How to Advance Alkyl Catalysis in Organic Reactions?
JUL 15, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Catalysis Evolution
Alkyl catalysis in organic reactions has undergone significant evolution over the past few decades, driven by the need for more efficient and sustainable synthetic processes. The journey began with traditional metal-based catalysts, which were effective but often suffered from issues such as toxicity and high costs. As environmental concerns grew, researchers shifted focus towards developing greener alternatives.
The 1990s saw a surge in interest in organocatalysis, with alkyl-based catalysts emerging as promising candidates. Early work focused on simple alkyl amines and thioureas, which demonstrated catalytic activity in various organic transformations. These initial successes paved the way for more complex alkyl-based catalytic systems.
A major breakthrough came in the early 2000s with the development of chiral alkyl catalysts. These catalysts enabled highly enantioselective reactions, opening up new possibilities in asymmetric synthesis. The field further expanded with the introduction of bifunctional alkyl catalysts, which could activate both electrophiles and nucleophiles simultaneously, leading to enhanced reaction rates and selectivities.
The mid-2000s witnessed the rise of alkyl-based phase-transfer catalysts, which proved particularly useful in biphasic reaction systems. These catalysts facilitated the transfer of reactants between immiscible phases, enabling reactions that were previously challenging or impossible.
In the 2010s, the focus shifted towards developing more sustainable and recyclable alkyl catalysts. This led to the exploration of supported alkyl catalysts, where the catalytic moieties were anchored onto solid supports or polymers. Such systems offered easier catalyst recovery and reuse, addressing one of the key limitations of homogeneous catalysis.
Recent years have seen the integration of alkyl catalysis with other cutting-edge technologies. For instance, the combination of alkyl catalysts with photocatalysis has led to novel visible-light-driven organic transformations. Similarly, the merger of alkyl catalysis with flow chemistry has enabled continuous-flow processes, enhancing productivity and scalability.
Looking ahead, the field of alkyl catalysis continues to evolve. Current research is exploring the potential of alkyl-based metal-organic frameworks (MOFs) as heterogeneous catalysts, offering high surface areas and tunable pore structures. Additionally, there is growing interest in developing alkyl catalysts capable of activating traditionally inert C-H bonds, which could revolutionize synthetic strategies in organic chemistry.
The 1990s saw a surge in interest in organocatalysis, with alkyl-based catalysts emerging as promising candidates. Early work focused on simple alkyl amines and thioureas, which demonstrated catalytic activity in various organic transformations. These initial successes paved the way for more complex alkyl-based catalytic systems.
A major breakthrough came in the early 2000s with the development of chiral alkyl catalysts. These catalysts enabled highly enantioselective reactions, opening up new possibilities in asymmetric synthesis. The field further expanded with the introduction of bifunctional alkyl catalysts, which could activate both electrophiles and nucleophiles simultaneously, leading to enhanced reaction rates and selectivities.
The mid-2000s witnessed the rise of alkyl-based phase-transfer catalysts, which proved particularly useful in biphasic reaction systems. These catalysts facilitated the transfer of reactants between immiscible phases, enabling reactions that were previously challenging or impossible.
In the 2010s, the focus shifted towards developing more sustainable and recyclable alkyl catalysts. This led to the exploration of supported alkyl catalysts, where the catalytic moieties were anchored onto solid supports or polymers. Such systems offered easier catalyst recovery and reuse, addressing one of the key limitations of homogeneous catalysis.
Recent years have seen the integration of alkyl catalysis with other cutting-edge technologies. For instance, the combination of alkyl catalysts with photocatalysis has led to novel visible-light-driven organic transformations. Similarly, the merger of alkyl catalysis with flow chemistry has enabled continuous-flow processes, enhancing productivity and scalability.
Looking ahead, the field of alkyl catalysis continues to evolve. Current research is exploring the potential of alkyl-based metal-organic frameworks (MOFs) as heterogeneous catalysts, offering high surface areas and tunable pore structures. Additionally, there is growing interest in developing alkyl catalysts capable of activating traditionally inert C-H bonds, which could revolutionize synthetic strategies in organic chemistry.
Market Demand Analysis
The market demand for advanced alkyl catalysis in organic reactions has been steadily growing, driven by the increasing need for more efficient and sustainable chemical processes across various industries. Pharmaceutical companies, in particular, are showing a keen interest in this technology as it offers potential for developing new drug candidates and improving existing synthetic routes. The ability to form carbon-carbon bonds through alkyl catalysis opens up possibilities for creating complex molecular structures with greater ease and precision.
