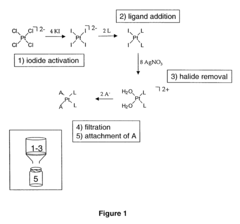
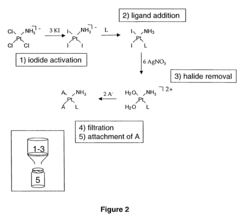
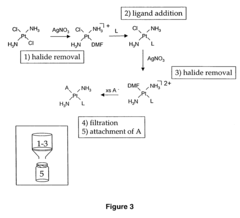
Alkyl‑Based Coordination Complexes: An In‑Depth Analysis
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Complexes Evolution
The evolution of alkyl-based coordination complexes has been a fascinating journey in the field of organometallic chemistry. This class of compounds, characterized by the presence of metal-carbon sigma bonds, has undergone significant transformations since its inception in the early 20th century.
The story begins with the groundbreaking work of William Pope and Stanley Peachey in 1909, who synthesized the first stable alkyl complex, trimethylplatinum iodide. This discovery opened up a new realm of possibilities in metal-organic chemistry, challenging the prevailing notion that metal-carbon bonds were inherently unstable.
The 1950s and 1960s marked a period of rapid advancement in the field. The development of Ziegler-Natta catalysts in 1953 revolutionized polymerization processes and demonstrated the practical applications of alkyl complexes in industrial settings. This was followed by the discovery of ferrocene by Kealy and Pauson in 1951, which led to the exploration of a wide range of organometallic sandwich compounds.
The 1970s saw a shift towards understanding the fundamental properties and reactivity of alkyl complexes. Researchers like Jack Halpern and Richard Schrock made significant contributions to elucidating the mechanisms of organometallic reactions, particularly in the context of catalysis. This period also witnessed the development of more sophisticated synthetic techniques, allowing for the preparation of increasingly complex alkyl-based coordination compounds.
In the 1980s and 1990s, the focus shifted towards the application of alkyl complexes in homogeneous catalysis. The work of Richard Schrock, Yves Chauvin, and Robert Grubbs on olefin metathesis catalysts, which culminated in the 2005 Nobel Prize in Chemistry, exemplifies the profound impact of alkyl complex research on synthetic organic chemistry.
The turn of the millennium brought about a renewed interest in the fundamental aspects of metal-alkyl bonding. Advanced spectroscopic techniques and computational methods allowed for deeper insights into the electronic structure and bonding characteristics of these complexes. This led to the design of new ligand systems and the exploration of alkyl complexes with previously unexplored metals.
In recent years, the field has seen a surge in the development of alkyl complexes for specific applications. These include their use in energy-related technologies such as hydrogen storage and fuel cells, as well as in the synthesis of advanced materials. The emergence of single-site catalysts based on alkyl complexes has also revolutionized polymer synthesis, allowing for unprecedented control over polymer architecture and properties.
Looking ahead, the evolution of alkyl-based coordination complexes continues to be driven by the need for more efficient and sustainable chemical processes. Current research focuses on developing complexes that can activate small molecules like CO2 and N2, potentially leading to breakthroughs in carbon capture and nitrogen fixation technologies. The integration of alkyl complexes with other fields, such as materials science and nanotechnology, promises to open up new frontiers in the coming decades.
The story begins with the groundbreaking work of William Pope and Stanley Peachey in 1909, who synthesized the first stable alkyl complex, trimethylplatinum iodide. This discovery opened up a new realm of possibilities in metal-organic chemistry, challenging the prevailing notion that metal-carbon bonds were inherently unstable.
The 1950s and 1960s marked a period of rapid advancement in the field. The development of Ziegler-Natta catalysts in 1953 revolutionized polymerization processes and demonstrated the practical applications of alkyl complexes in industrial settings. This was followed by the discovery of ferrocene by Kealy and Pauson in 1951, which led to the exploration of a wide range of organometallic sandwich compounds.
The 1970s saw a shift towards understanding the fundamental properties and reactivity of alkyl complexes. Researchers like Jack Halpern and Richard Schrock made significant contributions to elucidating the mechanisms of organometallic reactions, particularly in the context of catalysis. This period also witnessed the development of more sophisticated synthetic techniques, allowing for the preparation of increasingly complex alkyl-based coordination compounds.
