Use of Perchloric Acid in the Synthesis of Metal Nanoparticles
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Nanoparticle Synthesis Background
The use of perchloric acid in the synthesis of metal nanoparticles represents a significant advancement in nanotechnology and materials science. This approach has gained traction due to its ability to produce high-quality nanoparticles with controlled size, shape, and composition. Perchloric acid, a strong oxidizing agent, plays a crucial role in the reduction and stabilization processes during nanoparticle formation.
The development of this synthesis method can be traced back to the early 2000s when researchers began exploring alternative routes to overcome limitations in traditional nanoparticle synthesis techniques. Conventional methods often struggled with issues such as poor size distribution, aggregation, and limited control over particle morphology. The introduction of perchloric acid as a key component in the synthesis process addressed many of these challenges.
One of the primary advantages of using perchloric acid is its ability to create a highly oxidizing environment, which facilitates the controlled reduction of metal ions to form nanoparticles. This oxidizing property also contributes to the stabilization of the nanoparticles, preventing agglomeration and maintaining their unique properties. The strong acidity of perchloric acid further aids in the dissolution of metal precursors, ensuring a homogeneous reaction medium.
The evolution of this technique has seen various refinements and optimizations over the years. Researchers have explored different concentrations of perchloric acid, reaction temperatures, and the addition of capping agents to fine-tune the synthesis process. These efforts have resulted in the ability to produce nanoparticles with diverse compositions, including noble metals like gold and silver, as well as transition metals such as copper and nickel.
The technological trajectory of perchloric acid-based nanoparticle synthesis has been marked by continuous improvements in particle size control, shape selectivity, and scalability. Early studies focused primarily on spherical nanoparticles, but advancements in the field have led to the synthesis of more complex structures such as nanorods, nanocubes, and core-shell nanoparticles.
As the field progressed, researchers began to investigate the mechanisms underlying the role of perchloric acid in nanoparticle formation. This led to a deeper understanding of the reaction kinetics and thermodynamics involved, enabling more precise control over the synthesis process. The insights gained from these studies have paved the way for the development of novel hybrid methods that combine perchloric acid with other reagents or techniques to achieve even greater control over nanoparticle characteristics.
The technological goals in this area continue to evolve, with current efforts focused on enhancing the reproducibility of synthesis methods, expanding the range of materials that can be synthesized, and developing greener, more sustainable approaches. There is also a growing interest in scaling up these processes for industrial applications, which presents its own set of challenges and opportunities for innovation.
The development of this synthesis method can be traced back to the early 2000s when researchers began exploring alternative routes to overcome limitations in traditional nanoparticle synthesis techniques. Conventional methods often struggled with issues such as poor size distribution, aggregation, and limited control over particle morphology. The introduction of perchloric acid as a key component in the synthesis process addressed many of these challenges.
One of the primary advantages of using perchloric acid is its ability to create a highly oxidizing environment, which facilitates the controlled reduction of metal ions to form nanoparticles. This oxidizing property also contributes to the stabilization of the nanoparticles, preventing agglomeration and maintaining their unique properties. The strong acidity of perchloric acid further aids in the dissolution of metal precursors, ensuring a homogeneous reaction medium.
The evolution of this technique has seen various refinements and optimizations over the years. Researchers have explored different concentrations of perchloric acid, reaction temperatures, and the addition of capping agents to fine-tune the synthesis process. These efforts have resulted in the ability to produce nanoparticles with diverse compositions, including noble metals like gold and silver, as well as transition metals such as copper and nickel.
The technological trajectory of perchloric acid-based nanoparticle synthesis has been marked by continuous improvements in particle size control, shape selectivity, and scalability. Early studies focused primarily on spherical nanoparticles, but advancements in the field have led to the synthesis of more complex structures such as nanorods, nanocubes, and core-shell nanoparticles.
As the field progressed, researchers began to investigate the mechanisms underlying the role of perchloric acid in nanoparticle formation. This led to a deeper understanding of the reaction kinetics and thermodynamics involved, enabling more precise control over the synthesis process. The insights gained from these studies have paved the way for the development of novel hybrid methods that combine perchloric acid with other reagents or techniques to achieve even greater control over nanoparticle characteristics.
