How to Master Alkylation in Laboratory Settings?
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkylation Background and Objectives
Alkylation, a fundamental process in organic chemistry, has been a cornerstone of laboratory synthesis for over a century. This reaction involves the transfer of an alkyl group from one molecule to another, enabling the creation of carbon-carbon and carbon-heteroatom bonds. The evolution of alkylation techniques has been driven by the need for more efficient, selective, and environmentally friendly methods in both academic research and industrial applications.
The primary objective of mastering alkylation in laboratory settings is to develop a comprehensive understanding of the reaction mechanisms, optimize reaction conditions, and expand the scope of substrates and alkylating agents. This mastery is crucial for synthesizing complex organic molecules, pharmaceuticals, and materials with tailored properties. As the demand for novel compounds continues to grow, the ability to perform precise and controlled alkylation reactions becomes increasingly valuable.
Historically, alkylation methods have progressed from simple SN1 and SN2 reactions to more sophisticated approaches involving transition metal catalysis, phase-transfer catalysis, and asymmetric alkylation. Each advancement has brought new possibilities and challenges, pushing researchers to explore innovative strategies for achieving desired transformations with higher yields, improved selectivity, and milder reaction conditions.
The current landscape of alkylation research focuses on several key areas: developing greener alkylation protocols, enhancing regioselectivity and stereoselectivity, expanding the range of compatible functional groups, and improving the overall efficiency of the process. These efforts are driven by the increasing emphasis on sustainable chemistry and the need for more atom-economical synthetic routes.
Mastering alkylation in laboratory settings requires a multifaceted approach. This includes a deep understanding of reaction kinetics and thermodynamics, proficiency in handling air- and moisture-sensitive reagents, and expertise in modern analytical techniques for product characterization. Additionally, researchers must be adept at optimizing reaction parameters such as temperature, solvent choice, and catalyst loading to achieve optimal results.
The future of alkylation research holds exciting possibilities, including the development of novel catalytic systems, the application of flow chemistry for continuous alkylation processes, and the integration of computational methods for predicting and designing new alkylation reactions. As we strive to master this fundamental transformation, the goal is not only to refine existing methodologies but also to discover entirely new paradigms in carbon-carbon and carbon-heteroatom bond formation.
The primary objective of mastering alkylation in laboratory settings is to develop a comprehensive understanding of the reaction mechanisms, optimize reaction conditions, and expand the scope of substrates and alkylating agents. This mastery is crucial for synthesizing complex organic molecules, pharmaceuticals, and materials with tailored properties. As the demand for novel compounds continues to grow, the ability to perform precise and controlled alkylation reactions becomes increasingly valuable.
Historically, alkylation methods have progressed from simple SN1 and SN2 reactions to more sophisticated approaches involving transition metal catalysis, phase-transfer catalysis, and asymmetric alkylation. Each advancement has brought new possibilities and challenges, pushing researchers to explore innovative strategies for achieving desired transformations with higher yields, improved selectivity, and milder reaction conditions.
The current landscape of alkylation research focuses on several key areas: developing greener alkylation protocols, enhancing regioselectivity and stereoselectivity, expanding the range of compatible functional groups, and improving the overall efficiency of the process. These efforts are driven by the increasing emphasis on sustainable chemistry and the need for more atom-economical synthetic routes.
Mastering alkylation in laboratory settings requires a multifaceted approach. This includes a deep understanding of reaction kinetics and thermodynamics, proficiency in handling air- and moisture-sensitive reagents, and expertise in modern analytical techniques for product characterization. Additionally, researchers must be adept at optimizing reaction parameters such as temperature, solvent choice, and catalyst loading to achieve optimal results.
The future of alkylation research holds exciting possibilities, including the development of novel catalytic systems, the application of flow chemistry for continuous alkylation processes, and the integration of computational methods for predicting and designing new alkylation reactions. As we strive to master this fundamental transformation, the goal is not only to refine existing methodologies but also to discover entirely new paradigms in carbon-carbon and carbon-heteroatom bond formation.
Industrial Applications and Market Demand
Alkylation processes have become increasingly important in various industrial sectors, driving significant market demand for efficient and scalable laboratory techniques. The pharmaceutical industry, in particular, relies heavily on alkylation reactions for the synthesis of complex drug molecules. As the global pharmaceutical market continues to expand, projected to reach $1.5 trillion by 2023, the demand for advanced alkylation methods in laboratory settings is expected to grow proportionally.
