Alkyl Group Behavior in Aqueous Solutions
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Group Aqueous Behavior Research Background
The study of alkyl group behavior in aqueous solutions has been a subject of significant interest in the field of physical chemistry for several decades. This research area bridges the gap between organic chemistry and aqueous systems, providing crucial insights into the interactions between hydrophobic molecules and water. The importance of understanding these interactions extends far beyond academic curiosity, as it has profound implications for various industrial and biological processes.
Historically, the investigation of alkyl group behavior in water can be traced back to the early 20th century, with pioneering work on the hydrophobic effect. As research progressed, scientists began to recognize the complexity of these interactions, which are governed by a delicate balance of enthalpic and entropic factors. The advent of advanced spectroscopic techniques and computational methods in the latter half of the 20th century significantly accelerated progress in this field, allowing for more detailed and accurate observations of molecular-level phenomena.
The primary goal of research in this area is to elucidate the fundamental principles governing the behavior of alkyl groups in aqueous environments. This includes understanding the thermodynamics and kinetics of hydrophobic hydration, the formation and stability of micelles and other self-assembled structures, and the role of alkyl groups in biological membranes and protein folding. Additionally, researchers aim to develop predictive models that can accurately describe the behavior of alkyl-containing compounds in water, which is crucial for applications in drug design, environmental science, and materials engineering.
Recent technological advancements have opened up new avenues for investigation. High-resolution nuclear magnetic resonance (NMR) spectroscopy, for instance, has enabled researchers to probe the local structure and dynamics of water molecules around alkyl groups with unprecedented detail. Similarly, advanced molecular dynamics simulations have provided valuable insights into the energetics and time-dependent behavior of these systems at the atomic level.
The evolution of this research field has been marked by several key milestones. These include the development of the hydrophobic collapse model for protein folding, the discovery of the hydrophobic effect's role in micelle formation, and the elucidation of the molecular basis for the Hofmeister series. Each of these breakthroughs has contributed to our current understanding of alkyl group behavior in aqueous solutions and has paved the way for further investigations.
As we look to the future, the study of alkyl group behavior in aqueous solutions continues to be a vibrant and dynamic field. Emerging areas of focus include the behavior of alkyl groups at interfaces, the role of alkyl groups in the formation of nanoscale assemblies, and the development of novel materials based on controlled hydrophobic interactions. The ongoing research in this field promises to yield new insights that will have far-reaching implications for diverse areas of science and technology.
Historically, the investigation of alkyl group behavior in water can be traced back to the early 20th century, with pioneering work on the hydrophobic effect. As research progressed, scientists began to recognize the complexity of these interactions, which are governed by a delicate balance of enthalpic and entropic factors. The advent of advanced spectroscopic techniques and computational methods in the latter half of the 20th century significantly accelerated progress in this field, allowing for more detailed and accurate observations of molecular-level phenomena.
The primary goal of research in this area is to elucidate the fundamental principles governing the behavior of alkyl groups in aqueous environments. This includes understanding the thermodynamics and kinetics of hydrophobic hydration, the formation and stability of micelles and other self-assembled structures, and the role of alkyl groups in biological membranes and protein folding. Additionally, researchers aim to develop predictive models that can accurately describe the behavior of alkyl-containing compounds in water, which is crucial for applications in drug design, environmental science, and materials engineering.
Recent technological advancements have opened up new avenues for investigation. High-resolution nuclear magnetic resonance (NMR) spectroscopy, for instance, has enabled researchers to probe the local structure and dynamics of water molecules around alkyl groups with unprecedented detail. Similarly, advanced molecular dynamics simulations have provided valuable insights into the energetics and time-dependent behavior of these systems at the atomic level.
The evolution of this research field has been marked by several key milestones. These include the development of the hydrophobic collapse model for protein folding, the discovery of the hydrophobic effect's role in micelle formation, and the elucidation of the molecular basis for the Hofmeister series. Each of these breakthroughs has contributed to our current understanding of alkyl group behavior in aqueous solutions and has paved the way for further investigations.
