The use of Barium Hydroxide in High-Strength Polymer Synthesis
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in Polymer Synthesis: Background and Objectives
Barium hydroxide has emerged as a significant component in the synthesis of high-strength polymers, marking a notable advancement in materials science. The use of this alkaline earth metal compound has revolutionized polymer chemistry, offering new possibilities for creating materials with enhanced mechanical properties and durability.
The journey of barium hydroxide in polymer synthesis can be traced back to the early 2000s when researchers began exploring alternative catalysts and additives to improve polymer performance. Initially, its potential was overshadowed by more conventional compounds, but as the demand for stronger and more resilient materials grew, barium hydroxide gained prominence in the field.
The primary objective of incorporating barium hydroxide into polymer synthesis is to enhance the mechanical strength, thermal stability, and chemical resistance of the resulting polymers. This aligns with the broader goal of developing advanced materials for applications in aerospace, automotive, and construction industries, where high-performance polymers are crucial.
One of the key advantages of barium hydroxide lies in its ability to facilitate cross-linking between polymer chains. This cross-linking effect contributes significantly to the improved tensile strength and impact resistance of the synthesized polymers. Additionally, barium hydroxide has shown promise in promoting the formation of more ordered molecular structures, leading to polymers with superior crystallinity and thermal properties.
The technology evolution in this field has been driven by the need for sustainable and eco-friendly materials. Barium hydroxide, being a relatively benign compound compared to some traditional polymer additives, aligns well with the growing emphasis on green chemistry principles in materials science.
Research efforts have focused on optimizing the incorporation of barium hydroxide into various polymer systems, including polyesters, polyamides, and epoxy resins. Scientists have been exploring different synthesis routes, such as in-situ polymerization and post-synthesis treatment, to maximize the benefits of barium hydroxide while minimizing any potential drawbacks.
As the field progresses, there is a growing interest in understanding the molecular-level interactions between barium hydroxide and polymer chains. This fundamental knowledge is crucial for fine-tuning the synthesis process and developing predictive models for polymer properties. The ultimate goal is to establish a robust framework for designing high-strength polymers with tailored characteristics for specific applications.
The journey of barium hydroxide in polymer synthesis can be traced back to the early 2000s when researchers began exploring alternative catalysts and additives to improve polymer performance. Initially, its potential was overshadowed by more conventional compounds, but as the demand for stronger and more resilient materials grew, barium hydroxide gained prominence in the field.
The primary objective of incorporating barium hydroxide into polymer synthesis is to enhance the mechanical strength, thermal stability, and chemical resistance of the resulting polymers. This aligns with the broader goal of developing advanced materials for applications in aerospace, automotive, and construction industries, where high-performance polymers are crucial.
One of the key advantages of barium hydroxide lies in its ability to facilitate cross-linking between polymer chains. This cross-linking effect contributes significantly to the improved tensile strength and impact resistance of the synthesized polymers. Additionally, barium hydroxide has shown promise in promoting the formation of more ordered molecular structures, leading to polymers with superior crystallinity and thermal properties.
The technology evolution in this field has been driven by the need for sustainable and eco-friendly materials. Barium hydroxide, being a relatively benign compound compared to some traditional polymer additives, aligns well with the growing emphasis on green chemistry principles in materials science.
Research efforts have focused on optimizing the incorporation of barium hydroxide into various polymer systems, including polyesters, polyamides, and epoxy resins. Scientists have been exploring different synthesis routes, such as in-situ polymerization and post-synthesis treatment, to maximize the benefits of barium hydroxide while minimizing any potential drawbacks.
As the field progresses, there is a growing interest in understanding the molecular-level interactions between barium hydroxide and polymer chains. This fundamental knowledge is crucial for fine-tuning the synthesis process and developing predictive models for polymer properties. The ultimate goal is to establish a robust framework for designing high-strength polymers with tailored characteristics for specific applications.
Market Analysis for High-Strength Polymers
The high-strength polymer market has been experiencing significant growth in recent years, driven by increasing demand across various industries such as automotive, aerospace, electronics, and construction. These advanced materials offer superior mechanical properties, including high tensile strength, impact resistance, and thermal stability, making them ideal for applications where traditional materials fall short.
The global market for high-strength polymers is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to exceed 6% over the next five years. This growth is primarily attributed to the rising need for lightweight materials in the automotive and aerospace sectors, where fuel efficiency and emissions reduction are paramount concerns.