In the fine chemicals sector, there is a rising demand for alkyl catalysis techniques that can enable the production of specialty chemicals with higher yields and lower environmental impact. This aligns with the global trend towards green chemistry and sustainable manufacturing practices. The agrochemical industry is another significant market driver, as alkyl catalysis can facilitate the synthesis of novel crop protection agents and fertilizers with enhanced efficacy and reduced ecological footprint.
The polymer and materials science sectors are also contributing to the market demand for advanced alkyl catalysis. These industries are seeking innovative ways to create new materials with tailored properties, and alkyl catalysis offers a versatile tool for polymer synthesis and modification. Additionally, the growing focus on circular economy principles is pushing researchers to explore alkyl catalysis for the upcycling of plastic waste into value-added products.
From a geographical perspective, North America and Europe currently lead in terms of market demand for alkyl catalysis research and applications. However, rapidly developing economies in Asia, particularly China and India, are expected to become significant markets as their chemical industries expand and modernize. The increasing emphasis on domestic innovation and self-reliance in these countries is likely to fuel further investment in advanced catalytic technologies.
The market size for alkyl catalysis is challenging to quantify precisely due to its integration into broader chemical processes. However, industry reports suggest that the global catalysis market, of which alkyl catalysis is a growing segment, is projected to experience substantial growth in the coming years. This growth is attributed to the rising demand for sustainable chemical processes and the continuous need for innovation in synthetic methodologies.
Investors and venture capital firms are showing increased interest in startups and research initiatives focused on developing novel alkyl catalysis technologies. This influx of capital is expected to accelerate the commercialization of new catalytic systems and expand their applications across various industrial sectors. As regulatory pressures mount to reduce the environmental impact of chemical manufacturing, the demand for more efficient and selective alkyl catalysis methods is likely to intensify, further driving market growth and technological advancement in this field.
In the fine chemicals sector, there is a rising demand for alkyl catalysis techniques that can enable the production of specialty chemicals with higher yields and lower environmental impact. This aligns with the global trend towards green chemistry and sustainable manufacturing practices. The agrochemical industry is another significant market driver, as alkyl catalysis can facilitate the synthesis of novel crop protection agents and fertilizers with enhanced efficacy and reduced ecological footprint.
The polymer and materials science sectors are also contributing to the market demand for advanced alkyl catalysis. These industries are seeking innovative ways to create new materials with tailored properties, and alkyl catalysis offers a versatile tool for polymer synthesis and modification. Additionally, the growing focus on circular economy principles is pushing researchers to explore alkyl catalysis for the upcycling of plastic waste into value-added products.
From a geographical perspective, North America and Europe currently lead in terms of market demand for alkyl catalysis research and applications. However, rapidly developing economies in Asia, particularly China and India, are expected to become significant markets as their chemical industries expand and modernize. The increasing emphasis on domestic innovation and self-reliance in these countries is likely to fuel further investment in advanced catalytic technologies.
The market size for alkyl catalysis is challenging to quantify precisely due to its integration into broader chemical processes. However, industry reports suggest that the global catalysis market, of which alkyl catalysis is a growing segment, is projected to experience substantial growth in the coming years. This growth is attributed to the rising demand for sustainable chemical processes and the continuous need for innovation in synthetic methodologies.
Investors and venture capital firms are showing increased interest in startups and research initiatives focused on developing novel alkyl catalysis technologies. This influx of capital is expected to accelerate the commercialization of new catalytic systems and expand their applications across various industrial sectors. As regulatory pressures mount to reduce the environmental impact of chemical manufacturing, the demand for more efficient and selective alkyl catalysis methods is likely to intensify, further driving market growth and technological advancement in this field.
Current Challenges
Alkyl catalysis in organic reactions faces several significant challenges that hinder its widespread adoption and advancement. One of the primary obstacles is the stability and reactivity of alkyl catalysts. Unlike their aryl counterparts, alkyl catalysts are often more susceptible to decomposition and side reactions, limiting their efficiency and applicability in various organic transformations.
The control of selectivity in alkyl-catalyzed reactions presents another major hurdle. Achieving high levels of chemo-, regio-, and stereoselectivity remains challenging, particularly in complex molecular systems. This lack of precise control can lead to unwanted by-products and reduced yields, making alkyl catalysis less attractive for industrial applications.