As we look to the future, the study of alkyl group behavior in aqueous solutions continues to be a vibrant and dynamic field. Emerging areas of focus include the behavior of alkyl groups at interfaces, the role of alkyl groups in the formation of nanoscale assemblies, and the development of novel materials based on controlled hydrophobic interactions. The ongoing research in this field promises to yield new insights that will have far-reaching implications for diverse areas of science and technology.
Market Applications of Alkyl Group Aqueous Solutions
The market applications of alkyl group aqueous solutions span various industries, leveraging their unique properties and behaviors in water-based environments. In the personal care and cosmetics sector, these solutions play a crucial role in formulating shampoos, conditioners, and body washes. The amphiphilic nature of alkyl groups allows for effective emulsification and stabilization of oil-water mixtures, enabling the creation of long-lasting and high-performance products.
In the pharmaceutical industry, alkyl group aqueous solutions are utilized in drug delivery systems. Their ability to form micelles and liposomes makes them ideal carriers for hydrophobic drugs, enhancing bioavailability and targeted delivery. This application has led to the development of more effective treatments for various diseases, including cancer and cardiovascular disorders.
The cleaning and detergent industry heavily relies on alkyl group aqueous solutions for their surfactant properties. These solutions are key components in household and industrial cleaning products, providing superior cleaning performance by reducing surface tension and facilitating the removal of dirt and grease. The biodegradability of certain alkyl groups also aligns with the growing demand for environmentally friendly cleaning solutions.
In the oil and gas sector, alkyl group aqueous solutions find applications in enhanced oil recovery (EOR) techniques. By altering the interfacial properties between oil and water, these solutions help improve oil displacement efficiency, leading to increased recovery rates from mature oil fields. This application has significant economic implications for the energy industry.
The agriculture industry benefits from alkyl group aqueous solutions in the formulation of pesticides and herbicides. These solutions act as adjuvants, improving the spreading, wetting, and penetration of active ingredients on plant surfaces. This enhances the efficacy of crop protection products while potentially reducing the overall amount of chemicals needed.
In the textile industry, alkyl group aqueous solutions are used as dyeing auxiliaries and fabric softeners. They facilitate uniform dye distribution and improve color fastness, resulting in higher quality textile products. Additionally, their softening properties enhance the comfort and feel of fabrics.
The food industry employs alkyl group aqueous solutions as emulsifiers and stabilizers in various products, including sauces, dressings, and beverages. These solutions help maintain product consistency and prevent separation of ingredients, extending shelf life and improving overall quality.
In the pharmaceutical industry, alkyl group aqueous solutions are utilized in drug delivery systems. Their ability to form micelles and liposomes makes them ideal carriers for hydrophobic drugs, enhancing bioavailability and targeted delivery. This application has led to the development of more effective treatments for various diseases, including cancer and cardiovascular disorders.
The cleaning and detergent industry heavily relies on alkyl group aqueous solutions for their surfactant properties. These solutions are key components in household and industrial cleaning products, providing superior cleaning performance by reducing surface tension and facilitating the removal of dirt and grease. The biodegradability of certain alkyl groups also aligns with the growing demand for environmentally friendly cleaning solutions.
In the oil and gas sector, alkyl group aqueous solutions find applications in enhanced oil recovery (EOR) techniques. By altering the interfacial properties between oil and water, these solutions help improve oil displacement efficiency, leading to increased recovery rates from mature oil fields. This application has significant economic implications for the energy industry.
The agriculture industry benefits from alkyl group aqueous solutions in the formulation of pesticides and herbicides. These solutions act as adjuvants, improving the spreading, wetting, and penetration of active ingredients on plant surfaces. This enhances the efficacy of crop protection products while potentially reducing the overall amount of chemicals needed.
In the textile industry, alkyl group aqueous solutions are used as dyeing auxiliaries and fabric softeners. They facilitate uniform dye distribution and improve color fastness, resulting in higher quality textile products. Additionally, their softening properties enhance the comfort and feel of fabrics.
The food industry employs alkyl group aqueous solutions as emulsifiers and stabilizers in various products, including sauces, dressings, and beverages. These solutions help maintain product consistency and prevent separation of ingredients, extending shelf life and improving overall quality.