In the agrochemical sector, alkylation plays a crucial role in the development of pesticides and herbicides. With the increasing global population and the need for enhanced crop yields, the agrochemical market is forecasted to reach $300 billion by 2025. This growth directly translates to a higher demand for sophisticated alkylation techniques in research and development laboratories.
The polymer industry also heavily utilizes alkylation processes for the production of specialty plastics and resins. As the demand for lightweight and durable materials increases across automotive, aerospace, and consumer goods sectors, the market for alkylation-derived polymers is expected to expand significantly. This trend is further accelerated by the growing focus on sustainable and bio-based materials, where alkylation serves as a key step in the modification of natural polymers.
In the flavor and fragrance industry, alkylation is essential for the synthesis of various aroma compounds. With the global flavor and fragrance market projected to reach $35 billion by 2024, there is a growing need for precise and efficient alkylation methods in laboratory settings to develop new and improved scent profiles.
The petrochemical industry, while traditionally focused on large-scale alkylation processes, is increasingly investing in laboratory-scale research to optimize and develop new catalysts and reaction conditions. This shift is driven by the need for more environmentally friendly and energy-efficient processes, as well as the exploration of alternative feedstocks.
The electronics industry also benefits from advancements in alkylation techniques, particularly in the development of organic semiconductors and advanced materials for display technologies. As the demand for more sophisticated electronic devices grows, so does the need for precise control over molecular structures through alkylation processes in research laboratories.
Given these diverse industrial applications, there is a clear market demand for improved alkylation techniques in laboratory settings. This demand is not only for more efficient and selective reactions but also for methods that align with principles of green chemistry and sustainability. As industries continue to evolve and face new challenges, mastering alkylation in laboratory settings becomes crucial for driving innovation and meeting market needs across multiple sectors.
In the agrochemical sector, alkylation plays a crucial role in the development of pesticides and herbicides. With the increasing global population and the need for enhanced crop yields, the agrochemical market is forecasted to reach $300 billion by 2025. This growth directly translates to a higher demand for sophisticated alkylation techniques in research and development laboratories.
The polymer industry also heavily utilizes alkylation processes for the production of specialty plastics and resins. As the demand for lightweight and durable materials increases across automotive, aerospace, and consumer goods sectors, the market for alkylation-derived polymers is expected to expand significantly. This trend is further accelerated by the growing focus on sustainable and bio-based materials, where alkylation serves as a key step in the modification of natural polymers.
In the flavor and fragrance industry, alkylation is essential for the synthesis of various aroma compounds. With the global flavor and fragrance market projected to reach $35 billion by 2024, there is a growing need for precise and efficient alkylation methods in laboratory settings to develop new and improved scent profiles.
The petrochemical industry, while traditionally focused on large-scale alkylation processes, is increasingly investing in laboratory-scale research to optimize and develop new catalysts and reaction conditions. This shift is driven by the need for more environmentally friendly and energy-efficient processes, as well as the exploration of alternative feedstocks.
The electronics industry also benefits from advancements in alkylation techniques, particularly in the development of organic semiconductors and advanced materials for display technologies. As the demand for more sophisticated electronic devices grows, so does the need for precise control over molecular structures through alkylation processes in research laboratories.
Given these diverse industrial applications, there is a clear market demand for improved alkylation techniques in laboratory settings. This demand is not only for more efficient and selective reactions but also for methods that align with principles of green chemistry and sustainability. As industries continue to evolve and face new challenges, mastering alkylation in laboratory settings becomes crucial for driving innovation and meeting market needs across multiple sectors.
Current Challenges in Laboratory Alkylation
Alkylation in laboratory settings presents several significant challenges that researchers and technicians must navigate to achieve optimal results. One of the primary difficulties lies in controlling the selectivity of the alkylation reaction. Achieving the desired regioselectivity and stereoselectivity often requires precise control over reaction conditions, including temperature, solvent choice, and catalyst selection. This level of control can be particularly challenging in complex molecular systems or when working with sensitive substrates.
Another major hurdle is managing the reactivity of alkylating agents. Many alkylating reagents are highly reactive and can lead to undesired side reactions or over-alkylation. This necessitates careful handling and storage procedures, as well as meticulous reaction monitoring to ensure the desired product is obtained without compromising yield or purity. The potential for these side reactions also complicates purification processes, adding an extra layer of complexity to the overall procedure.
