Use of Perchloric Acid in Investigating Absolute Reaction Rates
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Research Background and Objectives
Perchloric acid has played a pivotal role in the investigation of absolute reaction rates, marking a significant milestone in the field of chemical kinetics. The study of reaction rates has been a fundamental aspect of physical chemistry since the late 19th century, with researchers striving to understand the mechanisms and factors influencing the speed of chemical reactions. The use of perchloric acid in this context emerged as a breakthrough technique, offering unique advantages in probing reaction dynamics at a molecular level.
The primary objective of employing perchloric acid in reaction rate studies is to elucidate the intricate details of chemical processes, particularly in solutions where traditional methods fall short. Perchloric acid's exceptional properties, including its strong acidity and low nucleophilicity, make it an ideal medium for investigating fast reactions and unstable intermediates. These characteristics allow researchers to maintain a controlled environment while observing rapid chemical transformations that would otherwise be challenging to study.
The historical development of this technique can be traced back to the early 20th century when the limitations of conventional kinetic studies became apparent. Scientists recognized the need for a more versatile and powerful tool to explore reaction mechanisms in extreme conditions. Perchloric acid emerged as a solution, offering the ability to stabilize reactive species and provide a uniform reaction medium across a wide range of concentrations and temperatures.
As research in this area progressed, the use of perchloric acid expanded beyond simple rate measurements to include the study of complex reaction networks, catalytic processes, and the behavior of ions in solution. This versatility has made it an indispensable tool in fields ranging from organic synthesis to materials science and biochemistry. The technique has enabled researchers to probe reactions at timescales previously thought impossible, revealing new insights into the nature of chemical bonding and reactivity.
The evolution of perchloric acid usage in kinetic studies has been closely tied to advancements in analytical instrumentation. The development of sophisticated spectroscopic and electrochemical methods has enhanced the precision and scope of measurements, allowing for real-time monitoring of reaction progress and the detection of short-lived intermediates. This synergy between chemical methodology and instrumental technology has driven continuous innovation in the field, pushing the boundaries of our understanding of reaction dynamics.
Looking forward, the objectives of perchloric acid research in absolute reaction rate studies continue to expand. Current goals include unraveling the mechanisms of increasingly complex biological processes, developing more efficient catalytic systems, and exploring reactions under extreme conditions relevant to materials processing and environmental chemistry. The ongoing refinement of this technique promises to yield further insights into the fundamental principles governing chemical reactivity, potentially leading to breakthroughs in drug design, energy storage, and environmental remediation.
The primary objective of employing perchloric acid in reaction rate studies is to elucidate the intricate details of chemical processes, particularly in solutions where traditional methods fall short. Perchloric acid's exceptional properties, including its strong acidity and low nucleophilicity, make it an ideal medium for investigating fast reactions and unstable intermediates. These characteristics allow researchers to maintain a controlled environment while observing rapid chemical transformations that would otherwise be challenging to study.
The historical development of this technique can be traced back to the early 20th century when the limitations of conventional kinetic studies became apparent. Scientists recognized the need for a more versatile and powerful tool to explore reaction mechanisms in extreme conditions. Perchloric acid emerged as a solution, offering the ability to stabilize reactive species and provide a uniform reaction medium across a wide range of concentrations and temperatures.
As research in this area progressed, the use of perchloric acid expanded beyond simple rate measurements to include the study of complex reaction networks, catalytic processes, and the behavior of ions in solution. This versatility has made it an indispensable tool in fields ranging from organic synthesis to materials science and biochemistry. The technique has enabled researchers to probe reactions at timescales previously thought impossible, revealing new insights into the nature of chemical bonding and reactivity.
The evolution of perchloric acid usage in kinetic studies has been closely tied to advancements in analytical instrumentation. The development of sophisticated spectroscopic and electrochemical methods has enhanced the precision and scope of measurements, allowing for real-time monitoring of reaction progress and the detection of short-lived intermediates. This synergy between chemical methodology and instrumental technology has driven continuous innovation in the field, pushing the boundaries of our understanding of reaction dynamics.
Looking forward, the objectives of perchloric acid research in absolute reaction rate studies continue to expand. Current goals include unraveling the mechanisms of increasingly complex biological processes, developing more efficient catalytic systems, and exploring reactions under extreme conditions relevant to materials processing and environmental chemistry. The ongoing refinement of this technique promises to yield further insights into the fundamental principles governing chemical reactivity, potentially leading to breakthroughs in drug design, energy storage, and environmental remediation.
Market Analysis for Reaction Rate Studies
The market for reaction rate studies, particularly those involving perchloric acid, has shown significant growth in recent years. This expansion is driven by the increasing demand for precise kinetic data in various industries, including pharmaceuticals, materials science, and chemical manufacturing. The global market for analytical instruments used in reaction rate studies is projected to reach several billion dollars by 2025, with a compound annual growth rate exceeding 5%.
Perchloric acid, as a powerful oxidizing agent, plays a crucial role in investigating absolute reaction rates due to its unique properties. Its use in reaction rate studies has become more prevalent, especially in academic research and industrial R&D settings. The market for high-purity perchloric acid suitable for these studies has seen steady growth, with major suppliers reporting increased sales volumes year-over-year.
The pharmaceutical industry remains a key driver of demand for reaction rate studies. As drug development processes become more complex, understanding reaction kinetics is critical for optimizing synthesis routes and improving product quality. This has led to increased investment in advanced analytical equipment and methodologies, including those utilizing perchloric acid for rate determinations.
In the materials science sector, the need for precise reaction rate data has grown with the development of novel materials and nanomaterials. Researchers and manufacturers are increasingly relying on sophisticated kinetic studies to fine-tune material properties and production processes. This trend has contributed to the expansion of the market for specialized analytical services and equipment.
The chemical manufacturing industry has also shown a growing interest in absolute reaction rate studies. As companies strive for greater efficiency and sustainability in their processes, accurate kinetic data becomes essential for process optimization and catalyst development. This has led to increased adoption of advanced analytical techniques, including those involving perchloric acid.
