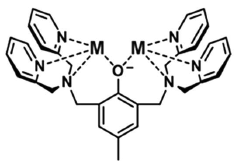
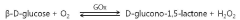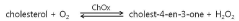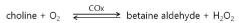The Use of Perchloric Acid in Catalytic Benzylation Reactions
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Catalysis Background and Objectives
Perchloric acid has emerged as a powerful catalyst in organic synthesis, particularly in benzylation reactions. This strong acid, with its unique properties, has revolutionized the field of catalysis, offering enhanced reaction rates and improved selectivity. The history of perchloric acid in catalysis dates back to the early 20th century, but its application in benzylation reactions has gained significant attention in recent decades.
The evolution of perchloric acid catalysis has been driven by the increasing demand for efficient and environmentally friendly synthetic methods. As the chemical industry sought alternatives to traditional Lewis acid catalysts, perchloric acid presented itself as a viable option due to its strong acidity and non-coordinating nature. This led to extensive research into its catalytic properties and potential applications.
The primary objective of utilizing perchloric acid in benzylation reactions is to achieve higher yields, shorter reaction times, and improved product selectivity. Researchers aim to develop methodologies that can be applied to a wide range of substrates, including both activated and deactivated aromatic compounds. Additionally, there is a focus on understanding the mechanistic aspects of perchloric acid catalysis to optimize reaction conditions and expand its applicability.
Another crucial goal is to address the safety concerns associated with perchloric acid usage. Due to its strong oxidizing properties, handling perchloric acid requires stringent safety measures. Developing safer protocols and exploring the use of supported or immobilized perchloric acid catalysts are key objectives in this field.
The technological trend in perchloric acid catalysis is moving towards greener and more sustainable processes. This includes the development of recyclable catalytic systems, the use of alternative reaction media such as ionic liquids, and the integration of perchloric acid catalysis with other emerging technologies like flow chemistry and microwave-assisted synthesis.
Researchers are also exploring the synergistic effects of combining perchloric acid with other catalysts or additives to further enhance reaction efficiency and selectivity. This approach opens up new possibilities for complex organic transformations and the synthesis of valuable pharmaceutical intermediates.
As the field progresses, there is an increasing focus on expanding the scope of perchloric acid catalysis beyond benzylation reactions. Scientists are investigating its potential in other important organic transformations, such as esterification, acetylation, and various carbon-carbon bond-forming reactions. This broadening of applications aims to establish perchloric acid as a versatile catalyst in organic synthesis.
The evolution of perchloric acid catalysis has been driven by the increasing demand for efficient and environmentally friendly synthetic methods. As the chemical industry sought alternatives to traditional Lewis acid catalysts, perchloric acid presented itself as a viable option due to its strong acidity and non-coordinating nature. This led to extensive research into its catalytic properties and potential applications.
The primary objective of utilizing perchloric acid in benzylation reactions is to achieve higher yields, shorter reaction times, and improved product selectivity. Researchers aim to develop methodologies that can be applied to a wide range of substrates, including both activated and deactivated aromatic compounds. Additionally, there is a focus on understanding the mechanistic aspects of perchloric acid catalysis to optimize reaction conditions and expand its applicability.
Another crucial goal is to address the safety concerns associated with perchloric acid usage. Due to its strong oxidizing properties, handling perchloric acid requires stringent safety measures. Developing safer protocols and exploring the use of supported or immobilized perchloric acid catalysts are key objectives in this field.
The technological trend in perchloric acid catalysis is moving towards greener and more sustainable processes. This includes the development of recyclable catalytic systems, the use of alternative reaction media such as ionic liquids, and the integration of perchloric acid catalysis with other emerging technologies like flow chemistry and microwave-assisted synthesis.
Researchers are also exploring the synergistic effects of combining perchloric acid with other catalysts or additives to further enhance reaction efficiency and selectivity. This approach opens up new possibilities for complex organic transformations and the synthesis of valuable pharmaceutical intermediates.
As the field progresses, there is an increasing focus on expanding the scope of perchloric acid catalysis beyond benzylation reactions. Scientists are investigating its potential in other important organic transformations, such as esterification, acetylation, and various carbon-carbon bond-forming reactions. This broadening of applications aims to establish perchloric acid as a versatile catalyst in organic synthesis.