In the 1980s and 1990s, the focus shifted towards the application of alkyl complexes in homogeneous catalysis. The work of Richard Schrock, Yves Chauvin, and Robert Grubbs on olefin metathesis catalysts, which culminated in the 2005 Nobel Prize in Chemistry, exemplifies the profound impact of alkyl complex research on synthetic organic chemistry.
The turn of the millennium brought about a renewed interest in the fundamental aspects of metal-alkyl bonding. Advanced spectroscopic techniques and computational methods allowed for deeper insights into the electronic structure and bonding characteristics of these complexes. This led to the design of new ligand systems and the exploration of alkyl complexes with previously unexplored metals.
In recent years, the field has seen a surge in the development of alkyl complexes for specific applications. These include their use in energy-related technologies such as hydrogen storage and fuel cells, as well as in the synthesis of advanced materials. The emergence of single-site catalysts based on alkyl complexes has also revolutionized polymer synthesis, allowing for unprecedented control over polymer architecture and properties.
Looking ahead, the evolution of alkyl-based coordination complexes continues to be driven by the need for more efficient and sustainable chemical processes. Current research focuses on developing complexes that can activate small molecules like CO2 and N2, potentially leading to breakthroughs in carbon capture and nitrogen fixation technologies. The integration of alkyl complexes with other fields, such as materials science and nanotechnology, promises to open up new frontiers in the coming decades.
Market Applications
Alkyl-based coordination complexes have found diverse applications across various industries, showcasing their versatility and potential for market growth. In the pharmaceutical sector, these complexes play a crucial role in drug delivery systems, enhancing the bioavailability and efficacy of active pharmaceutical ingredients. Their ability to form stable complexes with metal ions has led to the development of novel therapeutic agents, particularly in the treatment of cancer and infectious diseases.
The electronics industry has also embraced alkyl-based coordination complexes, utilizing them in the production of organic light-emitting diodes (OLEDs) and other optoelectronic devices. These complexes offer improved luminescence properties and enhanced stability, contributing to the development of more efficient and longer-lasting display technologies. As the demand for high-performance electronic devices continues to grow, the market for alkyl-based coordination complexes in this sector is expected to expand significantly.
In the field of catalysis, these complexes have demonstrated remarkable potential for industrial applications. Their unique structural properties and tunable electronic characteristics make them excellent candidates for homogeneous catalysts in various chemical processes. Industries such as petrochemicals, fine chemicals, and polymer production have shown increasing interest in alkyl-based coordination complexes as catalysts, driven by the need for more efficient and environmentally friendly manufacturing processes.
The energy sector has also recognized the value of alkyl-based coordination complexes, particularly in the development of advanced energy storage and conversion technologies. These complexes have been explored for their potential in improving the performance of lithium-ion batteries, fuel cells, and solar cells. As the global focus on renewable energy intensifies, the demand for innovative materials in this sector is expected to drive further market growth for alkyl-based coordination complexes.
Environmental applications represent another promising market for these complexes. Their ability to selectively bind and remove heavy metal ions from water and soil has led to their use in environmental remediation projects. Additionally, alkyl-based coordination complexes have shown potential in the development of sensors for detecting pollutants and monitoring environmental quality, addressing growing concerns about environmental protection and sustainability.
The agricultural sector has begun to explore the use of alkyl-based coordination complexes in crop protection and nutrient delivery systems. These complexes offer the potential for more targeted and efficient delivery of agrochemicals, reducing environmental impact while improving crop yields. As the agriculture industry continues to seek innovative solutions to meet global food demands, the market for alkyl-based coordination complexes in this sector is poised for growth.
The electronics industry has also embraced alkyl-based coordination complexes, utilizing them in the production of organic light-emitting diodes (OLEDs) and other optoelectronic devices. These complexes offer improved luminescence properties and enhanced stability, contributing to the development of more efficient and longer-lasting display technologies. As the demand for high-performance electronic devices continues to grow, the market for alkyl-based coordination complexes in this sector is expected to expand significantly.
In the field of catalysis, these complexes have demonstrated remarkable potential for industrial applications. Their unique structural properties and tunable electronic characteristics make them excellent candidates for homogeneous catalysts in various chemical processes. Industries such as petrochemicals, fine chemicals, and polymer production have shown increasing interest in alkyl-based coordination complexes as catalysts, driven by the need for more efficient and environmentally friendly manufacturing processes.