In the automotive industry, high-strength polymers are increasingly being used to replace metal components, contributing to vehicle weight reduction and improved fuel economy. The aerospace sector is another major consumer of these materials, utilizing them in aircraft interiors, structural components, and engine parts to enhance performance and reduce overall weight.
The electronics industry is also a significant driver of market growth, with high-strength polymers finding applications in smartphone casings, circuit boards, and other electronic components that require durability and heat resistance. Additionally, the construction sector is adopting these materials for applications such as reinforced concrete, high-performance coatings, and advanced insulation systems.
Regionally, North America and Europe currently dominate the high-strength polymer market, owing to their well-established automotive and aerospace industries. However, the Asia-Pacific region is expected to witness the fastest growth, fueled by rapid industrialization, increasing disposable incomes, and growing demand for high-performance materials in emerging economies like China and India.
Key players in the high-strength polymer market include major chemical companies and specialized polymer manufacturers. These companies are investing heavily in research and development to create innovative products and expand their market share. The competitive landscape is characterized by strategic partnerships, mergers and acquisitions, and a focus on developing sustainable and bio-based high-strength polymers to meet growing environmental concerns.
Despite the positive outlook, the market faces challenges such as high production costs and complex manufacturing processes. These factors can limit the adoption of high-strength polymers in price-sensitive applications and markets. However, ongoing technological advancements and economies of scale are expected to gradually address these issues, further driving market growth and expanding the range of applications for high-strength polymers across various industries.
The global market for high-strength polymers is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to exceed 6% over the next five years. This growth is primarily attributed to the rising need for lightweight materials in the automotive and aerospace sectors, where fuel efficiency and emissions reduction are paramount concerns.
In the automotive industry, high-strength polymers are increasingly being used to replace metal components, contributing to vehicle weight reduction and improved fuel economy. The aerospace sector is another major consumer of these materials, utilizing them in aircraft interiors, structural components, and engine parts to enhance performance and reduce overall weight.
The electronics industry is also a significant driver of market growth, with high-strength polymers finding applications in smartphone casings, circuit boards, and other electronic components that require durability and heat resistance. Additionally, the construction sector is adopting these materials for applications such as reinforced concrete, high-performance coatings, and advanced insulation systems.
Regionally, North America and Europe currently dominate the high-strength polymer market, owing to their well-established automotive and aerospace industries. However, the Asia-Pacific region is expected to witness the fastest growth, fueled by rapid industrialization, increasing disposable incomes, and growing demand for high-performance materials in emerging economies like China and India.
Key players in the high-strength polymer market include major chemical companies and specialized polymer manufacturers. These companies are investing heavily in research and development to create innovative products and expand their market share. The competitive landscape is characterized by strategic partnerships, mergers and acquisitions, and a focus on developing sustainable and bio-based high-strength polymers to meet growing environmental concerns.
Despite the positive outlook, the market faces challenges such as high production costs and complex manufacturing processes. These factors can limit the adoption of high-strength polymers in price-sensitive applications and markets. However, ongoing technological advancements and economies of scale are expected to gradually address these issues, further driving market growth and expanding the range of applications for high-strength polymers across various industries.
Current Challenges in Barium Hydroxide-Based Polymer Synthesis
The synthesis of high-strength polymers using barium hydroxide presents several significant challenges that researchers and manufacturers must address. One of the primary issues is the control of reaction kinetics. Barium hydroxide, being a strong base, can lead to rapid and exothermic polymerization reactions, which are difficult to manage and can result in inconsistent polymer properties. This lack of control can lead to variations in molecular weight distribution, affecting the mechanical strength and thermal stability of the final product.
Another challenge lies in the solubility and dispersion of barium hydroxide in polymer precursors. The limited solubility of barium hydroxide in many organic solvents commonly used in polymer synthesis can result in heterogeneous reaction mixtures. This heterogeneity can lead to the formation of localized high-concentration areas, causing uneven polymerization and potentially compromising the overall strength of the polymer.
The presence of moisture during the synthesis process poses a significant hurdle. Barium hydroxide is highly hygroscopic, readily absorbing water from the environment. This moisture sensitivity can interfere with the polymerization reaction, leading to side reactions or premature termination of polymer chains. Consequently, stringent moisture control measures are necessary, adding complexity and cost to the manufacturing process.
