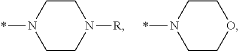Alkyl Influence on Modern Analytical Chemistry
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Groups in Analytical Chemistry: Evolution and Objectives
Alkyl groups have played a pivotal role in the evolution of analytical chemistry, shaping the field's development and expanding its capabilities over time. The journey of alkyl groups in analytical chemistry began in the early 20th century with the advent of chromatography techniques. Initially, alkyl-modified stationary phases were introduced to improve the separation of organic compounds, marking a significant milestone in the field's progression.
As analytical chemistry advanced, the importance of alkyl groups became increasingly apparent. Their unique properties, such as hydrophobicity and varying chain lengths, allowed for more precise and selective analyses. This led to the development of alkyl-based derivatization techniques, which enhanced the detection and quantification of various analytes, particularly in gas chromatography and high-performance liquid chromatography.
The 1970s and 1980s saw a surge in research focused on alkyl-modified surfaces and their applications in analytical separations. This period witnessed the emergence of reversed-phase chromatography, where alkyl-bonded silica became the stationary phase of choice for a wide range of analytical applications. The versatility of alkyl groups in modifying surface properties opened new avenues for analyzing complex mixtures and expanding the scope of analytical chemistry.
In recent decades, the role of alkyl groups has extended beyond traditional chromatography. Their incorporation into novel materials, such as molecularly imprinted polymers and nanoparticles, has led to the development of highly selective sensors and extraction media. These advancements have significantly improved the sensitivity and specificity of analytical methods, particularly in environmental monitoring and bioanalysis.
The ongoing evolution of alkyl groups in analytical chemistry aims to address several key objectives. Firstly, there is a continuous effort to enhance the selectivity and efficiency of separation techniques by fine-tuning alkyl chain properties. Secondly, researchers are exploring the potential of alkyl groups in developing more environmentally friendly and sustainable analytical methods, aligning with the principles of green chemistry. Lastly, there is a growing focus on leveraging alkyl chemistry to create multifunctional analytical platforms that can simultaneously perform separation, detection, and quantification tasks.
As we look to the future, the integration of alkyl groups with emerging technologies, such as microfluidics and miniaturized analytical devices, presents exciting opportunities for innovation. The ultimate goal is to develop more powerful, versatile, and accessible analytical tools that can meet the evolving demands of various scientific disciplines and industries.
As analytical chemistry advanced, the importance of alkyl groups became increasingly apparent. Their unique properties, such as hydrophobicity and varying chain lengths, allowed for more precise and selective analyses. This led to the development of alkyl-based derivatization techniques, which enhanced the detection and quantification of various analytes, particularly in gas chromatography and high-performance liquid chromatography.
The 1970s and 1980s saw a surge in research focused on alkyl-modified surfaces and their applications in analytical separations. This period witnessed the emergence of reversed-phase chromatography, where alkyl-bonded silica became the stationary phase of choice for a wide range of analytical applications. The versatility of alkyl groups in modifying surface properties opened new avenues for analyzing complex mixtures and expanding the scope of analytical chemistry.
In recent decades, the role of alkyl groups has extended beyond traditional chromatography. Their incorporation into novel materials, such as molecularly imprinted polymers and nanoparticles, has led to the development of highly selective sensors and extraction media. These advancements have significantly improved the sensitivity and specificity of analytical methods, particularly in environmental monitoring and bioanalysis.
The ongoing evolution of alkyl groups in analytical chemistry aims to address several key objectives. Firstly, there is a continuous effort to enhance the selectivity and efficiency of separation techniques by fine-tuning alkyl chain properties. Secondly, researchers are exploring the potential of alkyl groups in developing more environmentally friendly and sustainable analytical methods, aligning with the principles of green chemistry. Lastly, there is a growing focus on leveraging alkyl chemistry to create multifunctional analytical platforms that can simultaneously perform separation, detection, and quantification tasks.
As we look to the future, the integration of alkyl groups with emerging technologies, such as microfluidics and miniaturized analytical devices, presents exciting opportunities for innovation. The ultimate goal is to develop more powerful, versatile, and accessible analytical tools that can meet the evolving demands of various scientific disciplines and industries.
Market Demand for Alkyl-Based Analytical Techniques
The market demand for alkyl-based analytical techniques has been steadily growing in recent years, driven by advancements in chemical analysis and increasing applications across various industries. The global analytical chemistry market, which encompasses alkyl-based techniques, is projected to reach significant value in the coming years, with a substantial portion attributed to alkyl-related methodologies.