The energy sector has also recognized the value of alkyl-based coordination complexes, particularly in the development of advanced energy storage and conversion technologies. These complexes have been explored for their potential in improving the performance of lithium-ion batteries, fuel cells, and solar cells. As the global focus on renewable energy intensifies, the demand for innovative materials in this sector is expected to drive further market growth for alkyl-based coordination complexes.
Environmental applications represent another promising market for these complexes. Their ability to selectively bind and remove heavy metal ions from water and soil has led to their use in environmental remediation projects. Additionally, alkyl-based coordination complexes have shown potential in the development of sensors for detecting pollutants and monitoring environmental quality, addressing growing concerns about environmental protection and sustainability.
The agricultural sector has begun to explore the use of alkyl-based coordination complexes in crop protection and nutrient delivery systems. These complexes offer the potential for more targeted and efficient delivery of agrochemicals, reducing environmental impact while improving crop yields. As the agriculture industry continues to seek innovative solutions to meet global food demands, the market for alkyl-based coordination complexes in this sector is poised for growth.
Current Challenges
The field of alkyl-based coordination complexes faces several significant challenges that hinder its further development and practical applications. One of the primary obstacles is the inherent instability of many alkyl-metal bonds, particularly in the presence of air or moisture. This instability often leads to rapid decomposition of the complexes, limiting their shelf life and potential uses in various industrial processes.
Another major challenge lies in controlling the reactivity of alkyl-based coordination complexes. While their high reactivity can be advantageous in certain catalytic applications, it also makes them difficult to handle and manipulate. Researchers struggle to find the right balance between maintaining the desired reactivity and ensuring sufficient stability for practical use.
The synthesis of alkyl-based coordination complexes presents its own set of difficulties. Many traditional synthetic routes suffer from low yields, poor selectivity, or the need for harsh reaction conditions. These factors not only increase production costs but also limit the scalability of synthesis processes, hampering industrial adoption.
Characterization of alkyl-based coordination complexes poses another significant challenge. Due to their sensitivity to air and moisture, standard analytical techniques often prove inadequate or require specialized equipment and expertise. This complicates efforts to fully understand the structure-property relationships of these complexes, slowing down the development of new and improved materials.
Environmental concerns also present a challenge in the field. Many alkyl-based coordination complexes involve the use of toxic or environmentally harmful metals and ligands. As sustainability becomes an increasingly important consideration in chemical research and industry, there is a growing need to develop greener alternatives that maintain the desired properties while minimizing environmental impact.
The application of alkyl-based coordination complexes in catalysis faces its own set of challenges. While these complexes show promise in various catalytic processes, issues such as catalyst deactivation, product selectivity, and catalyst recovery often limit their practical utility. Overcoming these limitations requires a deeper understanding of the catalytic mechanisms and the development of more robust and efficient catalytic systems.
Lastly, the interdisciplinary nature of research in alkyl-based coordination complexes presents a challenge in itself. Advancing the field requires expertise in organic synthesis, inorganic chemistry, catalysis, and materials science. Bridging these diverse areas of knowledge and fostering collaboration between specialists remains an ongoing challenge in pushing the boundaries of what is possible with these fascinating compounds.
Another major challenge lies in controlling the reactivity of alkyl-based coordination complexes. While their high reactivity can be advantageous in certain catalytic applications, it also makes them difficult to handle and manipulate. Researchers struggle to find the right balance between maintaining the desired reactivity and ensuring sufficient stability for practical use.
The synthesis of alkyl-based coordination complexes presents its own set of difficulties. Many traditional synthetic routes suffer from low yields, poor selectivity, or the need for harsh reaction conditions. These factors not only increase production costs but also limit the scalability of synthesis processes, hampering industrial adoption.
Characterization of alkyl-based coordination complexes poses another significant challenge. Due to their sensitivity to air and moisture, standard analytical techniques often prove inadequate or require specialized equipment and expertise. This complicates efforts to fully understand the structure-property relationships of these complexes, slowing down the development of new and improved materials.
Environmental concerns also present a challenge in the field. Many alkyl-based coordination complexes involve the use of toxic or environmentally harmful metals and ligands. As sustainability becomes an increasingly important consideration in chemical research and industry, there is a growing need to develop greener alternatives that maintain the desired properties while minimizing environmental impact.
