The Role of Sulphanilic Acid in Photodynamic Therapy
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PDT and Sulphanilic Acid Background
Photodynamic therapy (PDT) has emerged as a promising non-invasive treatment modality for various cancers and other diseases. This light-activated therapeutic approach relies on the interaction between a photosensitizer, light of a specific wavelength, and oxygen to generate reactive oxygen species (ROS) that induce cellular damage and death in target tissues. The efficacy of PDT depends largely on the properties of the photosensitizer used, and researchers have been exploring various compounds to enhance the treatment's effectiveness.
Sulphanilic acid, a synthetic organic compound with the chemical formula C6H7NO3S, has recently gained attention in the field of PDT due to its unique properties and potential applications. Traditionally used in the production of dyes, pharmaceuticals, and other industrial processes, sulphanilic acid's role in PDT represents a novel and exciting area of research.
The interest in sulphanilic acid for PDT applications stems from its chemical structure and properties. As an aromatic compound containing both amino and sulfonic acid groups, sulphanilic acid exhibits characteristics that make it a promising candidate for photosensitizer development or enhancement. Its ability to form stable complexes with various metal ions and organic molecules opens up possibilities for creating new photosensitizer formulations or improving existing ones.
One of the key advantages of sulphanilic acid in the context of PDT is its potential to enhance the solubility and bioavailability of photosensitizers. Many photosensitizers suffer from poor water solubility, which can limit their effectiveness in biological systems. Sulphanilic acid's hydrophilic nature and ability to form conjugates with other molecules could help overcome this limitation, potentially leading to improved drug delivery and increased treatment efficacy.
Furthermore, sulphanilic acid's chemical properties suggest it may play a role in modulating the photophysical and photochemical properties of photosensitizers. This could lead to enhanced light absorption, improved ROS generation, or better targeting of specific cellular components, all of which are crucial factors in the success of PDT treatments.
The exploration of sulphanilic acid in PDT also aligns with the broader trend of repurposing known compounds for new therapeutic applications. This approach can potentially accelerate the development of new treatments by leveraging existing safety and toxicity data, thereby streamlining the path to clinical trials and eventual patient use.
As research in this area progresses, understanding the precise mechanisms by which sulphanilic acid influences PDT outcomes will be crucial. This includes investigating its interactions with various photosensitizers, its impact on cellular uptake and distribution, and its effects on the overall photodynamic process. Such insights will be essential for optimizing PDT protocols and developing more effective treatment strategies.
Sulphanilic acid, a synthetic organic compound with the chemical formula C6H7NO3S, has recently gained attention in the field of PDT due to its unique properties and potential applications. Traditionally used in the production of dyes, pharmaceuticals, and other industrial processes, sulphanilic acid's role in PDT represents a novel and exciting area of research.
The interest in sulphanilic acid for PDT applications stems from its chemical structure and properties. As an aromatic compound containing both amino and sulfonic acid groups, sulphanilic acid exhibits characteristics that make it a promising candidate for photosensitizer development or enhancement. Its ability to form stable complexes with various metal ions and organic molecules opens up possibilities for creating new photosensitizer formulations or improving existing ones.
One of the key advantages of sulphanilic acid in the context of PDT is its potential to enhance the solubility and bioavailability of photosensitizers. Many photosensitizers suffer from poor water solubility, which can limit their effectiveness in biological systems. Sulphanilic acid's hydrophilic nature and ability to form conjugates with other molecules could help overcome this limitation, potentially leading to improved drug delivery and increased treatment efficacy.
Furthermore, sulphanilic acid's chemical properties suggest it may play a role in modulating the photophysical and photochemical properties of photosensitizers. This could lead to enhanced light absorption, improved ROS generation, or better targeting of specific cellular components, all of which are crucial factors in the success of PDT treatments.
The exploration of sulphanilic acid in PDT also aligns with the broader trend of repurposing known compounds for new therapeutic applications. This approach can potentially accelerate the development of new treatments by leveraging existing safety and toxicity data, thereby streamlining the path to clinical trials and eventual patient use.
As research in this area progresses, understanding the precise mechanisms by which sulphanilic acid influences PDT outcomes will be crucial. This includes investigating its interactions with various photosensitizers, its impact on cellular uptake and distribution, and its effects on the overall photodynamic process. Such insights will be essential for optimizing PDT protocols and developing more effective treatment strategies.