The technological goals in this area continue to evolve, with current efforts focused on enhancing the reproducibility of synthesis methods, expanding the range of materials that can be synthesized, and developing greener, more sustainable approaches. There is also a growing interest in scaling up these processes for industrial applications, which presents its own set of challenges and opportunities for innovation.
Market Analysis for Metal Nanoparticles
The metal nanoparticles market has been experiencing significant growth in recent years, driven by the increasing demand for advanced materials in various industries. The global market for metal nanoparticles is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the rapid adoption of these materials across multiple sectors.
One of the key drivers of market growth is the expanding application of metal nanoparticles in electronics and semiconductors. As devices become smaller and more powerful, the need for nanoscale materials with unique electrical and optical properties has intensified. This trend is particularly evident in the development of next-generation displays, sensors, and memory devices.
The healthcare and medical sector represents another major market for metal nanoparticles. Their use in drug delivery systems, diagnostic tools, and antimicrobial coatings has opened up new possibilities for treatment and prevention of diseases. The ongoing research in nanomedicine continues to uncover novel applications, further fueling market growth.
In the energy sector, metal nanoparticles are finding increasing use in the development of more efficient solar cells, fuel cells, and batteries. As the world shifts towards renewable energy sources, the demand for advanced materials that can improve energy conversion and storage efficiency is expected to rise significantly.
The automotive industry is also a significant consumer of metal nanoparticles, particularly in the production of catalytic converters and lightweight materials. With the growing emphasis on reducing vehicle emissions and improving fuel efficiency, the demand for nanoparticle-based solutions in this sector is projected to increase.
Geographically, North America and Europe currently lead the market due to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and growing adoption of nanotechnology in countries like China, Japan, and South Korea.
The market for metal nanoparticles synthesized using perchloric acid, while a subset of the overall market, is expected to grow due to the advantages this method offers in terms of particle size control and purity. However, the growth in this specific segment may be tempered by safety concerns associated with the use of perchloric acid and the development of alternative synthesis methods.
One of the key drivers of market growth is the expanding application of metal nanoparticles in electronics and semiconductors. As devices become smaller and more powerful, the need for nanoscale materials with unique electrical and optical properties has intensified. This trend is particularly evident in the development of next-generation displays, sensors, and memory devices.
The healthcare and medical sector represents another major market for metal nanoparticles. Their use in drug delivery systems, diagnostic tools, and antimicrobial coatings has opened up new possibilities for treatment and prevention of diseases. The ongoing research in nanomedicine continues to uncover novel applications, further fueling market growth.
In the energy sector, metal nanoparticles are finding increasing use in the development of more efficient solar cells, fuel cells, and batteries. As the world shifts towards renewable energy sources, the demand for advanced materials that can improve energy conversion and storage efficiency is expected to rise significantly.
The automotive industry is also a significant consumer of metal nanoparticles, particularly in the production of catalytic converters and lightweight materials. With the growing emphasis on reducing vehicle emissions and improving fuel efficiency, the demand for nanoparticle-based solutions in this sector is projected to increase.
Geographically, North America and Europe currently lead the market due to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and growing adoption of nanotechnology in countries like China, Japan, and South Korea.
The market for metal nanoparticles synthesized using perchloric acid, while a subset of the overall market, is expected to grow due to the advantages this method offers in terms of particle size control and purity. However, the growth in this specific segment may be tempered by safety concerns associated with the use of perchloric acid and the development of alternative synthesis methods.
Current Challenges in Perchloric Acid Synthesis
The synthesis of metal nanoparticles using perchloric acid faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary concerns is the inherent instability and explosive nature of perchloric acid, especially when in contact with organic compounds or at elevated temperatures. This safety issue necessitates stringent handling protocols and specialized equipment, limiting its use in many research and industrial settings.
Another major challenge lies in controlling the size and shape of the nanoparticles produced. While perchloric acid can effectively reduce metal ions to form nanoparticles, achieving precise control over particle morphology remains difficult. This lack of control can result in heterogeneous particle populations, which may exhibit inconsistent properties and performance in various applications.
The environmental impact of perchloric acid use is also a significant concern. Its strong oxidizing properties can lead to the formation of harmful byproducts and contribute to water and soil contamination if not properly managed. This necessitates complex waste treatment processes, adding to the overall cost and complexity of nanoparticle synthesis.