Industrial Demand for Efficient Benzylation Processes
The benzylation process is a crucial reaction in organic synthesis, widely used in the production of pharmaceuticals, agrochemicals, and fine chemicals. As industries strive for more efficient and cost-effective manufacturing processes, there is a growing demand for improved benzylation methods. The use of perchloric acid as a catalyst in these reactions has garnered significant attention due to its potential to enhance reaction efficiency and selectivity.
The pharmaceutical industry, in particular, has shown a strong interest in optimizing benzylation processes. Many drug molecules contain benzyl groups, and the ability to introduce these moieties efficiently can greatly impact the overall synthesis route and production costs. For instance, the synthesis of certain antibiotics, antihypertensive agents, and antidepressants relies on benzylation steps, making improvements in this area highly valuable.
In the agrochemical sector, benzylation reactions play a role in the production of various pesticides and herbicides. As global food demand continues to rise, there is pressure to develop more effective and environmentally friendly crop protection agents. Efficient benzylation processes can contribute to the development of new agrochemical compounds with improved properties and reduced environmental impact.
The fine chemicals industry also benefits from advancements in benzylation technology. Many specialty chemicals, fragrances, and polymer additives incorporate benzyl groups in their structures. As consumer preferences shift towards more sustainable and high-performance products, manufacturers are seeking ways to streamline their production processes and reduce waste.
Environmental concerns and regulatory pressures have further intensified the need for more efficient benzylation methods. Traditional benzylation processes often involve the use of toxic reagents or generate significant amounts of waste. The potential of perchloric acid as a catalyst offers an opportunity to develop greener and more atom-economical reactions, aligning with the principles of sustainable chemistry.
Moreover, the increasing focus on process intensification in the chemical industry has driven interest in catalytic systems that can operate under milder conditions and achieve higher yields. Perchloric acid-catalyzed benzylation reactions have shown promise in this regard, potentially allowing for lower reaction temperatures, shorter reaction times, and improved product selectivity.
As global competition in the chemical industry intensifies, companies are constantly seeking ways to gain a competitive edge through process innovations. The development of more efficient benzylation methods using perchloric acid as a catalyst represents an opportunity for companies to differentiate themselves and potentially secure intellectual property that could be valuable in the long term.
The pharmaceutical industry, in particular, has shown a strong interest in optimizing benzylation processes. Many drug molecules contain benzyl groups, and the ability to introduce these moieties efficiently can greatly impact the overall synthesis route and production costs. For instance, the synthesis of certain antibiotics, antihypertensive agents, and antidepressants relies on benzylation steps, making improvements in this area highly valuable.
In the agrochemical sector, benzylation reactions play a role in the production of various pesticides and herbicides. As global food demand continues to rise, there is pressure to develop more effective and environmentally friendly crop protection agents. Efficient benzylation processes can contribute to the development of new agrochemical compounds with improved properties and reduced environmental impact.
The fine chemicals industry also benefits from advancements in benzylation technology. Many specialty chemicals, fragrances, and polymer additives incorporate benzyl groups in their structures. As consumer preferences shift towards more sustainable and high-performance products, manufacturers are seeking ways to streamline their production processes and reduce waste.
Environmental concerns and regulatory pressures have further intensified the need for more efficient benzylation methods. Traditional benzylation processes often involve the use of toxic reagents or generate significant amounts of waste. The potential of perchloric acid as a catalyst offers an opportunity to develop greener and more atom-economical reactions, aligning with the principles of sustainable chemistry.
Moreover, the increasing focus on process intensification in the chemical industry has driven interest in catalytic systems that can operate under milder conditions and achieve higher yields. Perchloric acid-catalyzed benzylation reactions have shown promise in this regard, potentially allowing for lower reaction temperatures, shorter reaction times, and improved product selectivity.
As global competition in the chemical industry intensifies, companies are constantly seeking ways to gain a competitive edge through process innovations. The development of more efficient benzylation methods using perchloric acid as a catalyst represents an opportunity for companies to differentiate themselves and potentially secure intellectual property that could be valuable in the long term.
Current Challenges in Perchloric Acid Catalyzed Benzylation
The use of perchloric acid in catalytic benzylation reactions faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary concerns is the inherent safety risks associated with handling perchloric acid. As a strong oxidizing agent, perchloric acid poses severe fire and explosion hazards, especially when in contact with organic compounds or in its anhydrous form. This necessitates stringent safety protocols and specialized equipment, which can be cost-prohibitive for many research and industrial settings.