Geographically, North America and Europe continue to dominate the market for reaction rate studies, with well-established research institutions and a strong presence of pharmaceutical and chemical companies. However, the Asia-Pacific region is experiencing the fastest growth, driven by rapid industrialization and increasing R&D investments in countries like China and India.
The market landscape is characterized by a mix of large analytical instrument manufacturers and specialized service providers. Key players are focusing on developing more sensitive and automated systems for reaction rate measurements, as well as offering comprehensive data analysis and interpretation services. This trend towards integrated solutions is expected to shape the market's future growth trajectory.
Perchloric acid, as a powerful oxidizing agent, plays a crucial role in investigating absolute reaction rates due to its unique properties. Its use in reaction rate studies has become more prevalent, especially in academic research and industrial R&D settings. The market for high-purity perchloric acid suitable for these studies has seen steady growth, with major suppliers reporting increased sales volumes year-over-year.
The pharmaceutical industry remains a key driver of demand for reaction rate studies. As drug development processes become more complex, understanding reaction kinetics is critical for optimizing synthesis routes and improving product quality. This has led to increased investment in advanced analytical equipment and methodologies, including those utilizing perchloric acid for rate determinations.
In the materials science sector, the need for precise reaction rate data has grown with the development of novel materials and nanomaterials. Researchers and manufacturers are increasingly relying on sophisticated kinetic studies to fine-tune material properties and production processes. This trend has contributed to the expansion of the market for specialized analytical services and equipment.
The chemical manufacturing industry has also shown a growing interest in absolute reaction rate studies. As companies strive for greater efficiency and sustainability in their processes, accurate kinetic data becomes essential for process optimization and catalyst development. This has led to increased adoption of advanced analytical techniques, including those involving perchloric acid.
Geographically, North America and Europe continue to dominate the market for reaction rate studies, with well-established research institutions and a strong presence of pharmaceutical and chemical companies. However, the Asia-Pacific region is experiencing the fastest growth, driven by rapid industrialization and increasing R&D investments in countries like China and India.
The market landscape is characterized by a mix of large analytical instrument manufacturers and specialized service providers. Key players are focusing on developing more sensitive and automated systems for reaction rate measurements, as well as offering comprehensive data analysis and interpretation services. This trend towards integrated solutions is expected to shape the market's future growth trajectory.
Current Challenges in Absolute Reaction Rate Determination
The determination of absolute reaction rates remains a challenging endeavor in chemical kinetics, with several significant obstacles hindering precise measurements and interpretations. One of the primary challenges is the complexity of reaction mechanisms, particularly in multi-step reactions. Isolating and quantifying individual rate constants within a complex reaction network often proves difficult, as intermediate species and parallel pathways can obscure the true reaction kinetics.
Another major hurdle is the influence of environmental factors on reaction rates. Temperature, pressure, and solvent effects can dramatically alter reaction kinetics, making it challenging to extrapolate laboratory results to real-world conditions. Furthermore, the presence of catalysts or inhibitors, even in trace amounts, can significantly impact reaction rates, leading to potential misinterpretations of kinetic data.
The time resolution of experimental techniques also poses a significant challenge, especially for fast reactions. While advanced spectroscopic methods have improved temporal resolution, capturing the kinetics of ultrafast reactions remains problematic. This limitation is particularly acute in the study of transition states and short-lived intermediates, which are crucial for understanding reaction mechanisms and determining accurate rate constants.
Measurement accuracy and precision present ongoing challenges. Even small errors in concentration measurements or timing can propagate to significant uncertainties in rate constants. This issue is compounded when dealing with reactions that have very low concentrations of reactants or products, pushing the limits of detection for many analytical techniques.
The use of perchloric acid in investigating absolute reaction rates introduces its own set of challenges. While perchloric acid is a powerful oxidizing agent and can facilitate certain reactions, its extreme reactivity can also lead to side reactions or decomposition of sensitive compounds. This can complicate kinetic analyses and potentially introduce artifacts in rate measurements.
Moreover, the strong acidity of perchloric acid can alter the reaction environment significantly, affecting protonation states of reactants and intermediates. This pH effect can dramatically change reaction pathways and rates, making it difficult to isolate the intrinsic kinetics of the reaction of interest from acid-catalyzed processes.
Safety considerations when working with perchloric acid also impose limitations on experimental designs and procedures. The potential for explosive reactions, especially with organic compounds, necessitates stringent safety protocols that may restrict the range of conditions under which experiments can be conducted.
Another major hurdle is the influence of environmental factors on reaction rates. Temperature, pressure, and solvent effects can dramatically alter reaction kinetics, making it challenging to extrapolate laboratory results to real-world conditions. Furthermore, the presence of catalysts or inhibitors, even in trace amounts, can significantly impact reaction rates, leading to potential misinterpretations of kinetic data.
The time resolution of experimental techniques also poses a significant challenge, especially for fast reactions. While advanced spectroscopic methods have improved temporal resolution, capturing the kinetics of ultrafast reactions remains problematic. This limitation is particularly acute in the study of transition states and short-lived intermediates, which are crucial for understanding reaction mechanisms and determining accurate rate constants.
Measurement accuracy and precision present ongoing challenges. Even small errors in concentration measurements or timing can propagate to significant uncertainties in rate constants. This issue is compounded when dealing with reactions that have very low concentrations of reactants or products, pushing the limits of detection for many analytical techniques.
The use of perchloric acid in investigating absolute reaction rates introduces its own set of challenges. While perchloric acid is a powerful oxidizing agent and can facilitate certain reactions, its extreme reactivity can also lead to side reactions or decomposition of sensitive compounds. This can complicate kinetic analyses and potentially introduce artifacts in rate measurements.