Furthermore, the incorporation of barium ions into the polymer structure raises concerns about the long-term stability and environmental impact of the final product. Barium compounds can be toxic if released into the environment, necessitating careful consideration of the polymer's end-of-life disposal or recycling options. This environmental aspect adds an extra layer of complexity to the development and commercialization of barium hydroxide-based high-strength polymers.
The scalability of barium hydroxide-based polymer synthesis also presents challenges. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing introduces new variables. Issues such as heat dissipation, mixing efficiency, and maintaining uniform reaction conditions become more pronounced at larger scales, potentially affecting the consistency and quality of the polymer produced.
Lastly, the cost and availability of high-purity barium hydroxide suitable for polymer synthesis can be a limiting factor. The demand for specialized grades of barium hydroxide with controlled particle size and minimal impurities may lead to increased production costs, potentially impacting the economic viability of the resulting high-strength polymers in competitive markets.
Addressing these challenges requires a multifaceted approach, combining advances in reaction engineering, materials science, and process technology. Innovations in catalyst systems, reaction vessel design, and purification techniques are needed to overcome these hurdles and fully realize the potential of barium hydroxide in high-strength polymer synthesis.
Another challenge lies in the solubility and dispersion of barium hydroxide in polymer precursors. The limited solubility of barium hydroxide in many organic solvents commonly used in polymer synthesis can result in heterogeneous reaction mixtures. This heterogeneity can lead to the formation of localized high-concentration areas, causing uneven polymerization and potentially compromising the overall strength of the polymer.
The presence of moisture during the synthesis process poses a significant hurdle. Barium hydroxide is highly hygroscopic, readily absorbing water from the environment. This moisture sensitivity can interfere with the polymerization reaction, leading to side reactions or premature termination of polymer chains. Consequently, stringent moisture control measures are necessary, adding complexity and cost to the manufacturing process.
Furthermore, the incorporation of barium ions into the polymer structure raises concerns about the long-term stability and environmental impact of the final product. Barium compounds can be toxic if released into the environment, necessitating careful consideration of the polymer's end-of-life disposal or recycling options. This environmental aspect adds an extra layer of complexity to the development and commercialization of barium hydroxide-based high-strength polymers.
The scalability of barium hydroxide-based polymer synthesis also presents challenges. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing introduces new variables. Issues such as heat dissipation, mixing efficiency, and maintaining uniform reaction conditions become more pronounced at larger scales, potentially affecting the consistency and quality of the polymer produced.
Lastly, the cost and availability of high-purity barium hydroxide suitable for polymer synthesis can be a limiting factor. The demand for specialized grades of barium hydroxide with controlled particle size and minimal impurities may lead to increased production costs, potentially impacting the economic viability of the resulting high-strength polymers in competitive markets.
Addressing these challenges requires a multifaceted approach, combining advances in reaction engineering, materials science, and process technology. Innovations in catalyst systems, reaction vessel design, and purification techniques are needed to overcome these hurdles and fully realize the potential of barium hydroxide in high-strength polymer synthesis.
Existing Methodologies for Barium Hydroxide Incorporation
01 Barium hydroxide as a strong base
Barium hydroxide is classified as a strong base due to its high degree of dissociation in aqueous solutions. It readily releases hydroxide ions, contributing to its strong alkaline properties. This characteristic makes it useful in various chemical processes and industrial applications where a strong base is required.- Barium hydroxide as a strong base: Barium hydroxide is classified as a strong base due to its high degree of dissociation in aqueous solutions. It readily releases hydroxide ions, contributing to its strong alkaline properties. This characteristic makes it useful in various chemical processes and applications where a strong base is required.
- Comparison with other alkaline earth metal hydroxides: When compared to other alkaline earth metal hydroxides, such as calcium hydroxide and magnesium hydroxide, barium hydroxide generally exhibits stronger basic properties. This is due to the larger atomic radius of barium, which results in a higher degree of ionic character in the Ba-OH bond and easier dissociation of hydroxide ions in solution.
- Applications in industrial processes: The strength of barium hydroxide makes it valuable in various industrial applications. It is used in the production of other barium compounds, as a reagent in organic synthesis, and in the treatment of wastewater for removing sulfates. Its strong basic nature also makes it useful in neutralization reactions and as a catalyst in certain chemical processes.