In the pharmaceutical sector, alkyl-based analytical techniques play a crucial role in drug discovery, development, and quality control processes. The rising demand for novel therapeutics and the stringent regulatory requirements for drug safety and efficacy have fueled the adoption of these techniques. Pharmaceutical companies are increasingly investing in advanced analytical tools to enhance their research capabilities and streamline drug development pipelines.
The environmental monitoring and analysis sector has also witnessed a surge in demand for alkyl-based analytical techniques. With growing concerns over pollution and environmental contamination, there is an increased need for accurate and sensitive detection methods for alkyl compounds in air, water, and soil samples. Government regulations and public awareness have further propelled the market growth in this segment.
The food and beverage industry represents another significant market for alkyl-based analytical techniques. As consumers become more health-conscious and demand transparency in food production, manufacturers are required to conduct thorough analyses of their products. Alkyl-based methods are essential for detecting and quantifying various additives, contaminants, and flavor compounds in food and beverages.
In the petrochemical industry, alkyl-based analytical techniques are indispensable for quality control and process optimization. The increasing complexity of refining processes and the need for more efficient production methods have driven the demand for advanced analytical tools. These techniques help in characterizing crude oil compositions, monitoring refining processes, and ensuring product quality.
The academic and research sector continues to be a significant contributor to the market demand for alkyl-based analytical techniques. Universities and research institutions are constantly exploring new applications and improving existing methodologies, driving innovation in the field. This sector also plays a crucial role in training future analysts and researchers, ensuring a steady supply of skilled professionals to meet industry demands.
Geographically, North America and Europe lead the market for alkyl-based analytical techniques, owing to their well-established pharmaceutical and chemical industries, stringent regulatory frameworks, and significant investments in research and development. However, the Asia-Pacific region is experiencing rapid growth in this market, driven by expanding industrial sectors, increasing environmental concerns, and growing investments in analytical infrastructure.
In the pharmaceutical sector, alkyl-based analytical techniques play a crucial role in drug discovery, development, and quality control processes. The rising demand for novel therapeutics and the stringent regulatory requirements for drug safety and efficacy have fueled the adoption of these techniques. Pharmaceutical companies are increasingly investing in advanced analytical tools to enhance their research capabilities and streamline drug development pipelines.
The environmental monitoring and analysis sector has also witnessed a surge in demand for alkyl-based analytical techniques. With growing concerns over pollution and environmental contamination, there is an increased need for accurate and sensitive detection methods for alkyl compounds in air, water, and soil samples. Government regulations and public awareness have further propelled the market growth in this segment.
The food and beverage industry represents another significant market for alkyl-based analytical techniques. As consumers become more health-conscious and demand transparency in food production, manufacturers are required to conduct thorough analyses of their products. Alkyl-based methods are essential for detecting and quantifying various additives, contaminants, and flavor compounds in food and beverages.
In the petrochemical industry, alkyl-based analytical techniques are indispensable for quality control and process optimization. The increasing complexity of refining processes and the need for more efficient production methods have driven the demand for advanced analytical tools. These techniques help in characterizing crude oil compositions, monitoring refining processes, and ensuring product quality.
The academic and research sector continues to be a significant contributor to the market demand for alkyl-based analytical techniques. Universities and research institutions are constantly exploring new applications and improving existing methodologies, driving innovation in the field. This sector also plays a crucial role in training future analysts and researchers, ensuring a steady supply of skilled professionals to meet industry demands.
Geographically, North America and Europe lead the market for alkyl-based analytical techniques, owing to their well-established pharmaceutical and chemical industries, stringent regulatory frameworks, and significant investments in research and development. However, the Asia-Pacific region is experiencing rapid growth in this market, driven by expanding industrial sectors, increasing environmental concerns, and growing investments in analytical infrastructure.
Current Challenges in Alkyl-Influenced Analytical Methods
The field of analytical chemistry faces several significant challenges related to the influence of alkyl groups on modern analytical methods. One of the primary issues is the complexity introduced by alkyl substituents in molecular structures, which can significantly affect the behavior of analytes during separation and detection processes. This complexity often leads to difficulties in achieving accurate and reproducible results, particularly in chromatographic techniques where alkyl groups can alter retention times and peak shapes.