Moreover, the strong acidity of perchloric acid can alter the reaction environment significantly, affecting protonation states of reactants and intermediates. This pH effect can dramatically change reaction pathways and rates, making it difficult to isolate the intrinsic kinetics of the reaction of interest from acid-catalyzed processes.
Safety considerations when working with perchloric acid also impose limitations on experimental designs and procedures. The potential for explosive reactions, especially with organic compounds, necessitates stringent safety protocols that may restrict the range of conditions under which experiments can be conducted.
Existing Methodologies Using Perchloric Acid
01 Reaction rate measurement techniques
Various techniques are employed to measure the reaction rates of perchloric acid, including spectroscopic methods, electrochemical analysis, and calorimetry. These methods allow for precise monitoring of reaction progress and kinetics in different experimental conditions.- Reaction rate measurement techniques: Various techniques are employed to measure the reaction rates of perchloric acid. These may include spectroscopic methods, electrochemical measurements, and calorimetry. Advanced analytical instruments and methodologies are utilized to accurately determine the kinetics of perchloric acid reactions under different conditions.
- Factors affecting perchloric acid reaction rates: The reaction rates of perchloric acid are influenced by several factors, including temperature, concentration, presence of catalysts, and the nature of the reactants. Understanding these factors is crucial for controlling and optimizing perchloric acid-based reactions in various applications.
- Applications in organic synthesis: Perchloric acid is used in various organic synthesis reactions, where its reaction rate plays a crucial role. It serves as a strong acid catalyst in certain transformations, and understanding its kinetics is essential for optimizing reaction conditions and yields in the production of organic compounds.
- Safety considerations in handling perchloric acid: Due to its highly reactive nature, special safety measures are required when handling perchloric acid. The reaction rates of perchloric acid with various materials, including organic compounds, are important considerations in developing safety protocols and designing appropriate equipment for its use and storage.
- Perchloric acid in analytical chemistry: Perchloric acid is widely used in analytical chemistry, where its reaction rates are important for various applications. These include sample preparation, digestion procedures, and as a component in mobile phases for chromatography. Understanding the kinetics of perchloric acid reactions is crucial for developing efficient analytical methods.
02 Catalysts for perchloric acid reactions
Catalysts play a crucial role in modifying the reaction rates of perchloric acid. Different types of catalysts, such as metal complexes and enzymes, can be used to accelerate or control the reaction kinetics, enabling more efficient and selective chemical processes.Expand Specific Solutions03 Temperature and pressure effects
The reaction rates of perchloric acid are significantly influenced by temperature and pressure. Studies have been conducted to understand how these parameters affect reaction kinetics, allowing for better control and optimization of chemical processes involving perchloric acid.Expand Specific Solutions04 Reaction mechanisms and intermediates
Research has been conducted to elucidate the reaction mechanisms and identify intermediates in perchloric acid reactions. Understanding these aspects helps in predicting reaction rates and developing more efficient chemical processes.Expand Specific Solutions05 Safety considerations in perchloric acid reactions
Due to the highly reactive nature of perchloric acid, safety measures are crucial when studying its reaction rates. Specialized equipment and protocols have been developed to ensure safe handling and accurate measurement of reaction kinetics.Expand Specific Solutions
Key Players in Chemical Kinetics Research
The use of perchloric acid in investigating absolute reaction rates is a niche area within chemical kinetics, currently in a mature research phase. The market size for this specific application is relatively small, primarily confined to academic and industrial research laboratories. Technologically, the field is well-established, with companies like Asahi Kasei Pharma Corp., NEC Corp., and Henkel AG & Co. KGaA having developed expertise in handling and applying perchloric acid for kinetic studies. These firms, along with others like Kobe Steel, Ltd. and Zhejiang Juhua Technology Center Co., Ltd., have contributed to refining methodologies and safety protocols, enhancing the precision and reliability of reaction rate measurements using perchloric acid.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed a microfluidic platform for using perchloric acid in high-throughput kinetic studies. Their system utilizes microfluidic chips with integrated mixing and detection chambers to perform rapid serial dilution experiments with perchloric acid solutions [5]. This allows for automated measurement of reaction rates across a wide range of concentrations. The platform incorporates in-line UV-vis spectroscopy for real-time monitoring of reaction progress. Machine learning algorithms are employed to analyze the large datasets and extract rate constants and mechanistic information [6].
Strengths: High-throughput capabilities; integration of advanced data analysis techniques. Weaknesses: Limited to reactions compatible with microfluidic format; potential for channel clogging with precipitates.
Oxford University Innovation Ltd.
Technical Solution: Oxford University Innovation has developed a cryogenic stopped-flow apparatus for studying reaction kinetics using perchloric acid at low temperatures. Their system allows for rapid mixing and spectroscopic analysis of perchloric acid solutions at temperatures down to -100°C [7]. This enables investigation of reaction intermediates and transition states that are only stable at low temperatures. The researchers have also developed specialized materials and coatings to protect the apparatus from the corrosive effects of perchloric acid at low temperatures [8].
Strengths: Unique low-temperature capabilities; ability to study unstable intermediates. Weaknesses: Limited to a narrow temperature range; requires expensive cryogenic equipment.