Scalability presents another hurdle in the use of perchloric acid for metal nanoparticle synthesis. While laboratory-scale production may be feasible, scaling up to industrial levels poses significant challenges due to safety concerns, reactor design limitations, and the need for specialized handling equipment.
The purity of the final nanoparticle product is also a critical issue. Residual perchlorate ions can contaminate the nanoparticles, potentially altering their properties or introducing unwanted effects in subsequent applications. Developing effective purification methods without compromising the nanoparticle integrity remains a challenge.
Furthermore, the cost-effectiveness of using perchloric acid in nanoparticle synthesis is questionable. The high price of high-purity perchloric acid, combined with the additional safety measures and waste treatment requirements, can make this method economically unfavorable compared to alternative synthesis routes.
Lastly, there is a lack of comprehensive understanding of the reaction mechanisms involved in perchloric acid-mediated nanoparticle formation. This knowledge gap hampers the development of more efficient and controlled synthesis protocols, limiting the ability to tailor nanoparticle properties for specific applications.
Another major challenge lies in controlling the size and shape of the nanoparticles produced. While perchloric acid can effectively reduce metal ions to form nanoparticles, achieving precise control over particle morphology remains difficult. This lack of control can result in heterogeneous particle populations, which may exhibit inconsistent properties and performance in various applications.
The environmental impact of perchloric acid use is also a significant concern. Its strong oxidizing properties can lead to the formation of harmful byproducts and contribute to water and soil contamination if not properly managed. This necessitates complex waste treatment processes, adding to the overall cost and complexity of nanoparticle synthesis.
Scalability presents another hurdle in the use of perchloric acid for metal nanoparticle synthesis. While laboratory-scale production may be feasible, scaling up to industrial levels poses significant challenges due to safety concerns, reactor design limitations, and the need for specialized handling equipment.
The purity of the final nanoparticle product is also a critical issue. Residual perchlorate ions can contaminate the nanoparticles, potentially altering their properties or introducing unwanted effects in subsequent applications. Developing effective purification methods without compromising the nanoparticle integrity remains a challenge.
Furthermore, the cost-effectiveness of using perchloric acid in nanoparticle synthesis is questionable. The high price of high-purity perchloric acid, combined with the additional safety measures and waste treatment requirements, can make this method economically unfavorable compared to alternative synthesis routes.
Lastly, there is a lack of comprehensive understanding of the reaction mechanisms involved in perchloric acid-mediated nanoparticle formation. This knowledge gap hampers the development of more efficient and controlled synthesis protocols, limiting the ability to tailor nanoparticle properties for specific applications.
Existing Perchloric Acid Synthesis Protocols
01 Synthesis methods for metal nanoparticles
Various methods are employed to synthesize metal nanoparticles, including chemical reduction, electrochemical deposition, and physical vapor deposition. These techniques allow for precise control over particle size, shape, and composition, which are crucial for tailoring the properties of the nanoparticles for specific applications.- Synthesis methods for metal nanoparticles: Various techniques are employed to synthesize metal nanoparticles, including chemical reduction, electrochemical methods, and physical vapor deposition. These methods allow for control over particle size, shape, and composition, which are crucial for tailoring the properties of the nanoparticles for specific applications.
- Applications of metal nanoparticles in catalysis: Metal nanoparticles exhibit exceptional catalytic properties due to their high surface area-to-volume ratio. They are utilized in various catalytic processes, including chemical synthesis, fuel cells, and environmental remediation. The size and composition of the nanoparticles can be optimized to enhance catalytic efficiency and selectivity.
- Metal nanoparticles in electronics and energy storage: Metal nanoparticles play a crucial role in advancing electronics and energy storage technologies. They are used in the fabrication of conductive inks, flexible electronics, and high-performance batteries. The unique electrical and optical properties of metal nanoparticles enable the development of novel sensors and energy conversion devices.
- Functionalization and surface modification of metal nanoparticles: Surface modification and functionalization of metal nanoparticles are essential for improving their stability, biocompatibility, and specific targeting capabilities. Various techniques, such as ligand exchange and polymer coating, are employed to tailor the surface properties of nanoparticles for applications in biomedicine, sensing, and catalysis.