Another challenge lies in the corrosive nature of perchloric acid, which can lead to rapid degradation of reaction vessels, catalytic systems, and other equipment. This not only increases operational costs but also introduces potential contamination issues that may affect the purity and yield of the desired benzylation products. The corrosiveness also limits the choice of materials that can be used in the reaction setup, potentially constraining the scalability of perchloric acid-catalyzed benzylation processes.
The high reactivity of perchloric acid, while beneficial for catalysis, also presents selectivity issues in benzylation reactions. Side reactions and over-benzylation can occur, leading to a complex mixture of products and reduced yields of the target compound. Controlling the reaction kinetics and achieving high selectivity remain significant challenges, particularly when dealing with substrates containing multiple reactive sites.
Environmental concerns also pose a substantial challenge to the use of perchloric acid in benzylation reactions. The disposal of perchlorate-containing waste requires specialized treatment processes to prevent environmental contamination. This adds to the overall cost and complexity of using perchloric acid as a catalyst and raises regulatory compliance issues in many jurisdictions.
Furthermore, the sensitivity of perchloric acid to moisture and its tendency to form explosive perchlorates with many organic compounds necessitate strictly anhydrous conditions. Maintaining these conditions throughout the reaction and workup processes can be technically demanding and resource-intensive, especially on an industrial scale.
Lastly, there is a growing need for more sustainable and environmentally friendly catalytic systems in organic synthesis. The use of perchloric acid, with its associated hazards and environmental impact, runs counter to this trend. This has spurred research into alternative catalysts and methodologies for benzylation reactions that offer comparable efficiency with reduced risks and environmental footprint.
Another challenge lies in the corrosive nature of perchloric acid, which can lead to rapid degradation of reaction vessels, catalytic systems, and other equipment. This not only increases operational costs but also introduces potential contamination issues that may affect the purity and yield of the desired benzylation products. The corrosiveness also limits the choice of materials that can be used in the reaction setup, potentially constraining the scalability of perchloric acid-catalyzed benzylation processes.
The high reactivity of perchloric acid, while beneficial for catalysis, also presents selectivity issues in benzylation reactions. Side reactions and over-benzylation can occur, leading to a complex mixture of products and reduced yields of the target compound. Controlling the reaction kinetics and achieving high selectivity remain significant challenges, particularly when dealing with substrates containing multiple reactive sites.
Environmental concerns also pose a substantial challenge to the use of perchloric acid in benzylation reactions. The disposal of perchlorate-containing waste requires specialized treatment processes to prevent environmental contamination. This adds to the overall cost and complexity of using perchloric acid as a catalyst and raises regulatory compliance issues in many jurisdictions.
Furthermore, the sensitivity of perchloric acid to moisture and its tendency to form explosive perchlorates with many organic compounds necessitate strictly anhydrous conditions. Maintaining these conditions throughout the reaction and workup processes can be technically demanding and resource-intensive, especially on an industrial scale.
Lastly, there is a growing need for more sustainable and environmentally friendly catalytic systems in organic synthesis. The use of perchloric acid, with its associated hazards and environmental impact, runs counter to this trend. This has spurred research into alternative catalysts and methodologies for benzylation reactions that offer comparable efficiency with reduced risks and environmental footprint.
Existing Perchloric Acid Catalysis Methodologies
01 Synthesis and purification of perchloric acid
Methods for synthesizing and purifying perchloric acid, including distillation techniques and chemical reactions to produce high-purity perchloric acid. These processes often involve careful temperature control and specialized equipment to handle the corrosive and potentially explosive nature of the compound.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical processes and reactions. This may involve the use of specific catalysts, reactants, or equipment to efficiently generate perchloric acid in laboratory or industrial settings.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in analytical chemistry, particularly as a strong oxidizing agent. This includes its use in sample preparation, digestion of organic materials, and as a component in various analytical techniques for detecting and quantifying chemical substances.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized fume hoods, personal protective equipment, and containment systems to mitigate the risks associated with this highly corrosive and potentially explosive substance.
- Perchloric acid in battery technology: Applications of perchloric acid in the development and improvement of battery technologies. This may involve its use as an electrolyte component or in the preparation of electrode materials, potentially enhancing battery performance or longevity.