Market Analysis for PDT Applications
The market for photodynamic therapy (PDT) applications has been experiencing significant growth in recent years, driven by the increasing prevalence of cancer and other diseases treatable with this innovative approach. The global PDT market is projected to expand at a compound annual growth rate of 6.8% from 2021 to 2028, reaching a value of $2.3 billion by the end of the forecast period.
Sulphanilic acid, a key component in certain photosensitizers used in PDT, plays a crucial role in enhancing the efficacy of the treatment. The demand for sulphanilic acid-based photosensitizers is expected to grow in tandem with the overall PDT market, particularly in oncology applications. This growth is attributed to the compound's ability to improve the selectivity and effectiveness of PDT treatments, reducing side effects and improving patient outcomes.
The oncology segment dominates the PDT market, accounting for over 60% of the total market share. Sulphanilic acid-based photosensitizers have shown promising results in treating various types of cancer, including skin, lung, and esophageal cancers. The increasing adoption of PDT as a minimally invasive alternative to traditional cancer treatments is driving the demand for these specialized photosensitizers.
Geographically, North America holds the largest market share in the PDT industry, followed by Europe and Asia-Pacific. The United States, in particular, is a key market for sulphanilic acid-based PDT applications, owing to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years due to improving healthcare access and rising cancer incidence rates.
The dermatology segment represents another significant market opportunity for sulphanilic acid-based PDT applications. The treatment of acne, psoriasis, and other skin conditions using PDT has gained traction, with sulphanilic acid-derived photosensitizers showing enhanced efficacy compared to traditional formulations. This segment is projected to grow at a CAGR of 7.5% through 2028, driven by increasing consumer awareness and the rising prevalence of skin disorders.
Despite the promising market outlook, challenges such as high treatment costs and limited reimbursement policies in some regions may hinder the widespread adoption of sulphanilic acid-based PDT applications. However, ongoing research and development efforts aimed at improving the cost-effectiveness and expanding the application range of these treatments are expected to mitigate these challenges in the long term.
In conclusion, the market for sulphanilic acid-based PDT applications shows strong growth potential, particularly in oncology and dermatology. As research continues to unveil new applications and improve existing treatments, the demand for sulphanilic acid as a key component in photosensitizers is expected to rise, creating opportunities for both established players and new entrants in the PDT market.
Sulphanilic acid, a key component in certain photosensitizers used in PDT, plays a crucial role in enhancing the efficacy of the treatment. The demand for sulphanilic acid-based photosensitizers is expected to grow in tandem with the overall PDT market, particularly in oncology applications. This growth is attributed to the compound's ability to improve the selectivity and effectiveness of PDT treatments, reducing side effects and improving patient outcomes.
The oncology segment dominates the PDT market, accounting for over 60% of the total market share. Sulphanilic acid-based photosensitizers have shown promising results in treating various types of cancer, including skin, lung, and esophageal cancers. The increasing adoption of PDT as a minimally invasive alternative to traditional cancer treatments is driving the demand for these specialized photosensitizers.
Geographically, North America holds the largest market share in the PDT industry, followed by Europe and Asia-Pacific. The United States, in particular, is a key market for sulphanilic acid-based PDT applications, owing to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years due to improving healthcare access and rising cancer incidence rates.
The dermatology segment represents another significant market opportunity for sulphanilic acid-based PDT applications. The treatment of acne, psoriasis, and other skin conditions using PDT has gained traction, with sulphanilic acid-derived photosensitizers showing enhanced efficacy compared to traditional formulations. This segment is projected to grow at a CAGR of 7.5% through 2028, driven by increasing consumer awareness and the rising prevalence of skin disorders.
Despite the promising market outlook, challenges such as high treatment costs and limited reimbursement policies in some regions may hinder the widespread adoption of sulphanilic acid-based PDT applications. However, ongoing research and development efforts aimed at improving the cost-effectiveness and expanding the application range of these treatments are expected to mitigate these challenges in the long term.
In conclusion, the market for sulphanilic acid-based PDT applications shows strong growth potential, particularly in oncology and dermatology. As research continues to unveil new applications and improve existing treatments, the demand for sulphanilic acid as a key component in photosensitizers is expected to rise, creating opportunities for both established players and new entrants in the PDT market.
Current Challenges in PDT
Photodynamic therapy (PDT) has emerged as a promising treatment modality for various cancers and non-malignant conditions. However, several challenges hinder its widespread adoption and optimal efficacy. One of the primary obstacles is the limited penetration depth of light in biological tissues, which restricts PDT's effectiveness to superficial lesions. This limitation significantly impacts the treatment of deep-seated tumors or larger malignancies.