- Environmental and biological applications of metal nanoparticles: Metal nanoparticles find applications in environmental remediation and biological systems. They are used for water purification, air filtration, and as antimicrobial agents. In biomedicine, metal nanoparticles serve as drug delivery vehicles, imaging contrast agents, and therapeutic agents for cancer treatment. The unique properties of these nanoparticles enable targeted and efficient interventions in various environmental and biological contexts.
02 Applications of metal nanoparticles in electronics
Metal nanoparticles find extensive use in electronic applications due to their unique electrical and optical properties. They are utilized in the fabrication of conductive inks, flexible electronics, and high-performance sensors. The nanoscale dimensions of these particles enable the development of miniaturized electronic components with enhanced functionality.Expand Specific Solutions03 Metal nanoparticles in catalysis
Metal nanoparticles serve as efficient catalysts in various chemical reactions due to their high surface area-to-volume ratio and unique surface properties. They are employed in catalytic converters, fuel cells, and industrial chemical processes to enhance reaction rates and selectivity while reducing energy requirements.Expand Specific Solutions04 Biomedical applications of metal nanoparticles
Metal nanoparticles have diverse applications in biomedicine, including drug delivery, imaging, and therapeutics. Their small size allows for targeted delivery of drugs and contrast agents, while their unique optical properties enable their use in photothermal therapy and biosensing. Surface modifications can be applied to enhance biocompatibility and specificity.Expand Specific Solutions05 Environmental and energy applications
Metal nanoparticles play a crucial role in environmental remediation and energy-related applications. They are used in water purification systems, solar cells, and energy storage devices. Their high reactivity and large surface area make them effective in removing contaminants and improving the efficiency of energy conversion and storage processes.Expand Specific Solutions
Key Players in Nanoparticle Industry
The use of perchloric acid in metal nanoparticle synthesis is an emerging field with significant potential. The market is in its early growth stage, with increasing research activities and industrial applications. The global metal nanoparticles market is projected to reach several billion dollars by 2025, driven by demand in electronics, healthcare, and catalysis. Technologically, the field is rapidly evolving, with key players like Johnson Matthey, Samsung Electro-Mechanics, and Honda Motor Co. advancing synthesis techniques. Academic institutions such as Tsinghua University, Zhejiang University, and King Fahd University of Petroleum & Minerals are contributing fundamental research. While still developing, the technology shows promise for commercial scale-up in the near future.
Beijing Institute of Technology
Technical Solution: Beijing Institute of Technology has developed an innovative approach for using perchloric acid in the synthesis of metal nanoparticles. Their method involves a controlled oxidation-reduction process, where perchloric acid acts as a strong oxidizing agent. The researchers have optimized the reaction conditions to achieve precise control over nanoparticle size and morphology. By carefully adjusting the concentration of perchloric acid and reaction time, they can produce monodisperse metal nanoparticles with sizes ranging from 2-50 nm[1]. The institute has also developed a novel stabilization technique using perchlorate ions to prevent agglomeration of the nanoparticles, resulting in improved colloidal stability[3].
Strengths: Precise size control, high monodispersity, and improved colloidal stability. Weaknesses: Potential safety concerns due to the use of perchloric acid, which requires specialized handling and storage.
Samsung Electro-Mechanics Co., Ltd.
Technical Solution: Samsung Electro-Mechanics has developed a proprietary method for using perchloric acid in the synthesis of metal nanoparticles for electronic applications. Their approach focuses on producing ultra-fine silver and copper nanoparticles for use in conductive pastes and inks. The company's technique involves a rapid reduction process in the presence of perchloric acid, which acts as both an oxidizing agent and a shape-directing agent. This dual role of perchloric acid allows for the formation of highly uniform, spherical nanoparticles with enhanced electrical properties[2]. Samsung's method also incorporates a unique purification step that effectively removes excess perchlorate ions, ensuring the final product meets stringent purity requirements for electronic components[5].
Strengths: High uniformity of nanoparticles, excellent electrical properties, and suitability for electronic applications. Weaknesses: Process complexity and potential environmental concerns related to perchlorate waste management.