Safety considerations pose a significant challenge in laboratory alkylation. Many alkylating agents are toxic, carcinogenic, or otherwise hazardous, requiring stringent safety protocols and specialized equipment for handling and disposal. This not only impacts the practical aspects of conducting experiments but also necessitates extensive training and safety measures to protect laboratory personnel.
Scale-up issues present another set of challenges when transitioning from small-scale laboratory reactions to larger quantities. Factors such as heat transfer, mixing efficiency, and reaction kinetics can behave differently at larger scales, potentially affecting yield, purity, and safety. Researchers must carefully optimize conditions and potentially redesign protocols when scaling up alkylation reactions.
The environmental impact of alkylation processes is an increasingly important consideration. Traditional alkylation methods often involve the use of stoichiometric amounts of reagents and generate significant waste. Developing more sustainable alkylation techniques, such as catalytic methods or the use of renewable feedstocks, is an ongoing challenge that requires innovative approaches and careful consideration of green chemistry principles.
Lastly, the development of new alkylation methodologies to address limitations in substrate scope and functional group tolerance remains a persistent challenge. Researchers continually strive to expand the toolkit of alkylation reactions to encompass a broader range of substrates and to achieve greater compatibility with sensitive functional groups. This ongoing effort requires a deep understanding of reaction mechanisms and creative problem-solving to overcome existing limitations in alkylation chemistry.
Another major hurdle is managing the reactivity of alkylating agents. Many alkylating reagents are highly reactive and can lead to undesired side reactions or over-alkylation. This necessitates careful handling and storage procedures, as well as meticulous reaction monitoring to ensure the desired product is obtained without compromising yield or purity. The potential for these side reactions also complicates purification processes, adding an extra layer of complexity to the overall procedure.
Safety considerations pose a significant challenge in laboratory alkylation. Many alkylating agents are toxic, carcinogenic, or otherwise hazardous, requiring stringent safety protocols and specialized equipment for handling and disposal. This not only impacts the practical aspects of conducting experiments but also necessitates extensive training and safety measures to protect laboratory personnel.
Scale-up issues present another set of challenges when transitioning from small-scale laboratory reactions to larger quantities. Factors such as heat transfer, mixing efficiency, and reaction kinetics can behave differently at larger scales, potentially affecting yield, purity, and safety. Researchers must carefully optimize conditions and potentially redesign protocols when scaling up alkylation reactions.
The environmental impact of alkylation processes is an increasingly important consideration. Traditional alkylation methods often involve the use of stoichiometric amounts of reagents and generate significant waste. Developing more sustainable alkylation techniques, such as catalytic methods or the use of renewable feedstocks, is an ongoing challenge that requires innovative approaches and careful consideration of green chemistry principles.
Lastly, the development of new alkylation methodologies to address limitations in substrate scope and functional group tolerance remains a persistent challenge. Researchers continually strive to expand the toolkit of alkylation reactions to encompass a broader range of substrates and to achieve greater compatibility with sensitive functional groups. This ongoing effort requires a deep understanding of reaction mechanisms and creative problem-solving to overcome existing limitations in alkylation chemistry.
State-of-the-Art Alkylation Methods
01 Catalytic alkylation processes
Various catalytic processes are employed for alkylation reactions, including the use of solid acid catalysts, zeolites, and ionic liquids. These catalysts enhance reaction efficiency, selectivity, and yield in the production of alkylated hydrocarbons, particularly in the petrochemical industry.- Catalytic alkylation processes: Various catalytic processes are employed for alkylation reactions, including the use of solid acid catalysts, zeolites, and ionic liquids. These catalysts enhance reaction efficiency, selectivity, and yield in the production of alkylated hydrocarbons, particularly in the petrochemical industry.
- Alkylation of aromatic compounds: Techniques for alkylating aromatic compounds, such as benzene and its derivatives, to produce valuable chemical intermediates and end products. These processes often involve the use of specific catalysts, reaction conditions, and alkylating agents to achieve desired product distributions and minimize unwanted side reactions.
- Isoparaffin-olefin alkylation: Methods for alkylating isoparaffins with olefins to produce high-octane gasoline components. This process is crucial in the petroleum refining industry and involves optimizing reaction conditions, catalyst selection, and feedstock composition to maximize product quality and yield.