Another critical challenge lies in the limited substrate scope of many alkyl-catalyzed reactions. While progress has been made in expanding the range of compatible substrates, there are still significant limitations compared to traditional transition metal catalysis. This restriction hampers the broader application of alkyl catalysis in diverse synthetic scenarios.
The mechanistic understanding of alkyl-catalyzed reactions is often less developed compared to well-established catalytic systems. This knowledge gap impedes rational catalyst design and optimization, making it difficult to predict and improve reaction outcomes systematically. Elucidating reaction mechanisms and identifying key intermediates remains a crucial challenge in advancing the field.
Furthermore, the development of sustainable and environmentally friendly alkyl catalysis processes poses a significant challenge. Many current systems rely on precious metals or harsh reaction conditions, which are not aligned with the principles of green chemistry. Finding alternatives that are both efficient and environmentally benign is a pressing need in the field.
The scalability of alkyl-catalyzed reactions is another area of concern. Many promising reactions demonstrated on a laboratory scale face difficulties when scaled up for industrial production. Issues such as heat transfer, mixing efficiency, and catalyst recovery become more pronounced at larger scales, necessitating innovative engineering solutions.
Lastly, the integration of alkyl catalysis with other emerging technologies, such as flow chemistry and artificial intelligence-driven reaction optimization, presents both opportunities and challenges. Harnessing these synergies effectively requires interdisciplinary approaches and overcoming technological barriers, which can be resource-intensive and time-consuming.
The control of selectivity in alkyl-catalyzed reactions presents another major hurdle. Achieving high levels of chemo-, regio-, and stereoselectivity remains challenging, particularly in complex molecular systems. This lack of precise control can lead to unwanted by-products and reduced yields, making alkyl catalysis less attractive for industrial applications.
Another critical challenge lies in the limited substrate scope of many alkyl-catalyzed reactions. While progress has been made in expanding the range of compatible substrates, there are still significant limitations compared to traditional transition metal catalysis. This restriction hampers the broader application of alkyl catalysis in diverse synthetic scenarios.
The mechanistic understanding of alkyl-catalyzed reactions is often less developed compared to well-established catalytic systems. This knowledge gap impedes rational catalyst design and optimization, making it difficult to predict and improve reaction outcomes systematically. Elucidating reaction mechanisms and identifying key intermediates remains a crucial challenge in advancing the field.
Furthermore, the development of sustainable and environmentally friendly alkyl catalysis processes poses a significant challenge. Many current systems rely on precious metals or harsh reaction conditions, which are not aligned with the principles of green chemistry. Finding alternatives that are both efficient and environmentally benign is a pressing need in the field.
The scalability of alkyl-catalyzed reactions is another area of concern. Many promising reactions demonstrated on a laboratory scale face difficulties when scaled up for industrial production. Issues such as heat transfer, mixing efficiency, and catalyst recovery become more pronounced at larger scales, necessitating innovative engineering solutions.
Lastly, the integration of alkyl catalysis with other emerging technologies, such as flow chemistry and artificial intelligence-driven reaction optimization, presents both opportunities and challenges. Harnessing these synergies effectively requires interdisciplinary approaches and overcoming technological barriers, which can be resource-intensive and time-consuming.
Existing Methodologies
01 Alkyl-substituted catalysts for organic synthesis
Alkyl-substituted catalysts are used in various organic synthesis reactions. These catalysts often contain alkyl groups attached to metal centers or organic frameworks, which can enhance catalytic activity, selectivity, and stability. The alkyl substituents can modify the electronic and steric properties of the catalyst, leading to improved performance in specific reactions.- Alkyl-based catalysts for organic synthesis: Alkyl catalysts are used in various organic synthesis reactions, including carbon-carbon bond formation and functionalization of organic compounds. These catalysts often involve alkyl metal complexes or organometallic compounds that facilitate specific chemical transformations.
- Alkyl catalysis in polymerization processes: Alkyl catalysts play a crucial role in polymerization reactions, particularly in the production of various polymers and copolymers. These catalysts can control the molecular weight, structure, and properties of the resulting polymeric materials.
- Alkyl-functionalized heterogeneous catalysts: Heterogeneous catalysts with alkyl functional groups are developed for applications in various chemical processes. These catalysts often involve supported alkyl species on solid surfaces, providing enhanced catalytic activity and selectivity.
- Alkyl catalysis in fine chemical production: Alkyl catalysts are employed in the synthesis of fine chemicals, pharmaceuticals, and specialty products. These catalysts enable selective transformations and can improve yield and efficiency in complex organic synthesis routes.