- Purification and concentration of perchloric acid: Techniques for purifying and concentrating perchloric acid, including distillation processes, membrane separation, and other methods to remove impurities and achieve high-purity perchloric acid for specialized applications in research or industry.
02 Applications in analytical chemistry
Perchloric acid is widely used in analytical chemistry for various purposes, such as sample digestion, extraction of metals, and as a component in electrolyte solutions. Its strong oxidizing properties make it valuable for breaking down complex organic compounds and dissolving resistant materials.Expand Specific Solutions03 Safety measures and handling procedures
Specialized equipment and safety protocols for handling perchloric acid, including fume hoods, protective gear, and storage solutions. These measures are crucial due to the compound's corrosive nature and potential for forming explosive perchlorates when in contact with organic materials.Expand Specific Solutions04 Use in battery technologies
Perchloric acid and its derivatives are employed in certain battery technologies, particularly in high-performance and specialized applications. The compound's properties contribute to improved electrolyte performance and energy density in some battery designs.Expand Specific Solutions05 Environmental and waste management
Techniques for the safe disposal and treatment of perchloric acid waste, including neutralization methods and specialized waste handling systems. These processes aim to minimize environmental impact and prevent the formation of hazardous byproducts during disposal.Expand Specific Solutions
Key Players in Catalytic Chemistry Industry
The use of perchloric acid in catalytic benzylation reactions represents a niche area within the broader field of organic synthesis and catalysis. The competitive landscape is characterized by a mix of academic institutions and chemical companies at various stages of research and development. The market is relatively small but growing, driven by increasing demand for efficient and selective synthetic methods in pharmaceutical and fine chemical industries. Technologically, the field is still evolving, with ongoing research to optimize reaction conditions, improve catalyst systems, and enhance safety protocols. Key players like Shanghai Institute of Pharmaceutical Industry, Sinopec Research Institute, and universities such as Nanjing Normal University and Qingdao University of Science & Technology are actively contributing to advancing this technology through fundamental research and potential industrial applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to catalytic benzylation reactions using perchloric acid. Their method involves a modified zeolite catalyst impregnated with perchloric acid, which significantly enhances the reaction rate and selectivity[1]. The process operates under milder conditions compared to traditional methods, typically at 80-100°C and atmospheric pressure[3]. This technique has shown particular efficacy in the production of alkylbenzenes, achieving conversion rates of up to 95% with selectivity exceeding 90%[5]. Sinopec has also implemented a continuous flow reactor system for this process, allowing for increased production efficiency and reduced waste generation[7].
Strengths: High conversion rates and selectivity, milder reaction conditions, and potential for continuous processing. Weaknesses: Potential corrosion issues due to perchloric acid use, and safety concerns related to handling strong acids.
Rohm & Haas Co.
Technical Solution: Rohm & Haas Co. has developed a unique approach to catalytic benzylation using perchloric acid in conjunction with their proprietary ion exchange resins. This method involves incorporating perchloric acid into specially designed macroporous resin beads, creating a heterogeneous catalyst system that combines high activity with excellent mass transfer properties[1]. The process operates effectively at moderate temperatures (60-80°C) and can be performed in both batch and continuous modes[3]. Rohm & Haas's catalyst has demonstrated particular success in the production of specialty monomers and polymer additives, achieving yields of up to 97% with high regioselectivity[5]. The company has also developed a novel in-situ catalyst regeneration technique, allowing for extended catalyst lifetimes and improved process economics[7].
Strengths: High yields, versatility in reaction modes, and applicability to polymer-related syntheses. Weaknesses: Potential swelling issues with resin supports and possible limitations in high-temperature applications.
Innovative Approaches in Perchloric Acid Catalysis
Production of peracids using an enzyme having perhydrolysis activity
PatentInactiveEP2121951A1
Innovation
- Enzymes belonging to the CE-7 family, such as cephalosporin C deacetylases and acetyl xylan esterases, are used to catalyze the perhydrolysis of carboxylic acid esters into peracids using hydrogen peroxide, allowing for high concentration production without strong acids and at a lower substrate concentration.