Another challenge lies in the development of sensitive and selective detection methods for alkyl-containing compounds. Many traditional analytical techniques struggle to differentiate between structurally similar alkyl derivatives, leading to potential misidentifications or incomplete characterizations. This is particularly problematic in environmental and pharmaceutical analyses, where precise identification of alkyl-substituted pollutants or drug metabolites is crucial.
The influence of alkyl groups on ionization efficiency in mass spectrometry presents another significant hurdle. Alkyl substituents can enhance or suppress ionization, depending on their position and nature, leading to variable response factors and complicating quantitative analysis. This variability necessitates careful method development and validation to ensure accurate quantification across a range of alkyl-containing analytes.
In spectroscopic techniques, such as NMR and IR, the presence of alkyl groups can lead to signal overlap and spectral congestion, making interpretation challenging, especially for complex mixtures. This issue is exacerbated in the analysis of large biomolecules or environmental samples where multiple alkyl-containing species may be present simultaneously.
The stability and reactivity of alkyl-containing compounds during sample preparation and analysis also pose significant challenges. Some alkyl groups are prone to oxidation or rearrangement under certain conditions, potentially leading to artifacts or degradation products that can interfere with accurate analysis. This necessitates the development of specialized sample handling and storage protocols to maintain the integrity of alkyl-containing analytes.
Furthermore, the hydrophobicity imparted by alkyl groups can lead to solubility issues and non-specific adsorption to surfaces, complicating sample preparation and potentially causing analyte losses. This is particularly problematic in trace analysis and when working with complex matrices, where efficient extraction and preconcentration of alkyl-containing analytes are essential.
Lastly, the environmental impact of alkyl-containing solvents and reagents used in analytical methods is an emerging concern. There is a growing need to develop greener analytical techniques that minimize the use of alkyl-based organic solvents while maintaining or improving analytical performance. This challenge requires innovative approaches to method development and a paradigm shift towards more sustainable analytical practices.
Another challenge lies in the development of sensitive and selective detection methods for alkyl-containing compounds. Many traditional analytical techniques struggle to differentiate between structurally similar alkyl derivatives, leading to potential misidentifications or incomplete characterizations. This is particularly problematic in environmental and pharmaceutical analyses, where precise identification of alkyl-substituted pollutants or drug metabolites is crucial.
The influence of alkyl groups on ionization efficiency in mass spectrometry presents another significant hurdle. Alkyl substituents can enhance or suppress ionization, depending on their position and nature, leading to variable response factors and complicating quantitative analysis. This variability necessitates careful method development and validation to ensure accurate quantification across a range of alkyl-containing analytes.
In spectroscopic techniques, such as NMR and IR, the presence of alkyl groups can lead to signal overlap and spectral congestion, making interpretation challenging, especially for complex mixtures. This issue is exacerbated in the analysis of large biomolecules or environmental samples where multiple alkyl-containing species may be present simultaneously.
The stability and reactivity of alkyl-containing compounds during sample preparation and analysis also pose significant challenges. Some alkyl groups are prone to oxidation or rearrangement under certain conditions, potentially leading to artifacts or degradation products that can interfere with accurate analysis. This necessitates the development of specialized sample handling and storage protocols to maintain the integrity of alkyl-containing analytes.
Furthermore, the hydrophobicity imparted by alkyl groups can lead to solubility issues and non-specific adsorption to surfaces, complicating sample preparation and potentially causing analyte losses. This is particularly problematic in trace analysis and when working with complex matrices, where efficient extraction and preconcentration of alkyl-containing analytes are essential.
Lastly, the environmental impact of alkyl-containing solvents and reagents used in analytical methods is an emerging concern. There is a growing need to develop greener analytical techniques that minimize the use of alkyl-based organic solvents while maintaining or improving analytical performance. This challenge requires innovative approaches to method development and a paradigm shift towards more sustainable analytical practices.
Existing Alkyl-Based Analytical Solutions
01 Alkyl group substitution in chemical compounds
Alkyl groups are often used to substitute hydrogen atoms in various chemical compounds, altering their properties and reactivity. This substitution can affect solubility, boiling point, and other physical characteristics of the resulting molecules.- Alkyl group substitution in chemical compounds: Alkyl groups are often used as substituents in various chemical compounds to modify their properties. These substitutions can affect the compound's reactivity, solubility, and other physical characteristics. The size and structure of the alkyl group can be tailored to achieve specific desired effects in the final product.