Current Challenges in Alkyl Group Aqueous Research
The research on alkyl group behavior in aqueous solutions faces several significant challenges that hinder progress in this field. One of the primary obstacles is the complexity of interactions between alkyl groups and water molecules. The hydrophobic nature of alkyl groups leads to intricate molecular arrangements and aggregation behaviors that are difficult to predict and model accurately. This complexity is further compounded by the influence of factors such as temperature, pressure, and the presence of other solutes, which can dramatically alter the behavior of alkyl groups in aqueous environments.
Another major challenge lies in the limitations of current analytical techniques. While advanced spectroscopic methods have improved our ability to study these systems, they often fall short in providing real-time, in-situ observations of alkyl group dynamics at the molecular level. This gap in observational capabilities hampers our understanding of the transient structures and interactions that play crucial roles in determining the overall behavior of alkyl groups in water.
The development of accurate computational models presents yet another hurdle. Existing molecular dynamics simulations and quantum mechanical calculations struggle to capture the full range of interactions and time scales relevant to alkyl group behavior in aqueous solutions. The multiscale nature of these systems, spanning from individual molecular interactions to macroscopic properties, demands sophisticated modeling approaches that are computationally intensive and often require simplifying assumptions that may compromise accuracy.
Furthermore, the field faces challenges in standardizing experimental protocols and data interpretation. The subtle effects of experimental conditions on alkyl group behavior can lead to inconsistencies in results across different research groups. This variability makes it difficult to establish a unified understanding of the underlying principles governing alkyl group-water interactions.
The interdisciplinary nature of this research area also poses challenges in integrating knowledge from various fields such as physical chemistry, biophysics, and materials science. Bridging the gaps between these disciplines and fostering collaborative efforts is essential for comprehensive insights into alkyl group behavior in aqueous solutions.
Lastly, there is a pressing need for developing novel materials and applications based on the unique properties of alkyl groups in water. Translating fundamental research findings into practical innovations remains a significant challenge, requiring not only scientific breakthroughs but also engineering solutions to scale up and implement these discoveries in real-world scenarios.
Another major challenge lies in the limitations of current analytical techniques. While advanced spectroscopic methods have improved our ability to study these systems, they often fall short in providing real-time, in-situ observations of alkyl group dynamics at the molecular level. This gap in observational capabilities hampers our understanding of the transient structures and interactions that play crucial roles in determining the overall behavior of alkyl groups in water.
The development of accurate computational models presents yet another hurdle. Existing molecular dynamics simulations and quantum mechanical calculations struggle to capture the full range of interactions and time scales relevant to alkyl group behavior in aqueous solutions. The multiscale nature of these systems, spanning from individual molecular interactions to macroscopic properties, demands sophisticated modeling approaches that are computationally intensive and often require simplifying assumptions that may compromise accuracy.
Furthermore, the field faces challenges in standardizing experimental protocols and data interpretation. The subtle effects of experimental conditions on alkyl group behavior can lead to inconsistencies in results across different research groups. This variability makes it difficult to establish a unified understanding of the underlying principles governing alkyl group-water interactions.
The interdisciplinary nature of this research area also poses challenges in integrating knowledge from various fields such as physical chemistry, biophysics, and materials science. Bridging the gaps between these disciplines and fostering collaborative efforts is essential for comprehensive insights into alkyl group behavior in aqueous solutions.
Lastly, there is a pressing need for developing novel materials and applications based on the unique properties of alkyl groups in water. Translating fundamental research findings into practical innovations remains a significant challenge, requiring not only scientific breakthroughs but also engineering solutions to scale up and implement these discoveries in real-world scenarios.
Existing Methodologies for Alkyl Group Analysis
01 Reactivity and substitution patterns
Alkyl groups exhibit varying reactivity and substitution patterns depending on their structure and position. Their behavior in chemical reactions is influenced by factors such as chain length, branching, and the presence of other functional groups. Understanding these patterns is crucial for predicting and controlling reactions involving alkyl groups.- Chemical reactivity of alkyl groups: Alkyl groups exhibit varying degrees of chemical reactivity depending on their structure and size. Their behavior in different chemical reactions, such as substitution and elimination, is influenced by factors like steric hindrance and electronic effects. Understanding these behaviors is crucial for predicting and controlling reactions involving alkyl-containing compounds.