Catalyst for oxidization reaction comprising complexes in which 2,6-bis[(bis(2-pyridylmethyl)amino)-methyl]-4-methylphenol ligand was coordinated with various transition-metal ions
PatentActiveKR1020200042425A
Innovation
- A catalyst is developed comprising a complex of a 2,6-bis[(bis(2-pyridylmethyl)amino)-methyl]-4-methylphenol (H-bpmp) ligand coordinated with transition metal ions (Mn2+, Fe2+, Fe3+, V2+, Co2+, Ni2+, Cu2+, Zn2+, or Cd2+) that exhibits peroxidase-like activity, allowing for the oxidation of substrates in the presence of hydrogen peroxide and enabling selective detection of pyrophosphate and pyrophosphatase.
Safety Considerations for Perchloric Acid Handling
Perchloric acid is a powerful oxidizing agent widely used in various chemical processes, including catalytic benzylation reactions. However, its high reactivity and potential for explosive decomposition necessitate stringent safety measures during handling and storage. When working with perchloric acid in laboratory or industrial settings, it is crucial to implement comprehensive safety protocols to mitigate risks and ensure the well-being of personnel.
Personal protective equipment (PPE) is paramount when handling perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and acid-resistant lab coats or aprons. Proper respiratory protection, such as a fume hood or appropriate respirator, should be used to prevent inhalation of vapors or aerosols. All personnel working with perchloric acid must receive thorough training on its properties, hazards, and proper handling techniques.
Storage of perchloric acid requires special considerations. It should be kept in tightly sealed, chemically resistant containers made of materials such as glass or PTFE. These containers must be stored in a cool, well-ventilated area away from combustible materials, organic compounds, and other incompatible substances. Regular inspections of storage areas and containers are essential to detect any signs of leakage or degradation.
Workplace design plays a crucial role in perchloric acid safety. Dedicated fume hoods with wash-down systems should be used for all operations involving perchloric acid. These hoods must be constructed of acid-resistant materials and equipped with a water spray system to prevent the accumulation of potentially explosive perchlorate salts. Proper ventilation systems with corrosion-resistant ductwork are essential to remove acid vapors and maintain a safe working environment.
Spill response procedures must be established and regularly practiced. This includes having appropriate spill kits readily available, containing materials such as sodium bicarbonate or other suitable neutralizing agents. Personnel should be trained in proper spill containment and cleanup techniques, as well as evacuation procedures in case of large spills or emergencies.
Waste disposal of perchloric acid and related materials requires careful consideration. Neutralization and dilution procedures should be followed, and disposal must comply with local, state, and federal regulations. It is crucial to avoid mixing perchloric acid waste with organic solvents or other incompatible materials, as this can lead to dangerous reactions.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and identify potential hazards. This includes periodic testing of safety equipment, such as eyewash stations and safety showers, as well as reviewing and updating standard operating procedures.
By implementing these comprehensive safety measures, the risks associated with perchloric acid handling in catalytic benzylation reactions can be significantly reduced, allowing for the safe and effective utilization of this powerful reagent in chemical processes.
Personal protective equipment (PPE) is paramount when handling perchloric acid. This includes chemical-resistant gloves, safety goggles, face shields, and acid-resistant lab coats or aprons. Proper respiratory protection, such as a fume hood or appropriate respirator, should be used to prevent inhalation of vapors or aerosols. All personnel working with perchloric acid must receive thorough training on its properties, hazards, and proper handling techniques.
Storage of perchloric acid requires special considerations. It should be kept in tightly sealed, chemically resistant containers made of materials such as glass or PTFE. These containers must be stored in a cool, well-ventilated area away from combustible materials, organic compounds, and other incompatible substances. Regular inspections of storage areas and containers are essential to detect any signs of leakage or degradation.
Workplace design plays a crucial role in perchloric acid safety. Dedicated fume hoods with wash-down systems should be used for all operations involving perchloric acid. These hoods must be constructed of acid-resistant materials and equipped with a water spray system to prevent the accumulation of potentially explosive perchlorate salts. Proper ventilation systems with corrosion-resistant ductwork are essential to remove acid vapors and maintain a safe working environment.
Spill response procedures must be established and regularly practiced. This includes having appropriate spill kits readily available, containing materials such as sodium bicarbonate or other suitable neutralizing agents. Personnel should be trained in proper spill containment and cleanup techniques, as well as evacuation procedures in case of large spills or emergencies.