- Alkyl groups in polymer chemistry: In polymer chemistry, alkyl groups play a crucial role in determining the properties of the resulting materials. They can be incorporated into the polymer backbone or used as side chains to influence factors such as flexibility, thermal stability, and chemical resistance. The length and branching of alkyl groups can be adjusted to fine-tune polymer characteristics.
- Alkyl groups in surfactants and detergents: Alkyl groups are essential components in the design of surfactants and detergents. The hydrophobic nature of alkyl chains contributes to the amphiphilic properties of these molecules, allowing them to interact with both water and oil-based substances. This makes them effective in cleaning and emulsification applications.
- Alkyl groups in pharmaceutical compounds: In pharmaceutical research and development, alkyl groups are often incorporated into drug molecules to modify their pharmacokinetic properties. These modifications can affect factors such as lipophilicity, metabolic stability, and binding affinity to target receptors, ultimately influencing the drug's efficacy and bioavailability.
- Alkyl groups in organic synthesis: Alkyl groups are widely used in organic synthesis as building blocks and reagents. They can be introduced through various reactions such as alkylation, reduction, and organometallic coupling. The choice of alkyl group and its method of introduction can significantly impact the outcome of synthetic processes and the properties of the final products.
02 Alkyl groups in polymer synthesis
Alkyl groups play a crucial role in polymer synthesis, influencing the properties of the resulting materials. They can be incorporated into polymer chains or used as side groups, affecting characteristics such as flexibility, thermal stability, and chemical resistance.Expand Specific Solutions03 Alkyl groups in surfactants and detergents
Alkyl groups are essential components of many surfactants and detergents. The length and structure of the alkyl chain affect the surface-active properties, determining factors such as foaming ability, emulsification, and cleaning efficiency.Expand Specific Solutions04 Alkyl groups in pharmaceutical compounds
In pharmaceutical research and development, alkyl groups are often used to modify drug molecules. These modifications can alter the drug's pharmacokinetics, including absorption, distribution, metabolism, and excretion, potentially improving its therapeutic efficacy.Expand Specific Solutions05 Alkyl groups in organometallic compounds
Alkyl groups are frequently used in the synthesis of organometallic compounds. These compounds have applications in catalysis, material science, and organic synthesis. The nature of the alkyl group can significantly influence the reactivity and selectivity of the organometallic complex.Expand Specific Solutions
Key Players in Alkyl-Related Analytical Instrumentation
The competitive landscape for "Alkyl Influence on Modern Analytical Chemistry" is characterized by a mature industry in an advanced stage of development. The market size is substantial, driven by the widespread application of analytical chemistry across various sectors. Technologically, the field is well-established but continues to evolve, with major players like Gilead Sciences, Janssen Pharmaceutica, and Bristol Myers Squibb leading innovation. Academic institutions such as MIT, Harvard, and Yale contribute significantly to research advancements. The involvement of diverse companies, from pharmaceutical giants to specialized research organizations, indicates a highly competitive and collaborative environment, with ongoing efforts to enhance analytical techniques and applications in modern chemistry.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced analytical techniques for studying alkyl influences in modern chemistry. They have pioneered the use of high-resolution mass spectrometry coupled with chromatographic separation to analyze complex alkyl-containing molecules[1]. Their approach combines multi-dimensional NMR spectroscopy with computational modeling to elucidate alkyl group effects on molecular structure and reactivity[2]. MIT researchers have also developed novel alkyl-sensitive fluorescent probes for real-time imaging of lipid dynamics in living cells[3]. These methods enable precise characterization of alkyl moieties in pharmaceuticals, environmental samples, and biological systems.
Strengths: Cutting-edge instrumentation and interdisciplinary expertise. Weaknesses: High cost and complexity of techniques may limit widespread adoption.
President & Fellows of Harvard College
Technical Solution: Harvard has made significant contributions to understanding alkyl influences through innovative analytical approaches. They have developed alkyl-specific isotope analysis techniques using gas chromatography-isotope ratio mass spectrometry (GC-IRMS) to trace the origin and transformation of organic compounds in the environment[4]. Harvard researchers have also pioneered the use of alkyl-sensitive chemical probes for protein profiling and drug discovery[5]. Their work on alkyl-mediated cross-coupling reactions has led to new analytical methods for monitoring these transformations in complex mixtures[6]. Additionally, Harvard has developed advanced NMR techniques for studying alkyl group dynamics in macromolecules.
Strengths: Strong focus on environmental and biological applications. Weaknesses: Some techniques may be limited to specialized research settings.