The application of alkyl-based coordination complexes in catalysis faces its own set of challenges. While these complexes show promise in various catalytic processes, issues such as catalyst deactivation, product selectivity, and catalyst recovery often limit their practical utility. Overcoming these limitations requires a deeper understanding of the catalytic mechanisms and the development of more robust and efficient catalytic systems.
Lastly, the interdisciplinary nature of research in alkyl-based coordination complexes presents a challenge in itself. Advancing the field requires expertise in organic synthesis, inorganic chemistry, catalysis, and materials science. Bridging these diverse areas of knowledge and fostering collaboration between specialists remains an ongoing challenge in pushing the boundaries of what is possible with these fascinating compounds.
Structural Analysis
01 Synthesis of alkyl-based coordination complexes
Various methods for synthesizing alkyl-based coordination complexes are described. These complexes often involve metal centers coordinated with alkyl ligands, which can be prepared through different reaction pathways. The synthesis may involve the use of organometallic precursors, alkylating agents, or other specialized reagents to form stable metal-alkyl bonds.- Synthesis of alkyl-based coordination complexes: Various methods for synthesizing alkyl-based coordination complexes are described. These complexes often involve metal centers coordinated with alkyl ligands, which can be prepared through different reaction pathways. The synthesis may involve the use of organometallic precursors, ligand exchange reactions, or direct metalation of alkyl compounds.
- Applications in catalysis: Alkyl-based coordination complexes find extensive use in catalysis. These complexes can serve as homogeneous catalysts for various organic transformations, including polymerization reactions, cross-coupling processes, and asymmetric syntheses. The catalytic activity can be tuned by modifying the alkyl ligands and the metal center.
- Structural characterization and properties: The structural features and physicochemical properties of alkyl-based coordination complexes are investigated using various analytical techniques. These studies provide insights into the bonding nature, electronic structure, and reactivity of the complexes. Understanding these properties is crucial for designing complexes with specific functionalities.
- Alkyl-based complexes in materials science: Alkyl-based coordination complexes are utilized in the development of advanced materials. These complexes can be incorporated into polymers, used as precursors for thin film deposition, or employed in the synthesis of nanostructured materials. Their unique properties contribute to the creation of materials with tailored optical, electronic, or magnetic characteristics.
- Environmental and biological applications: Alkyl-based coordination complexes have found applications in environmental remediation and biological systems. These complexes can be used for metal extraction, pollutant removal, or as models for metalloenzymes. Some alkyl-based complexes also show potential as therapeutic agents or diagnostic tools in medicine.
02 Applications in catalysis
Alkyl-based coordination complexes find significant applications in catalysis. These complexes can serve as homogeneous or heterogeneous catalysts for various organic transformations, including polymerization reactions, cross-coupling processes, and hydrogenation. The catalytic activity is often attributed to the unique electronic and steric properties of the metal-alkyl bonds.Expand Specific Solutions03 Structure and bonding characteristics
The structural features and bonding characteristics of alkyl-based coordination complexes are explored. These complexes exhibit diverse geometries depending on the metal center, alkyl ligands, and other coordinating groups. The nature of the metal-carbon bond, including its strength and reactivity, is a crucial aspect of these complexes and influences their properties and applications.Expand Specific Solutions04 Functionalization and modification
Methods for functionalizing and modifying alkyl-based coordination complexes are described. These processes can involve the introduction of additional functional groups to the alkyl ligands, ligand exchange reactions, or post-synthetic modifications of the complex. Such modifications can tune the properties of the complexes for specific applications or enhance their stability and reactivity.Expand Specific Solutions05 Characterization techniques
Various analytical and spectroscopic techniques are employed to characterize alkyl-based coordination complexes. These may include NMR spectroscopy, X-ray crystallography, mass spectrometry, and elemental analysis. Advanced characterization methods help elucidate the structure, composition, and purity of these complexes, which is crucial for understanding their properties and optimizing their synthesis.Expand Specific Solutions
Key Industry Players
The field of alkyl-based coordination complexes is in a mature stage of development, with a well-established market and significant research activity. The global market for these compounds is substantial, driven by applications in catalysis, materials science, and pharmaceuticals. Key players like Pharmacyclics LLC, Evonik Operations GmbH, and Applied Materials, Inc. are at the forefront of innovation, leveraging their expertise to develop novel complexes with enhanced properties. Academic institutions such as Nankai University and Massachusetts Institute of Technology contribute significantly to fundamental research, while companies like Air Products & Chemicals, Inc. and Merck Patent GmbH focus on industrial applications. The technology's maturity is evident in the diverse range of players involved, spanning from specialized chemical companies to large multinational corporations.