- Alkylation reactor design and operation: Innovations in alkylation reactor design and operation, including continuous flow reactors, fixed-bed reactors, and multi-stage systems. These advancements aim to improve process efficiency, reduce catalyst deactivation, and enhance product selectivity in various alkylation applications.
- Alkylation in fine chemical synthesis: Application of alkylation reactions in the synthesis of fine chemicals, pharmaceuticals, and specialty products. This includes the development of selective alkylation methods, use of protecting groups, and stereoselective alkylation techniques to produce complex organic molecules with desired properties.
02 Alkylation of aromatic compounds
Techniques for alkylating aromatic compounds, such as benzene and its derivatives, to produce valuable chemical intermediates and end products. These methods often involve the use of specific catalysts, reaction conditions, and alkylating agents to achieve desired product distributions and minimize side reactions.Expand Specific Solutions03 Isoparaffin alkylation
Processes for the alkylation of isoparaffins with olefins to produce high-octane gasoline components. This includes various reactor designs, catalyst systems, and operating conditions to optimize the production of branched alkanes with improved fuel properties.Expand Specific Solutions04 Alkylation in fine chemical synthesis
Application of alkylation reactions in the synthesis of fine chemicals, pharmaceuticals, and specialty products. This involves selective alkylation strategies, protection-deprotection sequences, and the use of specific reagents to introduce alkyl groups at desired positions in complex molecules.Expand Specific Solutions05 Continuous flow alkylation processes
Development of continuous flow processes for alkylation reactions, offering advantages such as improved heat and mass transfer, enhanced safety, and increased productivity. These processes often involve specialized reactor designs, in-line monitoring, and automated control systems for consistent product quality.Expand Specific Solutions
Key Players in Alkylation Research
The alkylation technology landscape is in a mature phase, with established players dominating the market. The global alkylation market size is projected to reach several billion dollars by 2025, driven by increasing demand for high-octane fuels. Key players like ExxonMobil Chemical Patents, UOP LLC, and China Petroleum & Chemical Corp. have developed advanced alkylation processes, demonstrating high technical maturity. These companies, along with others such as Chevron Oronite and Total Petrochemicals & Refining USA, are continuously innovating to improve efficiency and reduce environmental impact. The competitive landscape is characterized by strategic partnerships, licensing agreements, and ongoing R&D efforts to maintain market positions and address evolving regulatory requirements.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced alkylation technologies for laboratory settings, focusing on improving catalyst efficiency and reducing environmental impact. Their approach involves the use of ionic liquid catalysts, which offer several advantages over traditional sulfuric acid catalysts. The process utilizes a biphasic system where the ionic liquid catalyst and hydrocarbon reactants form separate phases, allowing for easier product separation and catalyst recovery[1]. ExxonMobil's technology also incorporates continuous flow microreactors, enabling precise control over reaction conditions and improved safety in laboratory environments[2]. Additionally, they have implemented in-situ spectroscopic monitoring techniques to optimize reaction parameters in real-time, enhancing the overall efficiency of the alkylation process[3].
Strengths: Improved catalyst efficiency, reduced environmental impact, easier product separation, and enhanced process control. Weaknesses: Potentially higher initial setup costs and the need for specialized equipment and training.
UOP LLC
Technical Solution: UOP LLC has developed the AlkyClean® solid acid alkylation technology for laboratory-scale applications. This innovative process utilizes a proprietary solid acid catalyst that eliminates the need for liquid acids, significantly improving safety and environmental performance[4]. The technology employs a fixed-bed reactor system, allowing for continuous operation and easier catalyst handling. UOP's approach incorporates advanced process control systems to maintain optimal reaction conditions, resulting in high-quality alkylate production[5]. The company has also developed miniaturized versions of their commercial-scale equipment for laboratory use, enabling researchers to conduct experiments under conditions that closely mimic industrial processes[6]. Furthermore, UOP's technology includes integrated analytical tools for real-time product quality monitoring and process optimization.
Strengths: Enhanced safety, improved environmental performance, and scalability from laboratory to industrial applications. Weaknesses: Potential limitations in handling certain feedstocks and higher initial investment compared to traditional liquid acid systems.