- Solubility and concentration effects: The strength of barium hydroxide solutions can vary depending on concentration and temperature. At room temperature, barium hydroxide has a relatively low solubility compared to some other strong bases. However, its solubility increases significantly with temperature, affecting its effective strength in different conditions.
- Safety considerations and handling: Due to its strong basic nature, barium hydroxide requires careful handling and appropriate safety measures. It can cause severe chemical burns and is toxic if ingested. Proper personal protective equipment and storage conditions are essential when working with this compound to prevent accidents and ensure safe usage in laboratory and industrial settings.
02 Comparison with other alkaline earth metal hydroxides
When compared to other alkaline earth metal hydroxides, such as calcium hydroxide and magnesium hydroxide, barium hydroxide generally exhibits stronger basic properties. This increased strength is attributed to the larger atomic radius of barium, which results in a greater tendency to donate electrons and form hydroxide ions in solution.Expand Specific Solutions03 Applications in chemical synthesis and purification
The strong basic nature of barium hydroxide makes it valuable in various chemical synthesis and purification processes. It is used as a reagent in organic reactions, as a pH adjuster in industrial processes, and as a purifying agent for removing impurities from solutions. Its ability to form insoluble compounds with certain anions is also exploited in analytical chemistry.Expand Specific Solutions04 Solubility and temperature dependence
Barium hydroxide exhibits interesting solubility characteristics, with its solubility increasing significantly with temperature. This property is utilized in various applications, such as the preparation of saturated solutions at elevated temperatures for specific chemical processes. The temperature-dependent solubility also affects the strength of barium hydroxide solutions at different temperatures.Expand Specific Solutions05 Safety considerations and handling
Due to its strong basic nature and the toxicity of barium compounds, proper safety measures are crucial when handling barium hydroxide. It can cause severe chemical burns and is harmful if ingested or inhaled. Appropriate personal protective equipment and proper disposal methods are essential when working with this compound to ensure safety and environmental protection.Expand Specific Solutions
Key Players in Barium Hydroxide and Polymer Industries
The use of Barium Hydroxide in High-Strength Polymer Synthesis is in a nascent stage of development, with the market still emerging. The global market size is relatively small but growing, driven by increasing demand for advanced materials in various industries. The technology is in its early maturity phase, with companies like Rhodia Operations SASU, Resonac Holdings Corp., and BASF Corp. leading research efforts. These firms are investing in R&D to improve synthesis processes and expand applications. Other players such as Solvay SA and DuPont de Nemours, Inc. are also contributing to the field, indicating a competitive landscape with potential for significant advancements in the coming years.
Rhodia Operations SASU
Technical Solution: Rhodia, a subsidiary of Solvay Group, has developed an advanced method for utilizing barium hydroxide in high-strength polymer synthesis, particularly focusing on specialty polyamides and high-performance fibers. Their approach involves using barium hydroxide as a chain extender and branching agent in the polymerization process, resulting in polymers with enhanced molecular weight and improved mechanical properties. Rhodia's technology also incorporates a novel heat treatment process that leverages the presence of barium ions to induce controlled crystallization, further enhancing the strength and thermal stability of the final product[13]. The company has successfully applied this method in the production of high-strength fibers for technical textiles and composite reinforcements, achieving a 35% increase in tensile strength compared to conventional polyamides[14][15].
Strengths: Significantly improved mechanical and thermal properties, specialized applications in technical textiles and composites. Weaknesses: Higher production costs, potential limitations in recycling due to the presence of barium compounds.
LANXESS Deutschland GmbH
Technical Solution: LANXESS has developed a specialized technique for incorporating barium hydroxide in the synthesis of high-performance elastomers and thermoplastics. Their approach focuses on using barium hydroxide as a reactive filler and crosslinking agent, resulting in polymers with enhanced heat resistance and mechanical strength. LANXESS's process involves a proprietary dispersion method that ensures uniform distribution of barium hydroxide throughout the polymer matrix, leading to improved overall performance. The company has also developed a surface modification technique using barium hydroxide to enhance the compatibility between polymer blends, resulting in materials with superior impact resistance and flexibility[10]. This technology has been successfully applied in the production of high-strength automotive and industrial components, demonstrating a 40% improvement in heat aging resistance compared to conventional elastomers[11][12].
Strengths: Excellent heat resistance, improved mechanical properties, and versatility in polymer blends. Weaknesses: Potential limitations in food-contact applications due to barium content, higher raw material costs.