Innovations in Perchloric Acid-Based Rate Studies
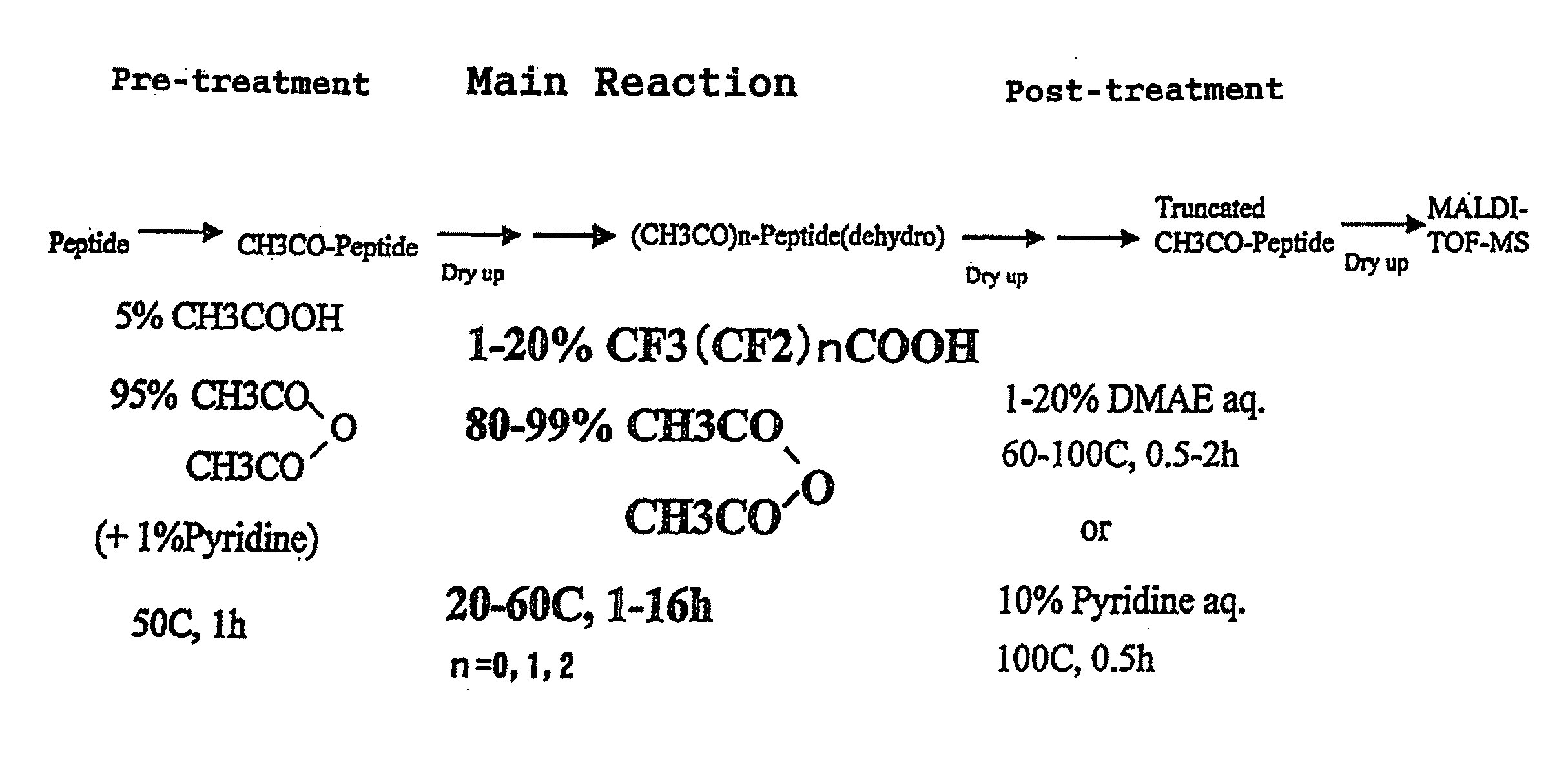
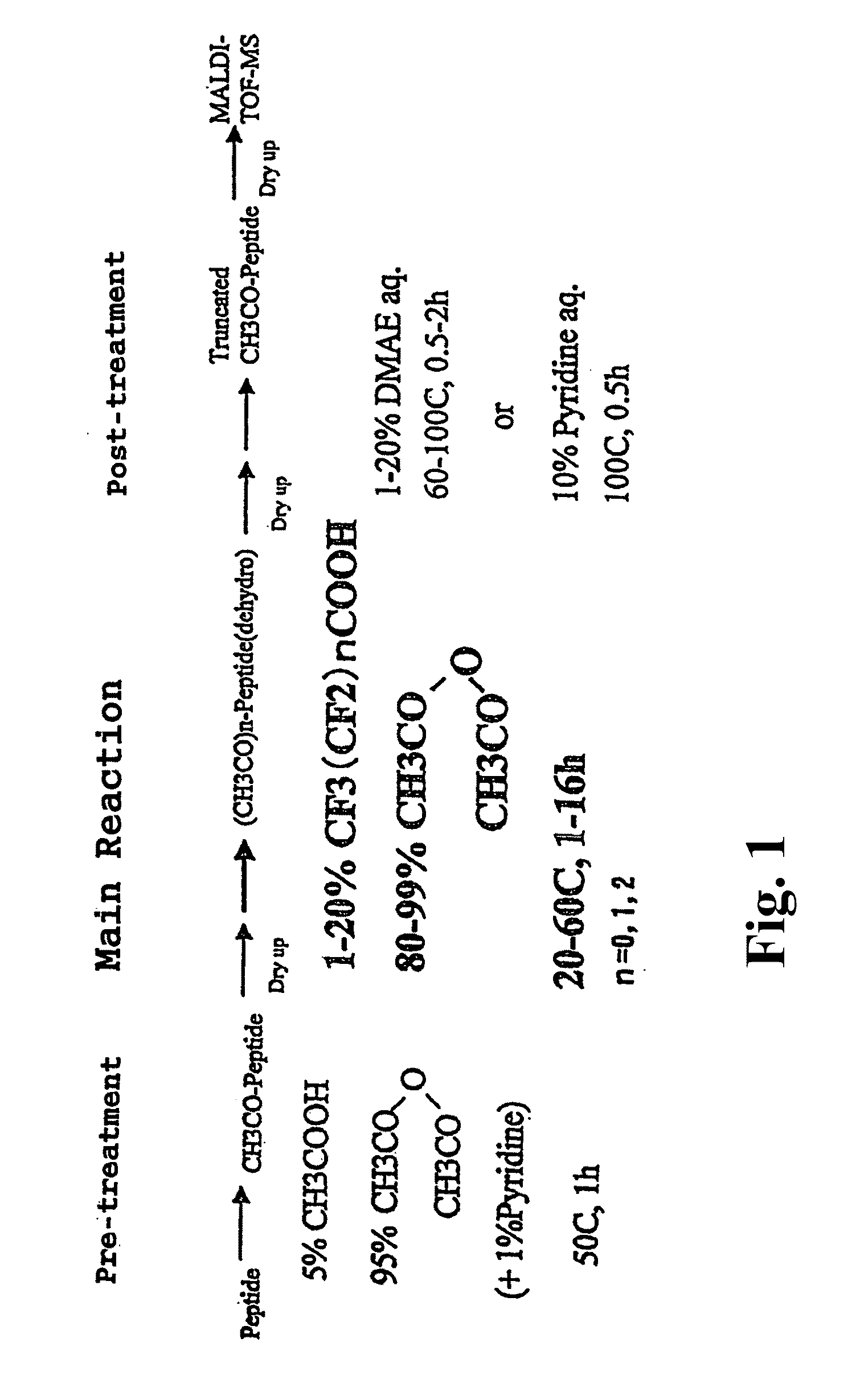
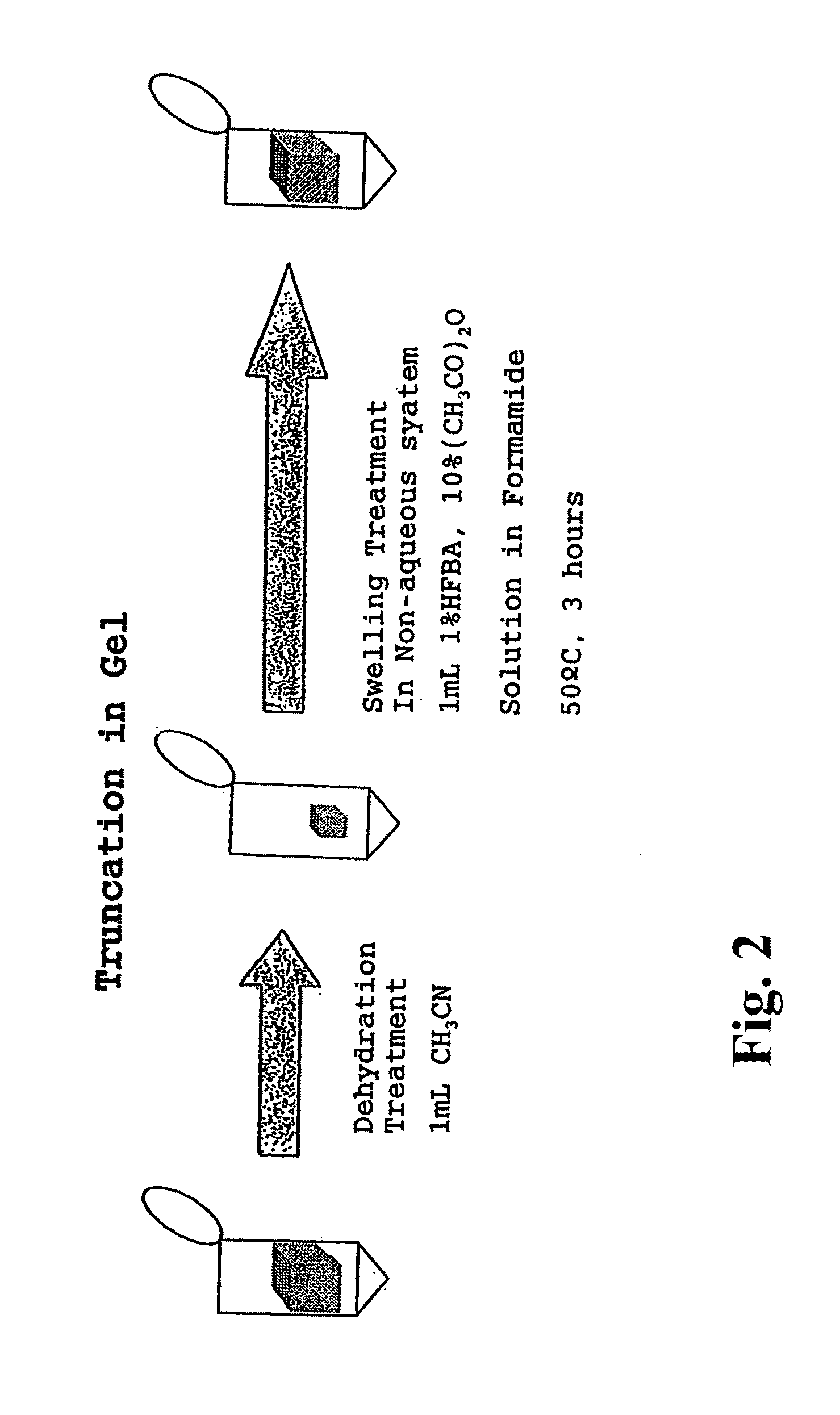
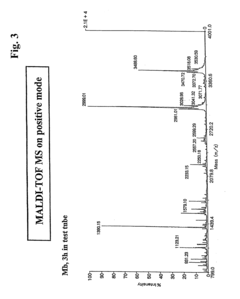
Method for analyzing c-terminal amino acid sequence of peptide using mass spectrometry
PatentInactiveUS20110183428A1
Innovation
- A method involving the use of a combination of alkanoic acid anhydride and a small amount of perfluoroalkanoic acid to form an oxazolone ring structure, allowing for selective release of C-terminal amino acids without side reactions, followed by enzymatic digestion with trypsin and MALDI-TOF-MS analysis to distinguish C-terminal from N-terminal peptide fragments.