- Alkyl group effects on physical properties: The presence and nature of alkyl groups can significantly impact the physical properties of molecules. These effects include changes in melting point, boiling point, solubility, and viscosity. The length and branching of alkyl chains play a crucial role in determining these properties, which is important in various applications, including the development of surfactants and lubricants.
- Alkyl groups in polymer chemistry: Alkyl groups play a significant role in polymer chemistry, affecting properties such as crystallinity, glass transition temperature, and mechanical strength. The incorporation of different alkyl groups can be used to tailor polymer characteristics for specific applications. This is particularly important in the development of specialty plastics and elastomers.
- Alkyl groups in organometallic compounds: Alkyl groups are important ligands in organometallic chemistry, influencing the reactivity and properties of metal complexes. The nature of the alkyl group can affect the stability, catalytic activity, and selectivity of these compounds. This behavior is crucial in the design of catalysts for various industrial processes and in the synthesis of novel materials.
- Environmental fate and biodegradation of alkyl compounds: The behavior of alkyl groups is important in understanding the environmental fate and biodegradation of organic compounds. Factors such as chain length and branching affect the rate of biodegradation and potential for bioaccumulation. This knowledge is crucial for assessing the environmental impact of alkyl-containing substances and developing more environmentally friendly products.
02 Influence on physical properties
The presence and nature of alkyl groups can significantly affect the physical properties of compounds. These groups can impact factors such as melting point, boiling point, solubility, and viscosity. The size and arrangement of alkyl substituents play a crucial role in determining intermolecular forces and overall molecular behavior.Expand Specific Solutions03 Role in catalytic processes
Alkyl groups play important roles in various catalytic processes. They can act as ligands in organometallic catalysts, influence the selectivity and activity of catalytic reactions, and participate in alkylation reactions. The behavior of alkyl groups in these processes is critical for optimizing catalytic performance and developing new catalytic systems.Expand Specific Solutions04 Conformational analysis and stereochemistry
The behavior of alkyl groups is closely tied to conformational analysis and stereochemistry. Rotation around single bonds, steric effects, and interactions with neighboring groups all contribute to the preferred conformations of alkyl-containing molecules. These factors influence the overall shape and reactivity of compounds, impacting their biological activity and material properties.Expand Specific Solutions05 Environmental and biological interactions
Alkyl groups significantly influence the environmental fate and biological interactions of compounds. Their hydrophobic nature affects biodegradation, bioaccumulation, and toxicity. Understanding the behavior of alkyl groups in these contexts is crucial for assessing environmental impact, designing safer chemicals, and developing pharmaceuticals with improved pharmacokinetic properties.Expand Specific Solutions
Key Players in Alkyl Group Research
The research on alkyl group behavior in aqueous solutions is currently in a developing stage, with a growing market driven by increasing applications in pharmaceuticals, chemicals, and materials science. The global market size for this technology is expanding, though exact figures are not readily available. Technologically, it is progressing from basic research to applied science, with companies like Sunshine Lake Pharma, UBE Corp., and Chugai Pharmaceutical leading in pharmaceutical applications. Academic institutions such as MIT are contributing significantly to fundamental research. The technology's maturity varies across sectors, with more established applications in chemical industries represented by companies like Kanto Chemical and Shin-Etsu Chemical, while newer applications are still emerging in fields like advanced materials and biotechnology.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced techniques for studying alkyl group behavior in aqueous solutions using nuclear magnetic resonance (NMR) spectroscopy and molecular dynamics simulations. Their approach combines high-resolution NMR experiments with computational modeling to elucidate the structure, dynamics, and interactions of alkyl groups in water[1][3]. They have successfully characterized the hydrophobic hydration of various alkyl chains, revealing how chain length and branching affect solvation properties[2]. MIT's research has also explored the impact of alkyl groups on protein folding and aggregation in aqueous environments, providing insights into biological processes[4].
Strengths: Cutting-edge research combining experimental and computational methods, strong interdisciplinary approach. Weaknesses: Primarily focused on fundamental research, which may limit immediate industrial applications.