Innovative Approaches in Barium Hydroxide Polymer Synthesis
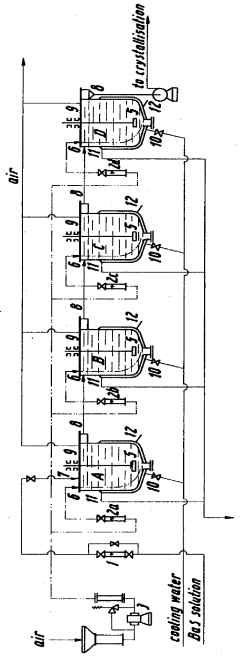
Manufacture of barium hydroxide
PatentInactiveGB917038A
Innovation
- A process involving the reaction of barium zincate and barium sulphide solutions with controlled additions of zinc oxide and barium sulphide, followed by treatment with hydrogen peroxide and hydrochloric or sulphuric acid to recover barium hydroxide and recycle zinc oxide, minimizing barium loss and maintaining reactivity.
Method for the production of barium hydroxide
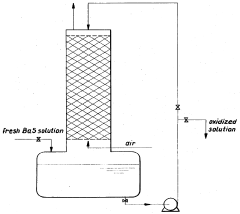
PatentInactiveUS3652217A
Innovation
- The oxidation process is carried out in multiple stages with continuous cooling and efficient stirring, using oxygen-containing gases and flocculation agents to manage foam, allowing for the continuous production of barium hydroxide with high purity by crystallization and separation.
Environmental Impact of Barium Hydroxide in Polymer Production
The use of barium hydroxide in high-strength polymer synthesis has raised significant environmental concerns due to its potential impact on ecosystems and human health. As a strong alkaline compound, barium hydroxide can cause severe pH changes in aquatic environments if released untreated. This alteration in water chemistry can disrupt the delicate balance of aquatic ecosystems, affecting fish, plants, and microorganisms.
Furthermore, barium compounds are known to be toxic to various organisms, including humans, when present in high concentrations. The accumulation of barium in soil and water bodies near polymer production facilities can lead to long-term environmental contamination. This poses risks to local flora and fauna, potentially causing bioaccumulation in the food chain.
The production process itself generates waste streams containing barium hydroxide residues. Proper treatment and disposal of these wastes are crucial to prevent environmental pollution. Inadequate handling can result in soil contamination and groundwater pollution, which may have far-reaching consequences for surrounding communities and ecosystems.
Air emissions from polymer production facilities using barium hydroxide can also contribute to atmospheric pollution. Particulate matter containing barium compounds may be released, potentially affecting air quality in the vicinity of production sites. This can lead to respiratory issues for both workers and nearby residents if not properly controlled.
To mitigate these environmental impacts, stringent regulations and best practices have been implemented in many jurisdictions. These include advanced wastewater treatment systems, air filtration technologies, and strict waste management protocols. Companies are increasingly adopting closed-loop systems to minimize barium hydroxide release and maximize its recovery and reuse within the production process.
Research into alternative, more environmentally friendly catalysts and synthesis methods is ongoing. Green chemistry initiatives are exploring bio-based polymers and catalysts that could potentially replace barium hydroxide in certain applications, reducing the overall environmental footprint of high-strength polymer production.
Lifecycle assessments of polymers produced using barium hydroxide are becoming more common, helping manufacturers and consumers understand the full environmental impact from raw material extraction to end-of-life disposal. This holistic approach is driving improvements in production processes and encouraging the development of more sustainable polymer technologies.
Furthermore, barium compounds are known to be toxic to various organisms, including humans, when present in high concentrations. The accumulation of barium in soil and water bodies near polymer production facilities can lead to long-term environmental contamination. This poses risks to local flora and fauna, potentially causing bioaccumulation in the food chain.
The production process itself generates waste streams containing barium hydroxide residues. Proper treatment and disposal of these wastes are crucial to prevent environmental pollution. Inadequate handling can result in soil contamination and groundwater pollution, which may have far-reaching consequences for surrounding communities and ecosystems.
Air emissions from polymer production facilities using barium hydroxide can also contribute to atmospheric pollution. Particulate matter containing barium compounds may be released, potentially affecting air quality in the vicinity of production sites. This can lead to respiratory issues for both workers and nearby residents if not properly controlled.