Method for analysis of reaction products
PatentInactiveUS7045360B2
Innovation
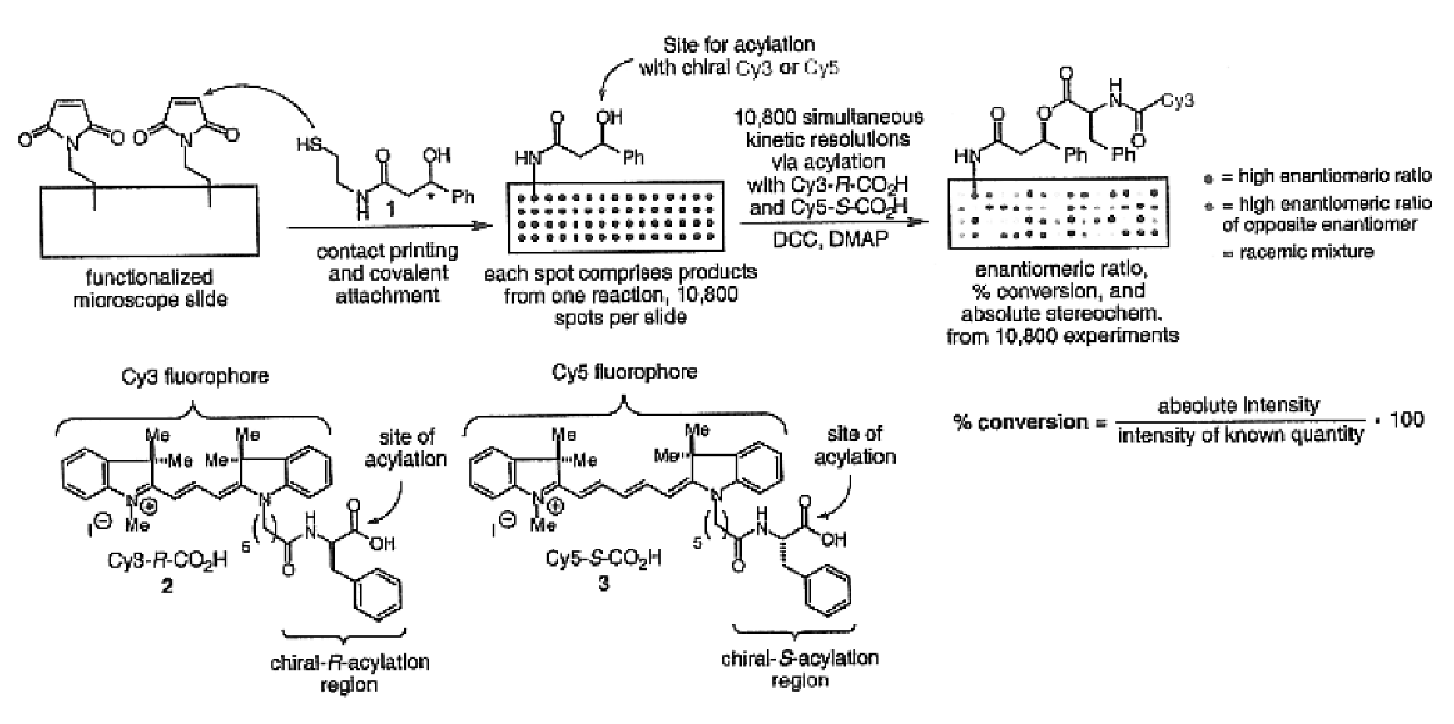
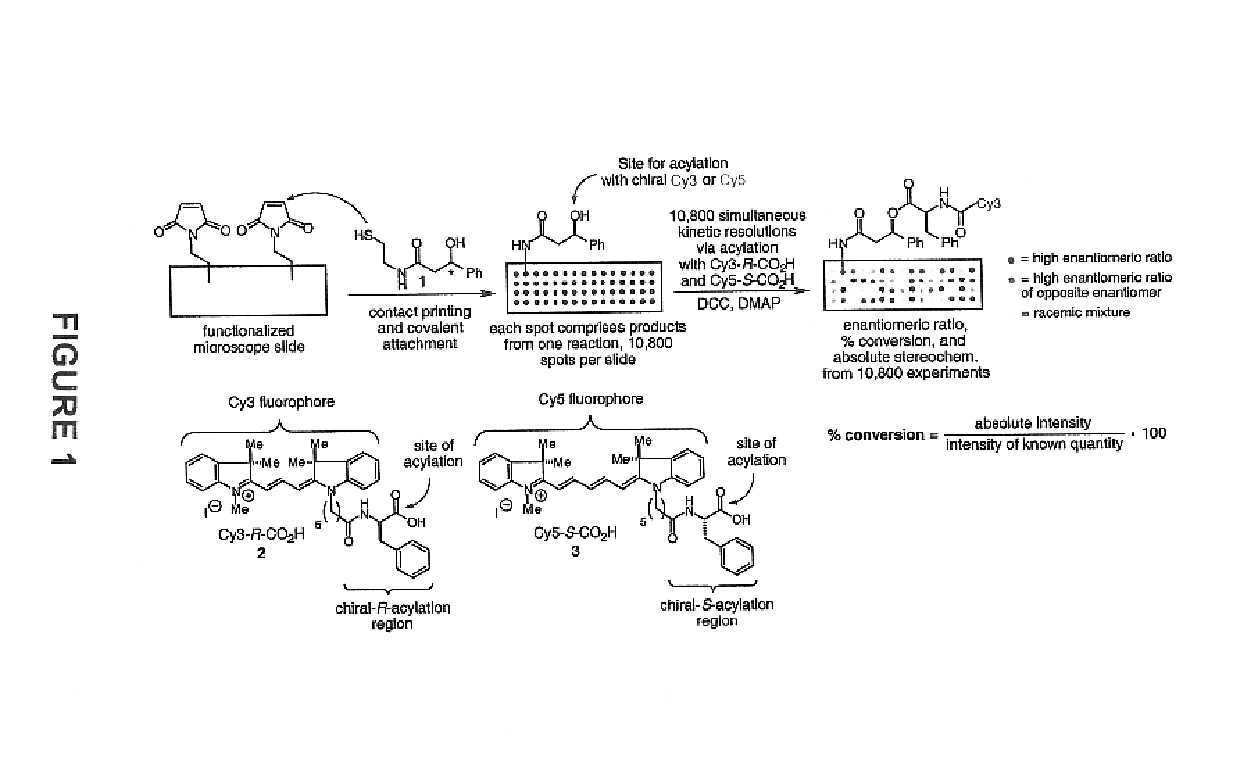
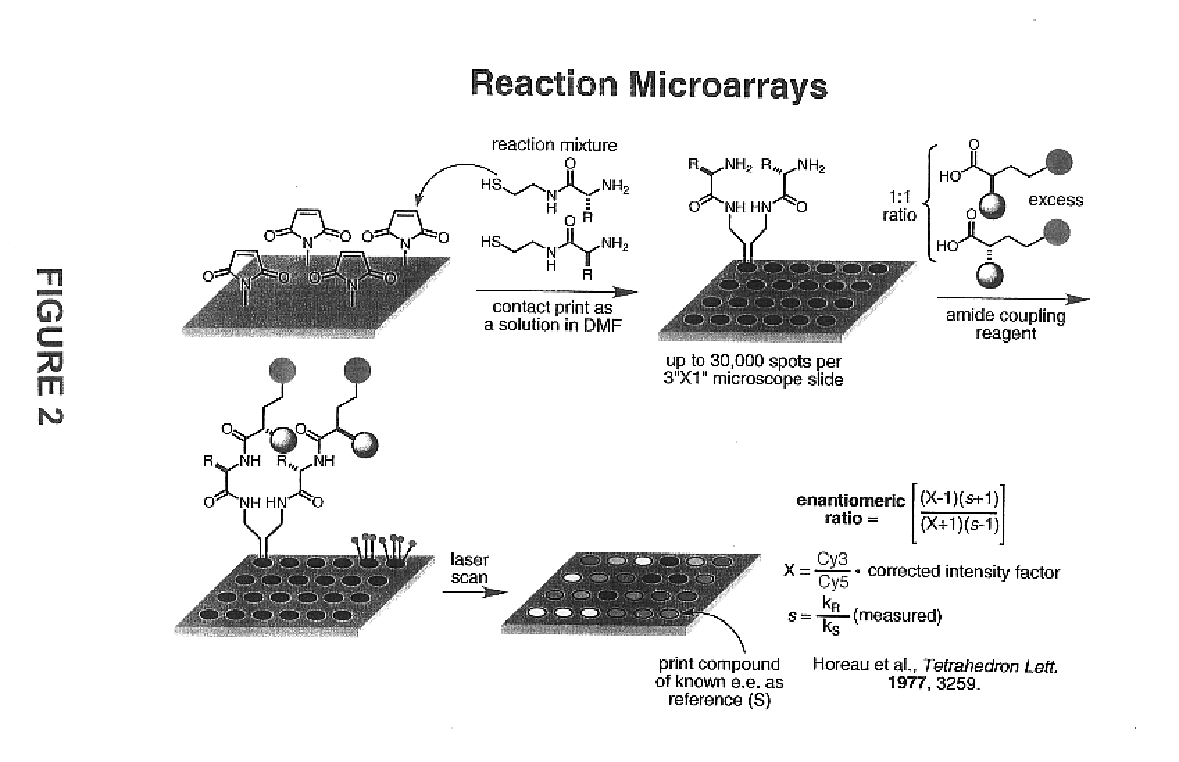
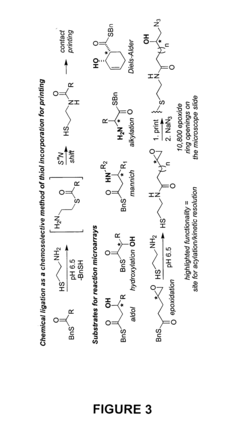
- A high-throughput method involving the arraying of reaction products on a surface and contacting them with sets of identification reagents, including chiral detecting agents, to rapidly determine enantiomeric ratios, absolute configurations, and functional group identities, enabling simultaneous analysis of thousands of reactions within a short period, such as 48 hours or less.
Safety Protocols for Perchloric Acid Handling
Perchloric acid is a highly reactive and potentially dangerous chemical compound that requires strict safety protocols for handling and use in laboratory settings. When investigating absolute reaction rates, researchers must adhere to comprehensive safety measures to mitigate risks associated with perchloric acid's explosive and oxidizing properties.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Laboratory personnel must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where perchloric acid vapors may be present, a properly fitted respirator with appropriate cartridges is essential. All PPE should be regularly inspected and replaced as needed to ensure optimal protection.
Proper storage of perchloric acid is critical to prevent accidents. The acid should be kept in a cool, dry, well-ventilated area, away from combustible materials and other incompatible substances. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Containers should be regularly inspected for signs of degradation or leakage.
When handling perchloric acid, researchers must work in a designated fume hood equipped with a wash-down system. This specialized fume hood helps contain and neutralize any vapors or spills. The work area should be free of organic materials and other substances that could react violently with perchloric acid. Additionally, a safety shower and eyewash station must be readily accessible in case of accidental exposure.
Dilution and waste disposal procedures require careful attention. Perchloric acid should always be added to water, never the reverse, to prevent dangerous splashing and heat generation. Waste solutions containing perchloric acid must be collected separately and neutralized before disposal, following local regulations and institutional guidelines.
Emergency response protocols must be established and communicated to all laboratory personnel. This includes procedures for spill containment, neutralization, and evacuation if necessary. A spill kit specifically designed for perchloric acid should be readily available, containing appropriate neutralizing agents and absorbent materials.
Regular training and education are essential components of safety protocols. All personnel working with perchloric acid should receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing compliance with safety standards.