Dow Global Technologies LLC
Technical Solution: Dow has developed proprietary technologies for manipulating alkyl group behavior in aqueous solutions, focusing on applications in surfactants, emulsifiers, and specialty chemicals. Their research includes the development of novel alkyl-based surfactants with enhanced performance in water treatment and personal care products[5]. Dow's scientists have also investigated the role of alkyl groups in controlling the solubility and stability of polymers in aqueous environments, leading to innovations in water-based coatings and adhesives[6]. Additionally, they have explored the use of alkyl-modified materials for improved oil-water separation technologies[7].
Strengths: Strong focus on practical applications, extensive industrial experience, and a wide range of patents. Weaknesses: May be less focused on fundamental research compared to academic institutions.
Core Innovations in Alkyl Group Aqueous Behavior
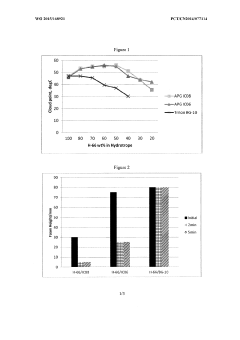
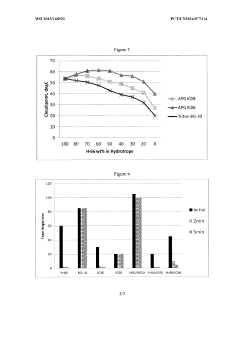
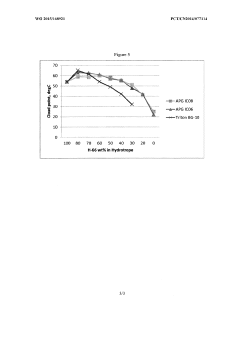
Low foaming and high stability hydrotrope formulation
PatentWO2015168921A1
Innovation
- A combination of alkyl phenoxy polyethoxy phosphate and alkyl glucoside, with an alkyl group having eight or fewer carbons, is used as a hydrotrope to enhance the cloud point temperature and reduce foaming in aqueous nonionic surfactant solutions, even in the presence of electrolytes, by optimizing the concentration of cumene sulfonic acid and alkyl glucoside.
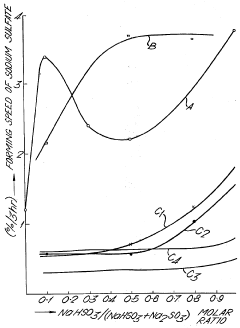
Antioxidant for the aqueous solutions of sulfite and/or bisulfite of sodium or potassium and process for preventing the oxidation of said aqueous solution
PatentInactiveUS3888969A
Innovation
- The use of specific antioxidants such as substituted phenols, tris(alkylphenyl) phosphates, trialkyl phosphites, and glycerin monofatty acid esters in aqueous solutions of sodium or potassium sulfites and bisulfites to prevent oxidation, which are added in concentrations ranging from 1-5000 ppm, effectively controlling the formation of sodium sulfate even when the solution composition changes.
Environmental Impact of Alkyl Group Solutions
The environmental impact of alkyl group solutions is a critical consideration in the research on alkyl group behavior in aqueous solutions. These solutions, widely used in various industrial processes and consumer products, can have significant effects on ecosystems and human health if not properly managed.
One of the primary environmental concerns is the potential for alkyl group solutions to contaminate water sources. When released into aquatic environments, these compounds can disrupt the delicate balance of ecosystems. They may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological consequences. Furthermore, the persistence of certain alkyl groups in the environment can result in prolonged exposure and chronic effects on wildlife.
The biodegradability of alkyl group solutions varies depending on their chemical structure and chain length. Shorter-chain alkyl groups tend to degrade more rapidly, while longer-chain compounds may persist in the environment for extended periods. This persistence can lead to the formation of harmful byproducts and contribute to the overall pollution load in ecosystems.
Atmospheric emissions from industrial processes involving alkyl group solutions can contribute to air pollution and potentially impact climate change. Some volatile organic compounds (VOCs) derived from these solutions may participate in photochemical reactions, leading to the formation of ground-level ozone and other secondary pollutants.