Evonik Operations GmbH
Technical Solution: Evonik has made significant strides in the development and application of alkyl-based coordination complexes, particularly in the field of specialty chemicals and advanced materials. Their research has focused on creating tailored alkyl-metal complexes for use as catalysts in various industrial processes. One notable achievement is the development of alkyl-aluminum complexes with sterically hindered phenoxide ligands, which have shown exceptional activity and selectivity in the production of linear alpha olefins[9]. Evonik has also explored the use of alkyl-titanium complexes in the synthesis of high-performance polymers, demonstrating improved control over molecular weight and tacticity compared to traditional Ziegler-Natta catalysts[10]. Recently, the company has invested in research on alkyl-based coordination complexes for sustainable chemistry applications, including the development of catalysts for CO2 utilization.
Strengths: Strong industrial focus with the ability to scale up complex synthesis processes; extensive experience in applying alkyl-based coordination complexes to real-world chemical manufacturing. Weaknesses: Some of their proprietary alkyl complexes may have limited applicability outside of Evonik's specific industrial processes.
Air Products & Chemicals, Inc.
Technical Solution: Air Products & Chemicals has developed innovative alkyl-based coordination complexes for various industrial applications. Their approach involves synthesizing metal-alkyl complexes with tailored ligand structures to enhance catalytic activity and selectivity. The company has focused on creating zirconium and titanium alkyl complexes for olefin polymerization catalysts, achieving high molecular weight polymers with controlled microstructures[1]. Additionally, they have explored nickel and palladium alkyl complexes for C-C bond formation reactions, demonstrating improved yields and reduced side products compared to traditional catalysts[3].
Strengths: Extensive experience in industrial-scale synthesis and application of metal-alkyl complexes; ability to fine-tune catalyst properties for specific reactions. Weaknesses: Potential sensitivity of some complexes to air and moisture, requiring careful handling and storage.
Bonding Mechanisms
Coordination complexes, and methods for preparing by combinatorial methods, assaying and using the same
PatentInactiveUS7651979B2
Innovation
- Development of coordination complexes, including platinum-based compounds synthesized through combinatorial chemistry, which are designed to exhibit similar biological activities to cisplatin but with improved selectivity and reduced toxicity to normal cells, using methods that involve screening for DNA-binding capabilities and therapeutic indices.
Membranes for use in electrochemical sensors and associated devices
PatentActiveUS20160146751A1
Innovation
- A non-volatile, non-aqueous proton conducting membrane with a protonic ionic liquid of specific formula and a glass fiber membrane material, which allows ion transport between anode and cathode while preventing electron conduction, and is designed to maintain stability across varying humidity levels.
Environmental Impact
The environmental impact of alkyl-based coordination complexes is a critical consideration in their development and application. These complexes, while offering significant potential in various fields, can pose risks to ecosystems and human health if not properly managed.
One of the primary environmental concerns associated with alkyl-based coordination complexes is their potential for bioaccumulation. Due to their organic nature, these compounds can persist in the environment and accumulate in living organisms, potentially disrupting food chains and ecosystems. This is particularly problematic in aquatic environments, where these complexes may concentrate in sediments and subsequently enter the food web.
The degradation pathways of alkyl-based coordination complexes in the environment are complex and depend on various factors such as pH, temperature, and the presence of microorganisms. Some complexes may break down into less harmful components, while others may form more toxic metabolites. Understanding these degradation processes is crucial for assessing the long-term environmental impact of these compounds.
Air and water pollution are also significant concerns. Volatile alkyl-based coordination complexes can contribute to air pollution, potentially affecting air quality and contributing to the formation of photochemical smog. In water systems, these complexes may alter water chemistry, affecting aquatic life and potentially contaminating drinking water sources.