Lastly, rigorous documentation and record-keeping practices are crucial. This includes maintaining up-to-date safety data sheets (SDS), logging usage and disposal of perchloric acid, and documenting any incidents or near-misses. Regular safety audits should be conducted to identify potential hazards and ensure compliance with established protocols.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Laboratory personnel must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where perchloric acid vapors may be present, a properly fitted respirator with appropriate cartridges is essential. All PPE should be regularly inspected and replaced as needed to ensure optimal protection.
Proper storage of perchloric acid is critical to prevent accidents. The acid should be kept in a cool, dry, well-ventilated area, away from combustible materials and other incompatible substances. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Containers should be regularly inspected for signs of degradation or leakage.
When handling perchloric acid, researchers must work in a designated fume hood equipped with a wash-down system. This specialized fume hood helps contain and neutralize any vapors or spills. The work area should be free of organic materials and other substances that could react violently with perchloric acid. Additionally, a safety shower and eyewash station must be readily accessible in case of accidental exposure.
Dilution and waste disposal procedures require careful attention. Perchloric acid should always be added to water, never the reverse, to prevent dangerous splashing and heat generation. Waste solutions containing perchloric acid must be collected separately and neutralized before disposal, following local regulations and institutional guidelines.
Emergency response protocols must be established and communicated to all laboratory personnel. This includes procedures for spill containment, neutralization, and evacuation if necessary. A spill kit specifically designed for perchloric acid should be readily available, containing appropriate neutralizing agents and absorbent materials.
Regular training and education are essential components of safety protocols. All personnel working with perchloric acid should receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing compliance with safety standards.
Lastly, rigorous documentation and record-keeping practices are crucial. This includes maintaining up-to-date safety data sheets (SDS), logging usage and disposal of perchloric acid, and documenting any incidents or near-misses. Regular safety audits should be conducted to identify potential hazards and ensure compliance with established protocols.
Environmental Impact of Perchloric Acid Usage
The use of perchloric acid in investigating absolute reaction rates has raised significant environmental concerns due to its potential impact on ecosystems and human health. Perchloric acid is a strong oxidizing agent and highly corrosive, making its handling and disposal a critical environmental issue. When released into the environment, it can contaminate soil and water sources, leading to adverse effects on flora and fauna.
One of the primary environmental risks associated with perchloric acid usage is its potential to form perchlorate salts. These salts are highly soluble and mobile in aquatic environments, allowing them to spread rapidly through groundwater and surface water systems. Perchlorate contamination has been detected in drinking water sources in various regions, raising concerns about its long-term effects on human health and wildlife.
The persistence of perchlorate in the environment is a significant challenge. Unlike many other contaminants, perchlorate does not readily degrade under natural conditions, leading to long-lasting environmental impacts. This persistence can result in bioaccumulation in plants and animals, potentially disrupting ecosystems and food chains.
In aquatic ecosystems, perchlorate contamination can affect the growth and development of various organisms. Studies have shown that exposure to perchlorate can interfere with thyroid hormone production in fish and amphibians, leading to developmental abnormalities and reduced survival rates. This can have cascading effects throughout the aquatic food web, impacting biodiversity and ecosystem balance.
Soil contamination by perchloric acid and its derivatives can alter soil chemistry and microbial communities. This may lead to reduced soil fertility and negatively impact plant growth, potentially affecting agricultural productivity in contaminated areas. Furthermore, the oxidizing nature of perchloric acid can contribute to the mobilization of heavy metals in soil, exacerbating environmental pollution.
To mitigate these environmental risks, strict protocols for the use, storage, and disposal of perchloric acid in research settings are essential. Proper containment systems, spill prevention measures, and waste treatment processes must be implemented to minimize the release of perchloric acid into the environment. Additionally, ongoing monitoring of water and soil quality in areas where perchloric acid is used extensively is crucial for early detection and remediation of contamination.
Research into alternative methods or less environmentally harmful substances for investigating absolute reaction rates is an important area of focus. Developing greener chemistry approaches that can achieve similar scientific outcomes without the environmental risks associated with perchloric acid usage is crucial for sustainable scientific practices.
One of the primary environmental risks associated with perchloric acid usage is its potential to form perchlorate salts. These salts are highly soluble and mobile in aquatic environments, allowing them to spread rapidly through groundwater and surface water systems. Perchlorate contamination has been detected in drinking water sources in various regions, raising concerns about its long-term effects on human health and wildlife.
The persistence of perchlorate in the environment is a significant challenge. Unlike many other contaminants, perchlorate does not readily degrade under natural conditions, leading to long-lasting environmental impacts. This persistence can result in bioaccumulation in plants and animals, potentially disrupting ecosystems and food chains.
In aquatic ecosystems, perchlorate contamination can affect the growth and development of various organisms. Studies have shown that exposure to perchlorate can interfere with thyroid hormone production in fish and amphibians, leading to developmental abnormalities and reduced survival rates. This can have cascading effects throughout the aquatic food web, impacting biodiversity and ecosystem balance.
Soil contamination by perchloric acid and its derivatives can alter soil chemistry and microbial communities. This may lead to reduced soil fertility and negatively impact plant growth, potentially affecting agricultural productivity in contaminated areas. Furthermore, the oxidizing nature of perchloric acid can contribute to the mobilization of heavy metals in soil, exacerbating environmental pollution.
To mitigate these environmental risks, strict protocols for the use, storage, and disposal of perchloric acid in research settings are essential. Proper containment systems, spill prevention measures, and waste treatment processes must be implemented to minimize the release of perchloric acid into the environment. Additionally, ongoing monitoring of water and soil quality in areas where perchloric acid is used extensively is crucial for early detection and remediation of contamination.
Research into alternative methods or less environmentally harmful substances for investigating absolute reaction rates is an important area of focus. Developing greener chemistry approaches that can achieve similar scientific outcomes without the environmental risks associated with perchloric acid usage is crucial for sustainable scientific practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!