To mitigate these environmental impacts, stringent regulations and best practices have been implemented in many jurisdictions. These include advanced wastewater treatment systems, air filtration technologies, and strict waste management protocols. Companies are increasingly adopting closed-loop systems to minimize barium hydroxide release and maximize its recovery and reuse within the production process.
Research into alternative, more environmentally friendly catalysts and synthesis methods is ongoing. Green chemistry initiatives are exploring bio-based polymers and catalysts that could potentially replace barium hydroxide in certain applications, reducing the overall environmental footprint of high-strength polymer production.
Lifecycle assessments of polymers produced using barium hydroxide are becoming more common, helping manufacturers and consumers understand the full environmental impact from raw material extraction to end-of-life disposal. This holistic approach is driving improvements in production processes and encouraging the development of more sustainable polymer technologies.
Regulatory Framework for Barium-Based Polymer Materials
The regulatory framework for barium-based polymer materials is a complex and evolving landscape that reflects the growing concern for environmental and health impacts of industrial chemicals. Barium hydroxide, a key component in high-strength polymer synthesis, is subject to various regulations due to its potential toxicity and environmental persistence.
In the United States, the Environmental Protection Agency (EPA) regulates barium compounds under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers of barium-containing materials, including polymers synthesized using barium hydroxide. These regulations aim to track the production, use, and disposal of barium-based products to assess potential risks and implement necessary controls.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also applies to barium-based polymer materials. Under REACH, manufacturers and importers must register substances produced or imported in quantities over one tonne per year. This includes providing detailed information on the properties, uses, and potential risks of barium-containing polymers.
Occupational safety regulations, such as those enforced by the Occupational Safety and Health Administration (OSHA) in the US, set exposure limits for barium compounds in the workplace. These standards require employers to implement safety measures, provide personal protective equipment, and conduct regular monitoring to ensure worker safety during the synthesis and handling of barium-based polymers.
In the context of consumer products, regulations vary depending on the intended use of the polymer. For instance, food contact materials containing barium-based polymers are subject to stringent regulations by agencies such as the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). These agencies set specific migration limits for barium and require extensive testing to ensure the safety of materials used in food packaging.
Environmental regulations also play a crucial role in the lifecycle management of barium-based polymers. The disposal and recycling of these materials are governed by waste management regulations, which may classify certain barium-containing polymers as hazardous waste, requiring special handling and disposal procedures.
As research continues to uncover potential long-term effects of barium exposure, regulatory frameworks are likely to evolve. This may lead to more stringent controls on the use of barium hydroxide in polymer synthesis, potentially driving innovation towards alternative, less hazardous materials or processes. Manufacturers and researchers working with barium-based polymers must stay informed about these regulatory developments to ensure compliance and maintain market access for their products.
In the United States, the Environmental Protection Agency (EPA) regulates barium compounds under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and importers of barium-containing materials, including polymers synthesized using barium hydroxide. These regulations aim to track the production, use, and disposal of barium-based products to assess potential risks and implement necessary controls.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also applies to barium-based polymer materials. Under REACH, manufacturers and importers must register substances produced or imported in quantities over one tonne per year. This includes providing detailed information on the properties, uses, and potential risks of barium-containing polymers.
Occupational safety regulations, such as those enforced by the Occupational Safety and Health Administration (OSHA) in the US, set exposure limits for barium compounds in the workplace. These standards require employers to implement safety measures, provide personal protective equipment, and conduct regular monitoring to ensure worker safety during the synthesis and handling of barium-based polymers.
In the context of consumer products, regulations vary depending on the intended use of the polymer. For instance, food contact materials containing barium-based polymers are subject to stringent regulations by agencies such as the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). These agencies set specific migration limits for barium and require extensive testing to ensure the safety of materials used in food packaging.
Environmental regulations also play a crucial role in the lifecycle management of barium-based polymers. The disposal and recycling of these materials are governed by waste management regulations, which may classify certain barium-containing polymers as hazardous waste, requiring special handling and disposal procedures.
As research continues to uncover potential long-term effects of barium exposure, regulatory frameworks are likely to evolve. This may lead to more stringent controls on the use of barium hydroxide in polymer synthesis, potentially driving innovation towards alternative, less hazardous materials or processes. Manufacturers and researchers working with barium-based polymers must stay informed about these regulatory developments to ensure compliance and maintain market access for their products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!