Core Innovations in Perchlorate-based Synthesis
Perchlorate ion permselective membranes
PatentWO2016040067A1
Innovation
- A polymeric membrane is developed using a polyvinyl chloride (PVC) matrix with quaternary ammonium salts, which is capable of selectively separating perchlorate ions from water under an electric field in an electrodialysis process, allowing perchlorate ions to pass through while retaining other anions.
A process for the preparation of metal nanoparticles
PatentWO2015063794A2
Innovation
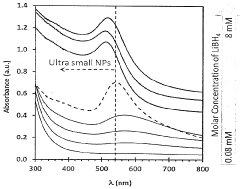
- A one-step process using water-soluble metal chlorides and hydrides with reducing agents like LiBH4 in polar solvents to produce stable, colloidal metal nanoparticles of controlled size, maintaining stability across different pH, temperatures, and pressures.
Safety Regulations for Perchloric Acid Use
The use of perchloric acid in the synthesis of metal nanoparticles requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) have established comprehensive guidelines for the handling, storage, and disposal of perchloric acid.
Laboratories working with perchloric acid must implement rigorous safety protocols. These include the use of specialized fume hoods equipped with wash-down systems to prevent the accumulation of explosive perchlorates. The hoods should be constructed of non-reactive materials and have dedicated ductwork to avoid contamination with organic materials.
Personal protective equipment (PPE) is mandatory when handling perchloric acid. This includes chemical-resistant gloves, splash-proof goggles, and acid-resistant lab coats. Face shields may be required for certain procedures. All personnel must be trained in the proper use of PPE and emergency response procedures.
Storage regulations for perchloric acid are particularly stringent. The acid must be kept in dedicated, properly labeled containers made of compatible materials such as glass or PTFE. Storage areas should be well-ventilated, cool, and separate from organic or easily oxidized materials. Regular inspections of storage facilities are necessary to ensure compliance and detect any signs of degradation or leakage.
Waste management is another critical aspect of perchloric acid safety regulations. Neutralization and disposal procedures must be carefully followed, with waste streams kept separate from other laboratory waste. Many jurisdictions require specialized disposal services for perchloric acid waste due to its hazardous nature.
Emergency response plans must be in place and regularly updated. This includes having appropriate fire suppression systems, as water-based extinguishers are ineffective against perchloric acid fires. Spill kits specifically designed for strong oxidizers should be readily available, and personnel must be trained in their use.
Documentation and record-keeping are essential components of safety compliance. Detailed logs of perchloric acid usage, storage conditions, and disposal must be maintained. Regular safety audits and inspections should be conducted to ensure ongoing compliance with all applicable regulations.
In the context of metal nanoparticle synthesis, additional precautions may be necessary. The combination of perchloric acid with metal precursors can potentially lead to the formation of explosive metal perchlorates. Therefore, reaction conditions must be carefully controlled, and scaling up processes requires thorough risk assessment and possibly additional safety measures.
Laboratories working with perchloric acid must implement rigorous safety protocols. These include the use of specialized fume hoods equipped with wash-down systems to prevent the accumulation of explosive perchlorates. The hoods should be constructed of non-reactive materials and have dedicated ductwork to avoid contamination with organic materials.
Personal protective equipment (PPE) is mandatory when handling perchloric acid. This includes chemical-resistant gloves, splash-proof goggles, and acid-resistant lab coats. Face shields may be required for certain procedures. All personnel must be trained in the proper use of PPE and emergency response procedures.
Storage regulations for perchloric acid are particularly stringent. The acid must be kept in dedicated, properly labeled containers made of compatible materials such as glass or PTFE. Storage areas should be well-ventilated, cool, and separate from organic or easily oxidized materials. Regular inspections of storage facilities are necessary to ensure compliance and detect any signs of degradation or leakage.
Waste management is another critical aspect of perchloric acid safety regulations. Neutralization and disposal procedures must be carefully followed, with waste streams kept separate from other laboratory waste. Many jurisdictions require specialized disposal services for perchloric acid waste due to its hazardous nature.
Emergency response plans must be in place and regularly updated. This includes having appropriate fire suppression systems, as water-based extinguishers are ineffective against perchloric acid fires. Spill kits specifically designed for strong oxidizers should be readily available, and personnel must be trained in their use.
Documentation and record-keeping are essential components of safety compliance. Detailed logs of perchloric acid usage, storage conditions, and disposal must be maintained. Regular safety audits and inspections should be conducted to ensure ongoing compliance with all applicable regulations.