Innovative Alkyl Chemistry in Modern Analysis
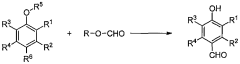
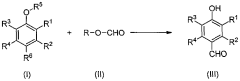
Process for production of vanillin and vanillin derivatives
PatentWO2013166642A1
Innovation
- A one-step regio- and chemoselective formylation process using a superacid to produce vanillin or vanillin derivatives from guaiacol or its derivatives, achieving high yields with minimal isomer formation by reacting guaiacol or its derivatives with a superacid, such as trifluoromethanesulfonic acid, in the presence of a formyl source like methyl formate, without the need for specific solvents or harsh conditions.
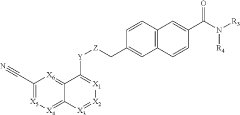
Novel CDK 8/19 inhibitors
PatentInactiveUS20220098160A1
Innovation
- Development of specific compounds, such as those represented by formulas I, II, and III, which inhibit the biological activity of CDK8/19 by interacting with these kinases, potentially used in pharmaceutical compositions for treating cancers mediated by CDK8/19 activation.
Environmental Impact of Alkyl-Based Analytical Methods
The environmental impact of alkyl-based analytical methods has become a growing concern in the field of modern analytical chemistry. These methods, while highly effective for various analytical purposes, can have significant implications for ecosystems and human health if not properly managed.
One of the primary environmental concerns associated with alkyl-based analytical techniques is the potential for chemical contamination. Many alkyl compounds used in these methods are persistent organic pollutants (POPs) that can accumulate in the environment and bioaccumulate in living organisms. This persistence can lead to long-term ecological damage, affecting biodiversity and ecosystem stability.
Water pollution is a particular area of concern. Alkyl-based analytical methods often involve the use of organic solvents and reagents that can contaminate water sources if not properly disposed of. This contamination can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and impacting water quality for both wildlife and human consumption.
Air quality is another environmental aspect affected by these analytical methods. Volatile organic compounds (VOCs) released during the analytical processes can contribute to air pollution and the formation of ground-level ozone. This not only impacts local air quality but can also contribute to broader atmospheric issues such as smog formation and climate change.
The production and disposal of alkyl-based chemicals used in analytical methods also raise environmental concerns. The manufacturing processes for these compounds often require significant energy inputs and can generate hazardous waste. Improper disposal of analytical waste containing alkyl compounds can lead to soil contamination, affecting terrestrial ecosystems and potentially entering the food chain.
To address these environmental challenges, the analytical chemistry community has been developing more sustainable approaches. Green chemistry principles are increasingly being applied to analytical methods, focusing on reducing the use of hazardous substances, minimizing waste generation, and improving energy efficiency. This includes the development of solvent-free extraction techniques, the use of less toxic alkyl alternatives, and the implementation of recycling and recovery systems for analytical waste.
Regulatory bodies have also responded to these environmental concerns by implementing stricter guidelines for the use and disposal of alkyl-based analytical chemicals. These regulations aim to minimize environmental exposure and promote more responsible practices in analytical laboratories.
As the field of analytical chemistry continues to evolve, there is a growing emphasis on balancing analytical performance with environmental stewardship. Researchers are exploring novel, environmentally friendly alternatives to traditional alkyl-based methods, such as the use of ionic liquids, supercritical fluids, and bio-based solvents. These innovations promise to maintain or even enhance analytical capabilities while significantly reducing the environmental footprint of analytical processes.
One of the primary environmental concerns associated with alkyl-based analytical techniques is the potential for chemical contamination. Many alkyl compounds used in these methods are persistent organic pollutants (POPs) that can accumulate in the environment and bioaccumulate in living organisms. This persistence can lead to long-term ecological damage, affecting biodiversity and ecosystem stability.
Water pollution is a particular area of concern. Alkyl-based analytical methods often involve the use of organic solvents and reagents that can contaminate water sources if not properly disposed of. This contamination can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and impacting water quality for both wildlife and human consumption.
Air quality is another environmental aspect affected by these analytical methods. Volatile organic compounds (VOCs) released during the analytical processes can contribute to air pollution and the formation of ground-level ozone. This not only impacts local air quality but can also contribute to broader atmospheric issues such as smog formation and climate change.
The production and disposal of alkyl-based chemicals used in analytical methods also raise environmental concerns. The manufacturing processes for these compounds often require significant energy inputs and can generate hazardous waste. Improper disposal of analytical waste containing alkyl compounds can lead to soil contamination, affecting terrestrial ecosystems and potentially entering the food chain.
