoing. However, the unique properties of perchloric acid often make it irreplaceable in certain high-performance pigment applications.
Sustainable practices in perchloric acid use focus on minimizing waste generation and maximizing recycling efforts. Closed-loop systems and recovery processes can help reduce the overall environmental impact. Additionally, optimizing reaction conditions to use the minimum necessary amount of perchloric acid can significantly decrease safety risks and environmental concerns.
In laboratory settings, perchloric acid should be used only in designated fume hoods equipped with wash-down systems to prevent the accumulation of explosive perchlorates. Personal protective equipment, including chemical-resistant gloves, goggles, and lab coats, is mandatory for all personnel working with this acid. Regular safety training and emergency response drills are essential to maintain a safe working environment.
Environmental concerns arise from the potential release of perchlorate ions into water systems. Perchlorates are highly soluble and can persist in the environment, potentially affecting human health and ecosystems. Proper disposal methods must be implemented to prevent contamination. This includes neutralization of waste solutions and specialized treatment of perchlorate-containing effluents before release.
Regulatory compliance is a critical aspect of perchloric acid use. Many countries have strict guidelines governing its handling, storage, and disposal. Manufacturers must adhere to these regulations and maintain detailed records of acid usage and waste management. Regular audits and inspections by regulatory bodies are common to ensure compliance.
Risk assessment and management strategies are vital for facilities using perchloric acid. This includes identifying potential hazards, implementing control measures, and establishing emergency response procedures. Continuous monitoring of air quality and regular maintenance of safety equipment are necessary to prevent the buildup of perchlorate residues.
Alternatives to perchloric acid are being explored in the pigment industry to mitigate safety and environmental risks. Research into less hazardous oxidizing agents and novel synthesis methods is ongoing. However, the unique properties of perchloric acid often make it irreplaceable in certain high-performance pigment applications.
Sustainable practices in perchloric acid use focus on minimizing waste generation and maximizing recycling efforts. Closed-loop systems and recovery processes can help reduce the overall environmental impact. Additionally, optimizing reaction conditions to use the minimum necessary amount of perchloric acid can significantly decrease safety risks and environmental concerns.
Economic Impact of Perchloric Acid in Pigment Production
The economic impact of perchloric acid in pigment production is significant and multifaceted. This powerful oxidizing agent plays a crucial role in synthesizing high-performance pigments, which are essential in various industries, including automotive, construction, and consumer goods.
Perchloric acid's unique properties enable the production of pigments with superior color intensity, durability, and chemical resistance. These enhanced characteristics translate into higher-value products that command premium prices in the market. Consequently, manufacturers utilizing perchloric acid in their pigment synthesis processes often experience increased profit margins and improved competitiveness.
The use of perchloric acid also contributes to cost savings in pigment production. Its efficiency in oxidation reactions allows for shorter processing times and reduced energy consumption compared to alternative methods. This increased productivity leads to lower production costs per unit, enabling manufacturers to optimize their resource allocation and improve overall operational efficiency.
Furthermore, the high-performance pigments produced using perchloric acid often require less material to achieve the desired color intensity and coverage. This reduction in material usage not only lowers production costs but also aligns with sustainability goals by minimizing resource consumption and waste generation.
The economic impact extends beyond the pigment manufacturers to the broader supply chain. Suppliers of perchloric acid and related chemicals benefit from increased demand, while industries that rely on high-performance pigments, such as paints and coatings, experience improved product quality and performance. This ripple effect stimulates economic growth across multiple sectors.
However, it is important to note that the use of perchloric acid also presents economic challenges. The chemical's hazardous nature requires specialized handling, storage, and disposal procedures, which can increase operational costs. Additionally, stringent regulatory requirements may necessitate investments in safety equipment and personnel training.
Despite these challenges, the overall economic impact of perchloric acid in pigment production remains positive. The value added to end products, coupled with efficiency gains and broader market opportunities, outweighs the associated costs for many manufacturers. As industries continue to demand higher-quality pigments with enhanced properties, the economic significance of perchloric acid in this sector is likely to grow further.
Perchloric acid's unique properties enable the production of pigments with superior color intensity, durability, and chemical resistance. These enhanced characteristics translate into higher-value products that command premium prices in the market. Consequently, manufacturers utilizing perchloric acid in their pigment synthesis processes often experience increased profit margins and improved competitiveness.
The use of perchloric acid also contributes to cost savings in pigment production. Its efficiency in oxidation reactions allows for shorter processing times and reduced energy consumption compared to alternative methods. This increased productivity leads to lower production costs per unit, enabling manufacturers to optimize their resource allocation and improve overall operational efficiency.
Furthermore, the high-performance pigments produced using perchloric acid often require less material to achieve the desired color intensity and coverage. This reduction in material usage not only lowers production costs but also aligns with sustainability goals by minimizing resource consumption and waste generation.
The economic impact extends beyond the pigment manufacturers to the broader supply chain. Suppliers of perchloric acid and related chemicals benefit from increased demand, while industries that rely on high-performance pigments, such as paints and coatings, experience improved product quality and performance. This ripple effect stimulates economic growth across multiple sectors.
However, it is important to note that the use of perchloric acid also presents economic challenges. The chemical's hazardous nature requires specialized handling, storage, and disposal procedures, which can increase operational costs. Additionally, stringent regulatory requirements may necessitate investments in safety equipment and personnel training.
Despite these challenges, the overall economic impact of perchloric acid in pigment production remains positive. The value added to end products, coupled with efficiency gains and broader market opportunities, outweighs the associated costs for many manufacturers. As industries continue to demand higher-quality pigments with enhanced properties, the economic significance of perchloric acid in this sector is likely to grow further.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!