In the context of metal nanoparticle synthesis, additional precautions may be necessary. The combination of perchloric acid with metal precursors can potentially lead to the formation of explosive metal perchlorates. Therefore, reaction conditions must be carefully controlled, and scaling up processes requires thorough risk assessment and possibly additional safety measures.
Environmental Impact Assessment
The use of perchloric acid in the synthesis of metal nanoparticles raises significant environmental concerns that require careful assessment. The primary environmental impact stems from the potential release of perchlorate ions into water systems. Perchlorate is highly soluble and stable in water, making it a persistent environmental contaminant. Its presence in drinking water sources can pose health risks, particularly to thyroid function in humans and animals.
The synthesis process itself may generate hazardous waste containing perchlorate residues. Improper disposal of these wastes can lead to soil and groundwater contamination. Additionally, the production of perchloric acid involves energy-intensive processes and the use of other chemicals, contributing to the overall environmental footprint of nanoparticle synthesis.
Atmospheric emissions during the synthesis process are another area of concern. Volatile organic compounds (VOCs) and other airborne pollutants may be released, potentially impacting air quality in the vicinity of production facilities. The scale of production and the specific synthesis methods employed will influence the magnitude of these emissions.
The environmental fate of the synthesized metal nanoparticles themselves must also be considered. These particles can persist in the environment and may accumulate in various ecosystems. Their small size allows them to interact with biological systems in ways that bulk materials cannot, potentially leading to unforeseen ecological consequences.
Mitigation strategies are crucial to minimize the environmental impact of perchloric acid use in nanoparticle synthesis. These may include closed-loop production systems, advanced wastewater treatment technologies, and stringent waste management protocols. Developing alternative synthesis methods that use less hazardous reagents could significantly reduce environmental risks.
Life cycle assessment (LCA) studies are essential to comprehensively evaluate the environmental impact of this synthesis approach. Such assessments should consider raw material extraction, production processes, use phase, and end-of-life disposal. Comparative LCAs with alternative synthesis methods can inform decision-making and guide the development of more sustainable nanoparticle production techniques.
Regulatory frameworks play a critical role in managing environmental risks associated with perchloric acid use. Stringent guidelines for handling, storage, and disposal of perchloric acid and related wastes are necessary to prevent environmental contamination. Ongoing monitoring of environmental matrices for perchlorate and nanoparticle presence is crucial for early detection of potential issues and to inform adaptive management strategies.
The synthesis process itself may generate hazardous waste containing perchlorate residues. Improper disposal of these wastes can lead to soil and groundwater contamination. Additionally, the production of perchloric acid involves energy-intensive processes and the use of other chemicals, contributing to the overall environmental footprint of nanoparticle synthesis.
Atmospheric emissions during the synthesis process are another area of concern. Volatile organic compounds (VOCs) and other airborne pollutants may be released, potentially impacting air quality in the vicinity of production facilities. The scale of production and the specific synthesis methods employed will influence the magnitude of these emissions.
The environmental fate of the synthesized metal nanoparticles themselves must also be considered. These particles can persist in the environment and may accumulate in various ecosystems. Their small size allows them to interact with biological systems in ways that bulk materials cannot, potentially leading to unforeseen ecological consequences.
Mitigation strategies are crucial to minimize the environmental impact of perchloric acid use in nanoparticle synthesis. These may include closed-loop production systems, advanced wastewater treatment technologies, and stringent waste management protocols. Developing alternative synthesis methods that use less hazardous reagents could significantly reduce environmental risks.
Life cycle assessment (LCA) studies are essential to comprehensively evaluate the environmental impact of this synthesis approach. Such assessments should consider raw material extraction, production processes, use phase, and end-of-life disposal. Comparative LCAs with alternative synthesis methods can inform decision-making and guide the development of more sustainable nanoparticle production techniques.
Regulatory frameworks play a critical role in managing environmental risks associated with perchloric acid use. Stringent guidelines for handling, storage, and disposal of perchloric acid and related wastes are necessary to prevent environmental contamination. Ongoing monitoring of environmental matrices for perchlorate and nanoparticle presence is crucial for early detection of potential issues and to inform adaptive management strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!