- Alkyl catalysis for sustainable chemical processes: Research focuses on developing alkyl catalysts for environmentally friendly and sustainable chemical processes. These catalysts aim to reduce waste, improve atom economy, and enable more efficient use of resources in various industrial applications.
02 Alkyl-based catalysts in polymerization processes
Alkyl-containing catalysts play a crucial role in polymerization reactions. These catalysts can initiate and control the polymerization of various monomers, leading to the production of polymers with specific properties. The alkyl groups on the catalyst can influence the molecular weight, distribution, and structure of the resulting polymers.Expand Specific Solutions03 Alkyl-modified zeolites and molecular sieves as catalysts
Zeolites and molecular sieves modified with alkyl groups are used as heterogeneous catalysts in various chemical processes. The alkyl modification can alter the surface properties, pore structure, and acidity of the zeolites, leading to enhanced catalytic performance in reactions such as cracking, isomerization, and alkylation.Expand Specific Solutions04 Alkyl-functionalized nanoparticles for catalysis
Nanoparticles functionalized with alkyl groups are employed as catalysts in various applications. The alkyl functionalization can improve the dispersion, stability, and catalytic activity of the nanoparticles. These catalysts are used in organic transformations, electrochemical reactions, and environmental remediation processes.Expand Specific Solutions05 Alkyl-based organocatalysts
Organic molecules containing alkyl groups are used as catalysts in various reactions. These organocatalysts can promote asymmetric transformations, C-C bond formations, and other organic reactions. The alkyl substituents on the organocatalyst can influence its solubility, reactivity, and stereoselectivity in the catalytic process.Expand Specific Solutions
Key Industry Players
The alkyl catalysis market in organic reactions is in a growth phase, driven by increasing demand for efficient and sustainable chemical processes. The market size is expanding, with major players like China Petroleum & Chemical Corp. and Chevron Oronite Co. LLC investing in research and development. Technological maturity varies, with established companies like Shell Internationale Research Maatschappij BV and ExxonMobil Chemical Patents, Inc. leading in innovation. Academic institutions such as California Institute of Technology and Massachusetts Institute of Technology contribute significantly to advancing the field. Collaboration between industry and academia is accelerating progress, as seen with partnerships involving the Centre National de la Recherche Scientifique and Japan Science & Technology Agency.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant advancements in alkyl catalysis for organic reactions. They have developed a novel alkylation catalyst system that combines solid acid catalysts with ionic liquids[1]. This system enhances the catalytic activity and selectivity in alkylation reactions, particularly in the production of high-quality gasoline components. The catalyst demonstrates improved stability and longer lifetimes compared to traditional liquid acid catalysts[2]. Sinopec has also explored the use of zeolite-based catalysts modified with rare earth elements to further improve the efficiency of alkylation processes[3]. Their research extends to the application of these catalysts in various petrochemical processes, including the production of linear alkylbenzenes and other valuable organic compounds[4].
Strengths: High catalytic activity, improved selectivity, and longer catalyst lifetimes. Weaknesses: Potential high costs associated with ionic liquids and rare earth elements, and possible limitations in large-scale industrial applications.
Sinopec Research Institute of Petroleum Processing
Technical Solution: The Sinopec Research Institute of Petroleum Processing has been at the forefront of advancing alkyl catalysis in organic reactions, particularly in the petroleum and petrochemical industries. They have developed a series of innovative solid acid catalysts for alkylation reactions, focusing on improving the octane number of gasoline and producing high-quality alkylate[1]. Their research includes the development of supported ionic liquid catalysts (SILCs) that combine the advantages of homogeneous and heterogeneous catalysis[2]. These catalysts show enhanced activity and selectivity in various alkylation processes, including isoparaffin-olefin alkylation[3]. The institute has also made progress in the field of shape-selective zeolite catalysts for alkylation reactions, which offer improved product distribution and reduced coke formation[4]. Additionally, they have explored the use of metal-organic frameworks (MOFs) as novel supports for alkylation catalysts, demonstrating promising results in terms of catalyst stability and reusability[5].
Strengths: Expertise in developing catalysts for industrial-scale applications, particularly in petroleum refining. Innovative approaches combining homogeneous and heterogeneous catalysis. Weaknesses: Potential challenges in scaling up novel catalyst systems, and possible environmental concerns related to some catalytic processes.