Innovative Catalysts and Reagents
Alkylation catalyzed by binary mixtures of acid and ionic liquid
PatentInactiveUS20100331599A1
Innovation
- The use of a catalyst composition comprising an acid ionic liquid and a strong Brønsted acid, specifically a binary mixture of certain acidic imidazolium ionic liquids and strong acids like sulfuric or trifluoromethanesulfonic acid, which reduces acid usage and enhances recyclability, selectivity, and safety.
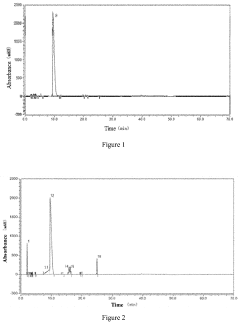
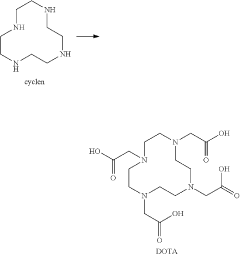
Method for preparing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
PatentActiveUS20200347023A1
Innovation
- A method involving an alkylation reaction between 1,4,7,10-tetraazacyclododecane and an alkylating agent in the presence of an acid-binding agent, followed by pH adjustment and recrystallization, which eliminates the need for ion exchange resins and low-temperature freezing, simplifying the process and reducing energy consumption.
Safety Protocols in Alkylation Experiments
Safety protocols are paramount in alkylation experiments due to the inherent risks associated with handling reactive chemicals and potentially hazardous substances. A comprehensive safety framework begins with proper personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and lab coats. Researchers must ensure that all PPE is in good condition and appropriate for the specific alkylating agents being used.
Ventilation is crucial in alkylation procedures. All experiments should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to volatile organic compounds and potentially toxic fumes. The fume hood should be regularly inspected and certified to ensure optimal performance.
Proper chemical storage and handling practices are essential. Alkylating agents should be stored in a cool, dry place, away from sources of heat or ignition. Incompatible chemicals must be segregated to prevent accidental reactions. When transferring alkylating agents, use appropriate techniques such as pipetting or pouring with a funnel to minimize spills and splashes.
Emergency preparedness is a critical component of safety protocols. Laboratories should have readily accessible eyewash stations, safety showers, and spill kits. All researchers must be trained in their use and location. A detailed emergency response plan should be in place, outlining procedures for chemical spills, fires, or personal injuries.
Waste management is another crucial aspect of alkylation safety. Proper disposal methods for alkylating agents and related waste must be established and strictly followed. This may include neutralization procedures, use of designated waste containers, and adherence to local and national regulations for hazardous waste disposal.
Regular safety training and education are vital for maintaining a safe laboratory environment. Researchers should receive comprehensive training on the specific hazards associated with alkylation reactions, proper handling techniques, and emergency procedures. This training should be documented and refreshed periodically.
Lastly, implementing a robust system for risk assessment and experiment planning is essential. Before conducting any alkylation experiment, researchers should perform a thorough risk assessment, identifying potential hazards and implementing appropriate control measures. This process should be documented and reviewed by supervisors or safety officers to ensure all necessary precautions are in place.
Ventilation is crucial in alkylation procedures. All experiments should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to volatile organic compounds and potentially toxic fumes. The fume hood should be regularly inspected and certified to ensure optimal performance.
Proper chemical storage and handling practices are essential. Alkylating agents should be stored in a cool, dry place, away from sources of heat or ignition. Incompatible chemicals must be segregated to prevent accidental reactions. When transferring alkylating agents, use appropriate techniques such as pipetting or pouring with a funnel to minimize spills and splashes.
Emergency preparedness is a critical component of safety protocols. Laboratories should have readily accessible eyewash stations, safety showers, and spill kits. All researchers must be trained in their use and location. A detailed emergency response plan should be in place, outlining procedures for chemical spills, fires, or personal injuries.
Waste management is another crucial aspect of alkylation safety. Proper disposal methods for alkylating agents and related waste must be established and strictly followed. This may include neutralization procedures, use of designated waste containers, and adherence to local and national regulations for hazardous waste disposal.
Regular safety training and education are vital for maintaining a safe laboratory environment. Researchers should receive comprehensive training on the specific hazards associated with alkylation reactions, proper handling techniques, and emergency procedures. This training should be documented and refreshed periodically.