To address these environmental challenges, the analytical chemistry community has been developing more sustainable approaches. Green chemistry principles are increasingly being applied to analytical methods, focusing on reducing the use of hazardous substances, minimizing waste generation, and improving energy efficiency. This includes the development of solvent-free extraction techniques, the use of less toxic alkyl alternatives, and the implementation of recycling and recovery systems for analytical waste.
Regulatory bodies have also responded to these environmental concerns by implementing stricter guidelines for the use and disposal of alkyl-based analytical chemicals. These regulations aim to minimize environmental exposure and promote more responsible practices in analytical laboratories.
As the field of analytical chemistry continues to evolve, there is a growing emphasis on balancing analytical performance with environmental stewardship. Researchers are exploring novel, environmentally friendly alternatives to traditional alkyl-based methods, such as the use of ionic liquids, supercritical fluids, and bio-based solvents. These innovations promise to maintain or even enhance analytical capabilities while significantly reducing the environmental footprint of analytical processes.
Regulatory Framework for Alkyl Use in Analytical Chemistry
The regulatory framework for alkyl use in analytical chemistry has evolved significantly in recent years, reflecting the growing importance of these compounds in modern analytical techniques. Regulatory bodies worldwide have established comprehensive guidelines to ensure the safe and responsible use of alkyl compounds in laboratory settings.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use of alkyl compounds. The Toxic Substances Control Act (TSCA) provides the EPA with the authority to require reporting, record-keeping, and testing requirements for chemical substances, including alkyl compounds used in analytical chemistry. The agency has implemented specific regulations for the handling, storage, and disposal of these substances to minimize environmental and health risks.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to alkyl compounds used in analytical chemistry. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring a high level of protection for human health and the environment. The European Chemicals Agency (ECHA) oversees the implementation of REACH and provides guidance on compliance.
In Japan, the Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including alkyl compounds. The law requires manufacturers and importers to notify the authorities of new chemical substances and conduct safety assessments before their introduction into the market.
International organizations, such as the International Organization for Standardization (ISO), have developed standards for analytical methods involving alkyl compounds. These standards provide guidelines for quality control, method validation, and reporting of results, ensuring consistency and reliability in analytical procedures across different laboratories and countries.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards. This system is particularly relevant for alkyl compounds used in analytical chemistry, as it ensures consistent labeling and safety data sheets across different jurisdictions.
Professional organizations, such as the American Chemical Society (ACS) and the Royal Society of Chemistry (RSC), have also developed guidelines and best practices for the use of alkyl compounds in analytical chemistry. These recommendations often complement regulatory requirements and provide practical guidance for laboratory professionals.
As analytical techniques continue to advance, regulatory frameworks are expected to evolve to address new challenges and emerging risks associated with alkyl compounds. This ongoing development ensures that the use of these substances in analytical chemistry remains safe, efficient, and environmentally responsible.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use of alkyl compounds. The Toxic Substances Control Act (TSCA) provides the EPA with the authority to require reporting, record-keeping, and testing requirements for chemical substances, including alkyl compounds used in analytical chemistry. The agency has implemented specific regulations for the handling, storage, and disposal of these substances to minimize environmental and health risks.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to alkyl compounds used in analytical chemistry. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring a high level of protection for human health and the environment. The European Chemicals Agency (ECHA) oversees the implementation of REACH and provides guidance on compliance.
In Japan, the Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including alkyl compounds. The law requires manufacturers and importers to notify the authorities of new chemical substances and conduct safety assessments before their introduction into the market.
International organizations, such as the International Organization for Standardization (ISO), have developed standards for analytical methods involving alkyl compounds. These standards provide guidelines for quality control, method validation, and reporting of results, ensuring consistency and reliability in analytical procedures across different laboratories and countries.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards. This system is particularly relevant for alkyl compounds used in analytical chemistry, as it ensures consistent labeling and safety data sheets across different jurisdictions.
Professional organizations, such as the American Chemical Society (ACS) and the Royal Society of Chemistry (RSC), have also developed guidelines and best practices for the use of alkyl compounds in analytical chemistry. These recommendations often complement regulatory requirements and provide practical guidance for laboratory professionals.
As analytical techniques continue to advance, regulatory frameworks are expected to evolve to address new challenges and emerging risks associated with alkyl compounds. This ongoing development ensures that the use of these substances in analytical chemistry remains safe, efficient, and environmentally responsible.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!