Innovative Mechanisms
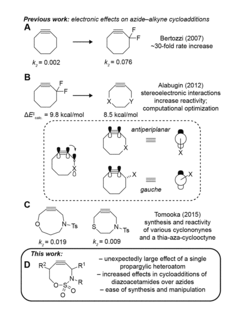
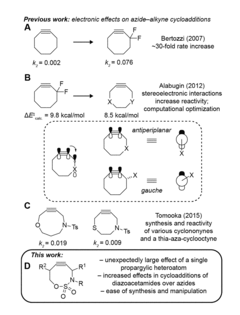
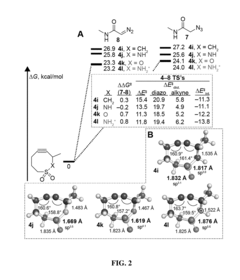
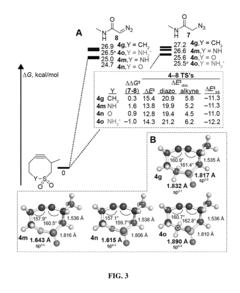
Electronically activated strained alkynes
PatentActiveUS20180201593A1
Innovation
- A new class of cycloalkynes incorporating heteroatoms into the ring, allowing for tunable reactivity and chemoselectivity through electronic and strain activation, synthesized using readily accessible silylated allenes and optimized for stability and ease of synthesis, enabling fast reaction rates and selective reactivity with various dipoles.
Process for the preparation of alkylene glycols
PatentWO2009013221A1
Innovation
- A catalytic process involving a heterogeneous system with a catalytic composition of active anions (metalates, carbonate, bicarbonate, or hydroxide) immobilized on solid supports and halides, allowing for high selectivity and activity in converting alkylene oxides to alkylene glycols without the need for high-temperature distillation, facilitating easy product separation and reducing catalyst degradation.
Green Chemistry Impact
The impact of alkyl catalysis on green chemistry is significant and multifaceted. This approach aligns closely with the principles of green chemistry by promoting more efficient and environmentally friendly organic reactions. Alkyl catalysis enables the use of milder reaction conditions, reducing energy consumption and minimizing the generation of hazardous waste. This contributes to the overall sustainability of chemical processes and helps to reduce the environmental footprint of organic synthesis.
One of the key benefits of alkyl catalysis in the context of green chemistry is its ability to improve atom economy. By facilitating more selective transformations, alkyl catalysts can help reduce the formation of unwanted by-products, thereby increasing the efficiency of chemical reactions. This not only leads to higher yields but also minimizes waste generation, a crucial aspect of green chemistry principles.
Furthermore, alkyl catalysis often allows for the use of less toxic and more benign reagents compared to traditional synthetic methods. This shift towards safer chemicals aligns with the green chemistry goal of reducing the use and generation of hazardous substances. In many cases, alkyl catalysts can replace more harmful metal-based catalysts, contributing to the development of cleaner and safer chemical processes.
The application of alkyl catalysis in organic reactions also supports the principle of catalysis as a fundamental pillar of green chemistry. Catalysts, by definition, are not consumed in the reaction and can often be recovered and reused. This characteristic of alkyl catalysts promotes resource efficiency and reduces the overall environmental impact of chemical production.
Additionally, alkyl catalysis can enable reactions to occur under ambient conditions or with renewable feedstocks, further enhancing its green chemistry credentials. This approach can lead to the development of more sustainable synthetic routes for pharmaceuticals, agrochemicals, and other important organic compounds, potentially revolutionizing industrial processes to be more environmentally friendly.
In the broader context of sustainable development, the advancement of alkyl catalysis contributes to the transition towards a circular economy in the chemical industry. By improving reaction efficiency, reducing waste, and enabling the use of renewable resources, this technology supports the goals of resource conservation and environmental protection.
One of the key benefits of alkyl catalysis in the context of green chemistry is its ability to improve atom economy. By facilitating more selective transformations, alkyl catalysts can help reduce the formation of unwanted by-products, thereby increasing the efficiency of chemical reactions. This not only leads to higher yields but also minimizes waste generation, a crucial aspect of green chemistry principles.
Furthermore, alkyl catalysis often allows for the use of less toxic and more benign reagents compared to traditional synthetic methods. This shift towards safer chemicals aligns with the green chemistry goal of reducing the use and generation of hazardous substances. In many cases, alkyl catalysts can replace more harmful metal-based catalysts, contributing to the development of cleaner and safer chemical processes.