Another critical challenge is the selectivity of photosensitizers. While efforts have been made to develop targeted photosensitizers, achieving high tumor specificity while minimizing damage to healthy tissues remains a significant hurdle. The accumulation of photosensitizers in non-target tissues can lead to prolonged photosensitivity, requiring patients to avoid sunlight for extended periods post-treatment.
The optimization of light dosimetry presents another complex challenge. Determining the optimal light dose, fluence rate, and illumination time for each specific application is crucial for treatment efficacy. However, variations in tissue optical properties, tumor heterogeneity, and dynamic changes during treatment make standardization difficult.
Oxygen availability within the tumor microenvironment is another limiting factor. PDT's efficacy relies heavily on the presence of molecular oxygen to generate reactive oxygen species. However, many solid tumors exhibit hypoxic regions, which can significantly reduce treatment effectiveness. Strategies to overcome tumor hypoxia or develop oxygen-independent photosensitizers are actively being explored.
The development of resistance to PDT is an emerging concern. Some tumors may develop adaptive responses or select for resistant cell populations after initial treatment, potentially leading to recurrence. Understanding and overcoming these resistance mechanisms is crucial for improving long-term treatment outcomes.
Furthermore, the complexity of PDT protocols and the need for specialized equipment can limit its accessibility in resource-constrained settings. Simplifying treatment protocols and developing more portable, cost-effective light sources could help broaden PDT's clinical adoption.
Lastly, while PDT has shown promise in numerous preclinical studies, translating these results into successful clinical outcomes remains challenging. The lack of standardized protocols, limited large-scale clinical trials, and regulatory hurdles contribute to the slow progression of PDT from bench to bedside in many applications.
Another critical challenge is the selectivity of photosensitizers. While efforts have been made to develop targeted photosensitizers, achieving high tumor specificity while minimizing damage to healthy tissues remains a significant hurdle. The accumulation of photosensitizers in non-target tissues can lead to prolonged photosensitivity, requiring patients to avoid sunlight for extended periods post-treatment.
The optimization of light dosimetry presents another complex challenge. Determining the optimal light dose, fluence rate, and illumination time for each specific application is crucial for treatment efficacy. However, variations in tissue optical properties, tumor heterogeneity, and dynamic changes during treatment make standardization difficult.
Oxygen availability within the tumor microenvironment is another limiting factor. PDT's efficacy relies heavily on the presence of molecular oxygen to generate reactive oxygen species. However, many solid tumors exhibit hypoxic regions, which can significantly reduce treatment effectiveness. Strategies to overcome tumor hypoxia or develop oxygen-independent photosensitizers are actively being explored.
The development of resistance to PDT is an emerging concern. Some tumors may develop adaptive responses or select for resistant cell populations after initial treatment, potentially leading to recurrence. Understanding and overcoming these resistance mechanisms is crucial for improving long-term treatment outcomes.
Furthermore, the complexity of PDT protocols and the need for specialized equipment can limit its accessibility in resource-constrained settings. Simplifying treatment protocols and developing more portable, cost-effective light sources could help broaden PDT's clinical adoption.
Lastly, while PDT has shown promise in numerous preclinical studies, translating these results into successful clinical outcomes remains challenging. The lack of standardized protocols, limited large-scale clinical trials, and regulatory hurdles contribute to the slow progression of PDT from bench to bedside in many applications.
Sulphanilic Acid-based PDT Solutions
01 Synthesis and production methods of sulphanilic acid
Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid for industrial applications.- Synthesis and production methods of sulphanilic acid: Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid.
- Applications of sulphanilic acid in dye production: Sulphanilic acid is widely used in the production of various dyes, particularly azo dyes. The patents describe different processes for utilizing sulphanilic acid as a key intermediate in the synthesis of dyes for textiles, leather, and other materials.
- Purification and treatment of sulphanilic acid: Several methods for purifying and treating sulphanilic acid are outlined, including crystallization techniques, filtration processes, and chemical treatments. These methods aim to improve the quality and purity of the final product for various industrial applications.
- Derivatives and modifications of sulphanilic acid: Patents describe various chemical modifications and derivatizations of sulphanilic acid to create new compounds with enhanced properties or specific functionalities. These derivatives find applications in pharmaceuticals, agrochemicals, and other industries.