Lastly, implementing a robust system for risk assessment and experiment planning is essential. Before conducting any alkylation experiment, researchers should perform a thorough risk assessment, identifying potential hazards and implementing appropriate control measures. This process should be documented and reviewed by supervisors or safety officers to ensure all necessary precautions are in place.
Environmental Impact of Alkylation Processes
Alkylation processes, while essential in many laboratory and industrial settings, can have significant environmental impacts that must be carefully considered and mitigated. The primary environmental concerns associated with alkylation include air pollution, water contamination, and waste generation.
Air pollution is a major issue in alkylation processes, particularly when volatile organic compounds (VOCs) are released. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on human health and ecosystems. Additionally, some alkylation reactions may produce greenhouse gases, further exacerbating climate change concerns.
Water contamination is another critical environmental impact of alkylation processes. Improper handling or disposal of alkylating agents and byproducts can lead to the contamination of groundwater and surface water sources. This pollution can have far-reaching consequences for aquatic ecosystems and potentially affect drinking water supplies.
Waste generation is an inherent aspect of alkylation processes, with both solid and liquid waste streams requiring proper management. Hazardous waste from these processes often contains toxic substances that pose risks to human health and the environment if not handled and disposed of correctly.
To address these environmental challenges, laboratories and industries employing alkylation techniques must implement robust pollution control measures. This includes the use of scrubbers and catalytic oxidizers to reduce air emissions, as well as advanced wastewater treatment systems to remove contaminants before discharge.
Furthermore, adopting green chemistry principles can significantly reduce the environmental footprint of alkylation processes. This involves developing alternative reagents and catalysts that are less toxic and more environmentally friendly, as well as optimizing reaction conditions to minimize waste generation and energy consumption.
Lifecycle assessments of alkylation processes are crucial for understanding their full environmental impact. These assessments consider all stages of the process, from raw material extraction to final product disposal, providing a comprehensive view of environmental effects and identifying areas for improvement.
Regulatory compliance is also a key aspect of managing the environmental impact of alkylation. Laboratories and industries must adhere to strict environmental regulations, which often require monitoring, reporting, and implementing best practices to minimize environmental harm.
In conclusion, mastering alkylation in laboratory settings necessitates a thorough understanding and proactive management of its environmental impacts. By implementing advanced pollution control technologies, adopting green chemistry principles, and maintaining regulatory compliance, laboratories can significantly reduce the environmental footprint of alkylation processes while still achieving their research and production goals.
Air pollution is a major issue in alkylation processes, particularly when volatile organic compounds (VOCs) are released. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on human health and ecosystems. Additionally, some alkylation reactions may produce greenhouse gases, further exacerbating climate change concerns.
Water contamination is another critical environmental impact of alkylation processes. Improper handling or disposal of alkylating agents and byproducts can lead to the contamination of groundwater and surface water sources. This pollution can have far-reaching consequences for aquatic ecosystems and potentially affect drinking water supplies.
Waste generation is an inherent aspect of alkylation processes, with both solid and liquid waste streams requiring proper management. Hazardous waste from these processes often contains toxic substances that pose risks to human health and the environment if not handled and disposed of correctly.
To address these environmental challenges, laboratories and industries employing alkylation techniques must implement robust pollution control measures. This includes the use of scrubbers and catalytic oxidizers to reduce air emissions, as well as advanced wastewater treatment systems to remove contaminants before discharge.
Furthermore, adopting green chemistry principles can significantly reduce the environmental footprint of alkylation processes. This involves developing alternative reagents and catalysts that are less toxic and more environmentally friendly, as well as optimizing reaction conditions to minimize waste generation and energy consumption.
Lifecycle assessments of alkylation processes are crucial for understanding their full environmental impact. These assessments consider all stages of the process, from raw material extraction to final product disposal, providing a comprehensive view of environmental effects and identifying areas for improvement.
Regulatory compliance is also a key aspect of managing the environmental impact of alkylation. Laboratories and industries must adhere to strict environmental regulations, which often require monitoring, reporting, and implementing best practices to minimize environmental harm.
In conclusion, mastering alkylation in laboratory settings necessitates a thorough understanding and proactive management of its environmental impacts. By implementing advanced pollution control technologies, adopting green chemistry principles, and maintaining regulatory compliance, laboratories can significantly reduce the environmental footprint of alkylation processes while still achieving their research and production goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!