The application of alkyl catalysis in organic reactions also supports the principle of catalysis as a fundamental pillar of green chemistry. Catalysts, by definition, are not consumed in the reaction and can often be recovered and reused. This characteristic of alkyl catalysts promotes resource efficiency and reduces the overall environmental impact of chemical production.
Additionally, alkyl catalysis can enable reactions to occur under ambient conditions or with renewable feedstocks, further enhancing its green chemistry credentials. This approach can lead to the development of more sustainable synthetic routes for pharmaceuticals, agrochemicals, and other important organic compounds, potentially revolutionizing industrial processes to be more environmentally friendly.
In the broader context of sustainable development, the advancement of alkyl catalysis contributes to the transition towards a circular economy in the chemical industry. By improving reaction efficiency, reducing waste, and enabling the use of renewable resources, this technology supports the goals of resource conservation and environmental protection.
Scalability Prospects
The scalability prospects for advancing alkyl catalysis in organic reactions are promising and multifaceted. As the demand for more efficient and sustainable chemical processes grows, the ability to scale up alkyl catalysis becomes increasingly important. One key aspect of scalability is the development of more robust and stable catalysts that can withstand industrial conditions. Current research is focusing on designing catalysts with improved thermal stability and resistance to deactivation, allowing for longer catalyst lifetimes and reduced replacement costs in large-scale operations.
Another crucial factor in scaling alkyl catalysis is the optimization of reaction conditions for industrial-scale production. This includes fine-tuning parameters such as temperature, pressure, and solvent systems to maximize yield and selectivity while minimizing waste and energy consumption. Continuous flow reactors are emerging as a promising technology for scaling up alkyl catalysis, offering better control over reaction conditions and improved heat and mass transfer compared to traditional batch processes.
The development of more efficient separation and purification techniques is also essential for the scalability of alkyl catalysis. As reactions are scaled up, the ability to effectively isolate and recover both products and catalysts becomes increasingly challenging. Advanced separation technologies, such as membrane-based systems and novel chromatographic techniques, are being explored to address these challenges and improve overall process efficiency.
Economic considerations play a significant role in the scalability of alkyl catalysis. As production scales increase, the cost-effectiveness of catalysts becomes a critical factor. Research is ongoing to develop catalysts based on more abundant and less expensive metals, reducing reliance on precious metal catalysts that can be cost-prohibitive at larger scales. Additionally, efforts are being made to improve catalyst recyclability and regeneration processes, further enhancing the economic viability of large-scale alkyl catalysis.
Environmental sustainability is another key aspect of scalability in alkyl catalysis. As industrial processes grow in scale, their environmental impact becomes more significant. Researchers are focusing on developing greener alkyl catalysis methods that minimize waste generation, reduce energy consumption, and utilize renewable feedstocks. This includes exploring bio-based catalysts and developing more atom-efficient reactions that maximize resource utilization.
Another crucial factor in scaling alkyl catalysis is the optimization of reaction conditions for industrial-scale production. This includes fine-tuning parameters such as temperature, pressure, and solvent systems to maximize yield and selectivity while minimizing waste and energy consumption. Continuous flow reactors are emerging as a promising technology for scaling up alkyl catalysis, offering better control over reaction conditions and improved heat and mass transfer compared to traditional batch processes.
The development of more efficient separation and purification techniques is also essential for the scalability of alkyl catalysis. As reactions are scaled up, the ability to effectively isolate and recover both products and catalysts becomes increasingly challenging. Advanced separation technologies, such as membrane-based systems and novel chromatographic techniques, are being explored to address these challenges and improve overall process efficiency.
Economic considerations play a significant role in the scalability of alkyl catalysis. As production scales increase, the cost-effectiveness of catalysts becomes a critical factor. Research is ongoing to develop catalysts based on more abundant and less expensive metals, reducing reliance on precious metal catalysts that can be cost-prohibitive at larger scales. Additionally, efforts are being made to improve catalyst recyclability and regeneration processes, further enhancing the economic viability of large-scale alkyl catalysis.
Environmental sustainability is another key aspect of scalability in alkyl catalysis. As industrial processes grow in scale, their environmental impact becomes more significant. Researchers are focusing on developing greener alkyl catalysis methods that minimize waste generation, reduce energy consumption, and utilize renewable feedstocks. This includes exploring bio-based catalysts and developing more atom-efficient reactions that maximize resource utilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!