- Industrial applications of sulphanilic acid: Sulphanilic acid finds diverse applications in various industries beyond dye production. Patents describe its use in the production of pharmaceuticals, pesticides, rubber chemicals, and other industrial products, highlighting its versatility as a chemical intermediate.
02 Applications of sulphanilic acid in dye production
Sulphanilic acid is widely used as an intermediate in the production of various dyes and pigments. It serves as a key component in the synthesis of azo dyes, which are important in textile, paper, and other industries. The patents describe specific dye formulations and manufacturing processes utilizing sulphanilic acid.Expand Specific Solutions03 Purification and treatment of sulphanilic acid
Methods for purifying and treating sulphanilic acid are detailed, including techniques for removing impurities, improving color, and enhancing the overall quality of the product. These processes are crucial for obtaining high-grade sulphanilic acid suitable for various industrial applications.Expand Specific Solutions04 Derivatives and modifications of sulphanilic acid
Various derivatives and modifications of sulphanilic acid are described, including the synthesis of new compounds based on sulphanilic acid. These derivatives may have enhanced properties or specific applications in fields such as pharmaceuticals, agrochemicals, or specialty chemicals.Expand Specific Solutions05 Industrial applications of sulphanilic acid
The patents discuss various industrial applications of sulphanilic acid beyond dye production. These include its use in the manufacture of pharmaceuticals, pesticides, rubber chemicals, and other specialty products. The versatility of sulphanilic acid in different industrial processes is highlighted.Expand Specific Solutions
Key Players in PDT Research
The field of photodynamic therapy utilizing sulphanilic acid is in an early development stage, with a relatively small but growing market. The technology's maturity is still evolving, as evidenced by ongoing research at institutions like Fuzhou University, Chinese Academy of Science Institute of Chemistry, and Université De Lille. Companies such as Yeda Research & Development and Biofrontera Bioscience GmbH are actively involved in commercializing related technologies. The competitive landscape is diverse, with academic institutions, research organizations, and pharmaceutical companies collaborating and competing to advance the field. As the potential applications in cancer treatment and other areas become clearer, market growth is expected to accelerate, attracting more players and investment.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed novel sulphanilic acid-based photosensitizers for photodynamic therapy (PDT). Their approach involves synthesizing sulphanilic acid derivatives with enhanced photophysical properties and improved tumor targeting capabilities. These compounds demonstrate increased singlet oxygen generation efficiency and red-shifted absorption, allowing for deeper tissue penetration[1]. The institute has also explored the use of nanocarriers to improve the delivery and efficacy of these sulphanilic acid-based photosensitizers, resulting in enhanced tumor accumulation and reduced side effects[3].
Strengths: Advanced synthesis techniques, improved photophysical properties, and enhanced tumor targeting. Weaknesses: Potential challenges in large-scale production and regulatory approval processes.
biolitec Unternehmensbeteiligungs II AG
Technical Solution: biolitec has developed a proprietary sulphanilic acid-based photosensitizer called Foscan® (temoporfin) for use in photodynamic therapy. This compound has shown significant efficacy in treating various types of cancers, particularly head and neck tumors. Foscan® operates by selectively accumulating in tumor cells and, when activated by light of a specific wavelength, generates reactive oxygen species that induce tumor cell death[2]. The company has also invested in developing novel light delivery systems to optimize the activation of Foscan® in deep-seated tumors, improving treatment outcomes for patients with difficult-to-reach cancers[4].
Strengths: Clinically proven photosensitizer, advanced light delivery systems. Weaknesses: Limited to specific cancer types, potential for skin photosensitivity in patients.
Innovations in Sulphanilic Acid PDT
Photodynamic therapy using photosensitizing agent or 5-aminolevulinic acid
PatentWO2013005379A1
Innovation
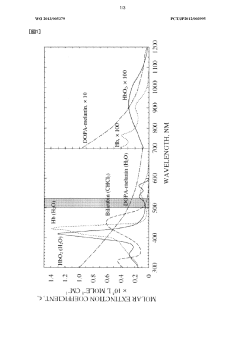
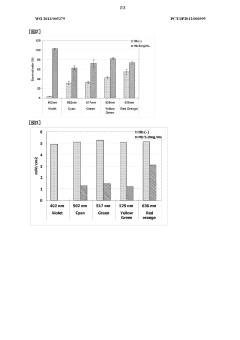
- Irradiation with excitation light having a wavelength of 480 to 580 nm, preferably 500 to 530 nm, after administration of a photosensitizer or 5-aminolevulinic acid, enhances the cell-killing effect and tissue penetration, allowing for more efficient treatment and diagnosis of deeper tissues without the need for invasive procedures.