The toxicity of alkyl group solutions to various organisms is another significant environmental concern. Exposure to these compounds can cause acute and chronic effects on aquatic life, including reduced growth rates, reproductive impairment, and altered behavior. In terrestrial ecosystems, soil contamination by alkyl group solutions can affect plant growth and soil microbial communities, potentially disrupting nutrient cycles and ecosystem functions.
Human health risks associated with environmental exposure to alkyl group solutions include potential carcinogenic effects, endocrine disruption, and respiratory issues. Occupational exposure in industries utilizing these compounds requires strict safety measures and proper handling protocols to minimize health risks to workers.
To mitigate the environmental impact of alkyl group solutions, research efforts are focused on developing more environmentally friendly alternatives and improving treatment technologies. Green chemistry approaches aim to design alkyl-based compounds with reduced toxicity and enhanced biodegradability. Advanced wastewater treatment methods, such as advanced oxidation processes and membrane filtration, are being explored to effectively remove these contaminants from industrial effluents.
Regulatory frameworks and environmental policies play a crucial role in managing the environmental impact of alkyl group solutions. Stringent guidelines for their production, use, and disposal are essential to minimize environmental contamination and protect public health. Ongoing monitoring and assessment of environmental concentrations and effects are necessary to inform policy decisions and guide future research directions in this field.
One of the primary environmental concerns is the potential for alkyl group solutions to contaminate water sources. When released into aquatic environments, these compounds can disrupt the delicate balance of ecosystems. They may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological consequences. Furthermore, the persistence of certain alkyl groups in the environment can result in prolonged exposure and chronic effects on wildlife.
The biodegradability of alkyl group solutions varies depending on their chemical structure and chain length. Shorter-chain alkyl groups tend to degrade more rapidly, while longer-chain compounds may persist in the environment for extended periods. This persistence can lead to the formation of harmful byproducts and contribute to the overall pollution load in ecosystems.
Atmospheric emissions from industrial processes involving alkyl group solutions can contribute to air pollution and potentially impact climate change. Some volatile organic compounds (VOCs) derived from these solutions may participate in photochemical reactions, leading to the formation of ground-level ozone and other secondary pollutants.
The toxicity of alkyl group solutions to various organisms is another significant environmental concern. Exposure to these compounds can cause acute and chronic effects on aquatic life, including reduced growth rates, reproductive impairment, and altered behavior. In terrestrial ecosystems, soil contamination by alkyl group solutions can affect plant growth and soil microbial communities, potentially disrupting nutrient cycles and ecosystem functions.
Human health risks associated with environmental exposure to alkyl group solutions include potential carcinogenic effects, endocrine disruption, and respiratory issues. Occupational exposure in industries utilizing these compounds requires strict safety measures and proper handling protocols to minimize health risks to workers.
To mitigate the environmental impact of alkyl group solutions, research efforts are focused on developing more environmentally friendly alternatives and improving treatment technologies. Green chemistry approaches aim to design alkyl-based compounds with reduced toxicity and enhanced biodegradability. Advanced wastewater treatment methods, such as advanced oxidation processes and membrane filtration, are being explored to effectively remove these contaminants from industrial effluents.
Regulatory frameworks and environmental policies play a crucial role in managing the environmental impact of alkyl group solutions. Stringent guidelines for their production, use, and disposal are essential to minimize environmental contamination and protect public health. Ongoing monitoring and assessment of environmental concentrations and effects are necessary to inform policy decisions and guide future research directions in this field.
Computational Modeling of Alkyl Group Behavior
Computational modeling has become an indispensable tool in the study of alkyl group behavior in aqueous solutions. These models provide valuable insights into the complex interactions between alkyl groups and water molecules at the molecular level, offering a deeper understanding of phenomena such as hydrophobic effects and self-assembly processes.
Molecular dynamics (MD) simulations are widely employed to investigate the behavior of alkyl groups in water. These simulations use classical mechanics principles to model the motion of atoms and molecules over time. By incorporating accurate force fields that describe the interactions between alkyl groups and water molecules, researchers can observe and analyze the dynamic behavior of these systems. MD simulations have been particularly useful in elucidating the formation and stability of micelles, as well as the conformational changes of alkyl chains in different aqueous environments.