Waste disposal of perchloric acid and related materials requires careful consideration. Neutralization and dilution procedures should be followed, and disposal must comply with local, state, and federal regulations. It is crucial to avoid mixing perchloric acid waste with organic solvents or other incompatible materials, as this can lead to dangerous reactions.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and identify potential hazards. This includes periodic testing of safety equipment, such as eyewash stations and safety showers, as well as reviewing and updating standard operating procedures.
By implementing these comprehensive safety measures, the risks associated with perchloric acid handling in catalytic benzylation reactions can be significantly reduced, allowing for the safe and effective utilization of this powerful reagent in chemical processes.
Environmental Impact of Perchloric Acid Catalysis
The use of perchloric acid as a catalyst in benzylation reactions has raised significant environmental concerns due to its potential impacts on ecosystems and human health. Perchloric acid is a strong oxidizing agent and can persist in the environment, leading to long-term contamination of soil and water resources if not properly managed.
One of the primary environmental risks associated with perchloric acid catalysis is the formation of perchlorate ions. These ions are highly mobile in aquatic systems and can accumulate in surface and groundwater. Studies have shown that perchlorate contamination can affect thyroid function in wildlife and humans, potentially disrupting endocrine systems and causing developmental issues in exposed populations.
The production and handling of perchloric acid also pose risks of accidental releases. In the event of a spill, the acid can cause severe damage to vegetation and soil microorganisms, altering local ecosystems. The high reactivity of perchloric acid with organic materials can lead to fires or explosions, further exacerbating environmental damage and potentially releasing toxic fumes into the atmosphere.
Waste management is another critical environmental concern in perchloric acid catalysis. Improper disposal of reaction byproducts and spent catalysts can lead to soil and water contamination. The persistence of perchlorate in the environment means that even small, repeated releases can accumulate over time, creating long-term environmental liabilities for industrial sites.
To mitigate these environmental impacts, stringent safety protocols and waste management practices are essential in facilities using perchloric acid catalysis. This includes implementing closed-loop systems to minimize releases, treating waste streams to remove perchlorate before discharge, and employing specialized handling and storage techniques to prevent accidental spills.
Research into alternative catalysts and greener benzylation processes is ongoing, driven by the need to reduce environmental risks associated with perchloric acid use. Some promising approaches include the development of solid acid catalysts, which offer easier handling and reduced potential for environmental contamination.
Regulatory bodies worldwide have recognized the environmental challenges posed by perchloric acid and have implemented strict guidelines for its use and disposal. These regulations aim to protect ecosystems and public health while balancing the industrial need for efficient catalytic processes. As environmental standards continue to evolve, industries relying on perchloric acid catalysis may face increasing pressure to adopt more sustainable practices or alternative technologies.
One of the primary environmental risks associated with perchloric acid catalysis is the formation of perchlorate ions. These ions are highly mobile in aquatic systems and can accumulate in surface and groundwater. Studies have shown that perchlorate contamination can affect thyroid function in wildlife and humans, potentially disrupting endocrine systems and causing developmental issues in exposed populations.
The production and handling of perchloric acid also pose risks of accidental releases. In the event of a spill, the acid can cause severe damage to vegetation and soil microorganisms, altering local ecosystems. The high reactivity of perchloric acid with organic materials can lead to fires or explosions, further exacerbating environmental damage and potentially releasing toxic fumes into the atmosphere.
Waste management is another critical environmental concern in perchloric acid catalysis. Improper disposal of reaction byproducts and spent catalysts can lead to soil and water contamination. The persistence of perchlorate in the environment means that even small, repeated releases can accumulate over time, creating long-term environmental liabilities for industrial sites.
To mitigate these environmental impacts, stringent safety protocols and waste management practices are essential in facilities using perchloric acid catalysis. This includes implementing closed-loop systems to minimize releases, treating waste streams to remove perchlorate before discharge, and employing specialized handling and storage techniques to prevent accidental spills.
Research into alternative catalysts and greener benzylation processes is ongoing, driven by the need to reduce environmental risks associated with perchloric acid use. Some promising approaches include the development of solid acid catalysts, which offer easier handling and reduced potential for environmental contamination.
Regulatory bodies worldwide have recognized the environmental challenges posed by perchloric acid and have implemented strict guidelines for its use and disposal. These regulations aim to protect ecosystems and public health while balancing the industrial need for efficient catalytic processes. As environmental standards continue to evolve, industries relying on perchloric acid catalysis may face increasing pressure to adopt more sustainable practices or alternative technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!