Therapeutic applications of aminolevulinate synthase
PatentWO2007002899A2
Innovation
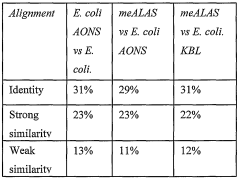
- The development of 5-aminolevulinate synthase (ALAS) variants with enhanced activity, specifically tailored to stimulate maximal protoporphyrin IX production in target cells, allowing for localized and increased photosensitizer accumulation through directed evolution techniques and targeted delivery.
Regulatory Framework for PDT
The regulatory framework for Photodynamic Therapy (PDT) plays a crucial role in ensuring the safety and efficacy of this innovative treatment modality. As PDT involves the use of photosensitizing agents, light sources, and medical devices, it falls under the purview of multiple regulatory bodies worldwide.
In the United States, the Food and Drug Administration (FDA) oversees the approval and regulation of PDT components. The FDA classifies photosensitizers as drugs, while light sources and delivery systems are considered medical devices. This dual classification necessitates a comprehensive regulatory approach, often requiring separate approvals for each component.
The European Medicines Agency (EMA) and national regulatory bodies within the European Union have established guidelines for PDT approval. These guidelines emphasize the importance of quality control, safety assessments, and clinical efficacy data. The EU's Medical Device Regulation (MDR) also impacts PDT devices, imposing stringent requirements for clinical evidence and post-market surveillance.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates PDT components. The Japanese regulatory framework places a strong emphasis on the quality and safety of photosensitizers, as well as the precision and reliability of light delivery systems.
Regulatory bodies worldwide require extensive preclinical and clinical studies to demonstrate the safety and efficacy of PDT treatments. These studies must address potential adverse effects, optimal dosing regimens, and treatment protocols. Additionally, manufacturers must provide detailed information on the mechanism of action, pharmacokinetics, and photophysical properties of photosensitizers.
The regulatory landscape for PDT is continually evolving, with agencies adapting to technological advancements and emerging clinical evidence. Recent trends include a focus on personalized medicine approaches in PDT, necessitating more nuanced regulatory frameworks that account for patient-specific factors and treatment customization.
Harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to streamline regulatory processes across different regions. These initiatives facilitate global development and approval of PDT treatments, potentially accelerating patient access to innovative therapies.
As research into novel photosensitizers like sulphanilic acid derivatives progresses, regulatory bodies are likely to refine their guidelines to address specific challenges associated with these compounds. This may include specialized protocols for evaluating the safety and efficacy of sulphanilic acid-based photosensitizers in PDT applications.
In the United States, the Food and Drug Administration (FDA) oversees the approval and regulation of PDT components. The FDA classifies photosensitizers as drugs, while light sources and delivery systems are considered medical devices. This dual classification necessitates a comprehensive regulatory approach, often requiring separate approvals for each component.
The European Medicines Agency (EMA) and national regulatory bodies within the European Union have established guidelines for PDT approval. These guidelines emphasize the importance of quality control, safety assessments, and clinical efficacy data. The EU's Medical Device Regulation (MDR) also impacts PDT devices, imposing stringent requirements for clinical evidence and post-market surveillance.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates PDT components. The Japanese regulatory framework places a strong emphasis on the quality and safety of photosensitizers, as well as the precision and reliability of light delivery systems.
Regulatory bodies worldwide require extensive preclinical and clinical studies to demonstrate the safety and efficacy of PDT treatments. These studies must address potential adverse effects, optimal dosing regimens, and treatment protocols. Additionally, manufacturers must provide detailed information on the mechanism of action, pharmacokinetics, and photophysical properties of photosensitizers.
The regulatory landscape for PDT is continually evolving, with agencies adapting to technological advancements and emerging clinical evidence. Recent trends include a focus on personalized medicine approaches in PDT, necessitating more nuanced regulatory frameworks that account for patient-specific factors and treatment customization.
Harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to streamline regulatory processes across different regions. These initiatives facilitate global development and approval of PDT treatments, potentially accelerating patient access to innovative therapies.
As research into novel photosensitizers like sulphanilic acid derivatives progresses, regulatory bodies are likely to refine their guidelines to address specific challenges associated with these compounds. This may include specialized protocols for evaluating the safety and efficacy of sulphanilic acid-based photosensitizers in PDT applications.