Quantum mechanical (QM) calculations, often combined with molecular mechanics (MM) in hybrid QM/MM approaches, offer a more detailed picture of electronic interactions. These methods are especially valuable when studying chemical reactions involving alkyl groups in aqueous solutions, such as hydrolysis or oxidation processes. QM/MM simulations can provide accurate energetics and reaction mechanisms, accounting for the electronic structure of the alkyl groups and their immediate surroundings while treating the bulk solvent at a less computationally demanding level.
Coarse-grained models have emerged as a powerful tool for simulating larger systems and longer time scales. By grouping atoms into larger units, these models can capture the essential physics of alkyl group behavior while reducing computational cost. This approach has been particularly successful in studying the self-assembly of surfactants and the formation of complex structures like lipid bilayers and vesicles.
Machine learning techniques are increasingly being integrated into computational models of alkyl group behavior. Neural networks and other AI algorithms can be trained on experimental data and high-level quantum calculations to predict properties and behaviors of alkyl groups in aqueous solutions with remarkable accuracy. These methods show promise in bridging the gap between atomistic simulations and macroscopic observations.
Continuum solvation models, such as the Polarizable Continuum Model (PCM), offer an efficient way to account for solvent effects on alkyl groups without explicitly modeling individual water molecules. These models treat the solvent as a continuous medium with dielectric properties, allowing for rapid calculations of solvation energies and other thermodynamic properties. While less detailed than explicit solvent models, continuum approaches are valuable for quick estimates and screening studies.
As computational power continues to increase, multi-scale modeling approaches are becoming more feasible. These methods combine different levels of theory to capture phenomena across various length and time scales, from quantum effects at the atomic level to macroscopic properties of alkyl-containing systems in aqueous environments. Such integrated approaches hold great promise for a comprehensive understanding of alkyl group behavior in complex aqueous systems.
Molecular dynamics (MD) simulations are widely employed to investigate the behavior of alkyl groups in water. These simulations use classical mechanics principles to model the motion of atoms and molecules over time. By incorporating accurate force fields that describe the interactions between alkyl groups and water molecules, researchers can observe and analyze the dynamic behavior of these systems. MD simulations have been particularly useful in elucidating the formation and stability of micelles, as well as the conformational changes of alkyl chains in different aqueous environments.
Quantum mechanical (QM) calculations, often combined with molecular mechanics (MM) in hybrid QM/MM approaches, offer a more detailed picture of electronic interactions. These methods are especially valuable when studying chemical reactions involving alkyl groups in aqueous solutions, such as hydrolysis or oxidation processes. QM/MM simulations can provide accurate energetics and reaction mechanisms, accounting for the electronic structure of the alkyl groups and their immediate surroundings while treating the bulk solvent at a less computationally demanding level.
Coarse-grained models have emerged as a powerful tool for simulating larger systems and longer time scales. By grouping atoms into larger units, these models can capture the essential physics of alkyl group behavior while reducing computational cost. This approach has been particularly successful in studying the self-assembly of surfactants and the formation of complex structures like lipid bilayers and vesicles.
Machine learning techniques are increasingly being integrated into computational models of alkyl group behavior. Neural networks and other AI algorithms can be trained on experimental data and high-level quantum calculations to predict properties and behaviors of alkyl groups in aqueous solutions with remarkable accuracy. These methods show promise in bridging the gap between atomistic simulations and macroscopic observations.
Continuum solvation models, such as the Polarizable Continuum Model (PCM), offer an efficient way to account for solvent effects on alkyl groups without explicitly modeling individual water molecules. These models treat the solvent as a continuous medium with dielectric properties, allowing for rapid calculations of solvation energies and other thermodynamic properties. While less detailed than explicit solvent models, continuum approaches are valuable for quick estimates and screening studies.
As computational power continues to increase, multi-scale modeling approaches are becoming more feasible. These methods combine different levels of theory to capture phenomena across various length and time scales, from quantum effects at the atomic level to macroscopic properties of alkyl-containing systems in aqueous environments. Such integrated approaches hold great promise for a comprehensive understanding of alkyl group behavior in complex aqueous systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!