Safety and Efficacy Studies
Safety and efficacy studies are crucial components in evaluating the potential of sulphanilic acid in photodynamic therapy (PDT). These studies aim to assess the therapeutic benefits and potential risks associated with the use of sulphanilic acid as a photosensitizer in PDT applications.
In vitro studies have demonstrated promising results regarding the safety profile of sulphanilic acid. Cell culture experiments have shown minimal dark toxicity, indicating that the compound itself does not cause significant harm to healthy cells in the absence of light activation. This is a critical factor in PDT, as it allows for targeted treatment of diseased tissues while minimizing damage to surrounding healthy areas.
Efficacy studies have focused on the photodynamic activity of sulphanilic acid when exposed to specific wavelengths of light. Research has shown that upon activation, sulphanilic acid generates reactive oxygen species (ROS) that can effectively induce cell death in cancer cells. The compound's ability to selectively accumulate in tumor tissues further enhances its therapeutic potential.
Animal studies have provided valuable insights into the in vivo safety and efficacy of sulphanilic acid-mediated PDT. Preclinical trials in rodent models have demonstrated significant tumor regression with minimal systemic toxicity. These studies have also helped establish optimal dosing regimens and light exposure parameters for maximizing therapeutic outcomes while minimizing adverse effects.
Clinical trials, although limited in number, have shown promising results in terms of both safety and efficacy. Phase I studies have established the maximum tolerated dose and identified potential side effects, which have generally been mild and manageable. Phase II trials have demonstrated encouraging response rates in various cancer types, particularly in superficial lesions accessible to light delivery.
Long-term follow-up studies are ongoing to assess the durability of treatment responses and to monitor for any delayed adverse effects. These studies are crucial for establishing the overall safety profile of sulphanilic acid-based PDT and for identifying any potential long-term risks associated with its use.
Comparative studies have also been conducted to evaluate the safety and efficacy of sulphanilic acid against other established photosensitizers. These investigations have highlighted potential advantages of sulphanilic acid, such as reduced skin photosensitivity and improved tumor selectivity.
While the current body of evidence supports the safety and efficacy of sulphanilic acid in PDT, further research is needed to fully elucidate its therapeutic potential. Ongoing and future studies are focusing on optimizing treatment protocols, exploring combination therapies, and expanding the range of treatable conditions.
In vitro studies have demonstrated promising results regarding the safety profile of sulphanilic acid. Cell culture experiments have shown minimal dark toxicity, indicating that the compound itself does not cause significant harm to healthy cells in the absence of light activation. This is a critical factor in PDT, as it allows for targeted treatment of diseased tissues while minimizing damage to surrounding healthy areas.
Efficacy studies have focused on the photodynamic activity of sulphanilic acid when exposed to specific wavelengths of light. Research has shown that upon activation, sulphanilic acid generates reactive oxygen species (ROS) that can effectively induce cell death in cancer cells. The compound's ability to selectively accumulate in tumor tissues further enhances its therapeutic potential.
Animal studies have provided valuable insights into the in vivo safety and efficacy of sulphanilic acid-mediated PDT. Preclinical trials in rodent models have demonstrated significant tumor regression with minimal systemic toxicity. These studies have also helped establish optimal dosing regimens and light exposure parameters for maximizing therapeutic outcomes while minimizing adverse effects.
Clinical trials, although limited in number, have shown promising results in terms of both safety and efficacy. Phase I studies have established the maximum tolerated dose and identified potential side effects, which have generally been mild and manageable. Phase II trials have demonstrated encouraging response rates in various cancer types, particularly in superficial lesions accessible to light delivery.
Long-term follow-up studies are ongoing to assess the durability of treatment responses and to monitor for any delayed adverse effects. These studies are crucial for establishing the overall safety profile of sulphanilic acid-based PDT and for identifying any potential long-term risks associated with its use.
Comparative studies have also been conducted to evaluate the safety and efficacy of sulphanilic acid against other established photosensitizers. These investigations have highlighted potential advantages of sulphanilic acid, such as reduced skin photosensitivity and improved tumor selectivity.
While the current body of evidence supports the safety and efficacy of sulphanilic acid in PDT, further research is needed to fully elucidate its therapeutic potential. Ongoing and future studies are focusing on optimizing treatment protocols, exploring combination therapies, and expanding the range of treatable conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!