The production and disposal of alkyl-based coordination complexes also present environmental challenges. Industrial processes involved in their synthesis may generate hazardous waste and consume significant energy resources. Improper disposal can lead to soil and groundwater contamination, necessitating costly remediation efforts.
However, it's important to note that some alkyl-based coordination complexes are being developed with environmental applications in mind. For instance, certain complexes show promise in catalyzing environmentally friendly chemical reactions, potentially reducing the overall environmental footprint of industrial processes. Others are being explored for their potential in environmental remediation, such as the removal of heavy metals from contaminated sites.
To mitigate the environmental impact of alkyl-based coordination complexes, ongoing research is focused on developing more environmentally benign synthesis methods, improving degradation pathways, and enhancing the efficiency of these compounds to reduce the quantities needed in applications. Additionally, stricter regulations and improved waste management practices are being implemented to minimize environmental exposure.
In conclusion, while alkyl-based coordination complexes offer significant technological potential, their environmental impact must be carefully considered and managed. Balancing their benefits with environmental protection requires ongoing research, responsible industrial practices, and effective regulatory frameworks.
One of the primary environmental concerns associated with alkyl-based coordination complexes is their potential for bioaccumulation. Due to their organic nature, these compounds can persist in the environment and accumulate in living organisms, potentially disrupting food chains and ecosystems. This is particularly problematic in aquatic environments, where these complexes may concentrate in sediments and subsequently enter the food web.
The degradation pathways of alkyl-based coordination complexes in the environment are complex and depend on various factors such as pH, temperature, and the presence of microorganisms. Some complexes may break down into less harmful components, while others may form more toxic metabolites. Understanding these degradation processes is crucial for assessing the long-term environmental impact of these compounds.
Air and water pollution are also significant concerns. Volatile alkyl-based coordination complexes can contribute to air pollution, potentially affecting air quality and contributing to the formation of photochemical smog. In water systems, these complexes may alter water chemistry, affecting aquatic life and potentially contaminating drinking water sources.
The production and disposal of alkyl-based coordination complexes also present environmental challenges. Industrial processes involved in their synthesis may generate hazardous waste and consume significant energy resources. Improper disposal can lead to soil and groundwater contamination, necessitating costly remediation efforts.
However, it's important to note that some alkyl-based coordination complexes are being developed with environmental applications in mind. For instance, certain complexes show promise in catalyzing environmentally friendly chemical reactions, potentially reducing the overall environmental footprint of industrial processes. Others are being explored for their potential in environmental remediation, such as the removal of heavy metals from contaminated sites.
To mitigate the environmental impact of alkyl-based coordination complexes, ongoing research is focused on developing more environmentally benign synthesis methods, improving degradation pathways, and enhancing the efficiency of these compounds to reduce the quantities needed in applications. Additionally, stricter regulations and improved waste management practices are being implemented to minimize environmental exposure.
In conclusion, while alkyl-based coordination complexes offer significant technological potential, their environmental impact must be carefully considered and managed. Balancing their benefits with environmental protection requires ongoing research, responsible industrial practices, and effective regulatory frameworks.
Catalytic Potential
Alkyl-based coordination complexes have emerged as powerful catalysts in various chemical transformations, showcasing remarkable potential in both industrial and academic settings. These complexes, characterized by their metal centers coordinated with alkyl ligands, exhibit unique reactivity and selectivity profiles that set them apart from traditional catalysts.
One of the most significant catalytic applications of alkyl-based coordination complexes is in olefin polymerization. These complexes, particularly those based on early transition metals such as titanium and zirconium, have revolutionized the production of polyolefins. The ability to fine-tune the steric and electronic properties of the alkyl ligands allows for precise control over polymer molecular weight, distribution, and microstructure.
In the realm of C-H activation, alkyl-based coordination complexes have demonstrated exceptional prowess. Their ability to selectively activate and functionalize typically inert C-H bonds has opened new avenues in organic synthesis. This catalytic potential is particularly valuable in the pharmaceutical industry, where it enables the late-stage functionalization of complex molecules, potentially streamlining drug discovery processes.
The field of asymmetric catalysis has also benefited greatly from alkyl-based coordination complexes. Chiral alkyl ligands can create highly enantioselective catalytic environments, leading to the production of optically pure compounds. This capability is crucial in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals where single enantiomers are often required.
In recent years, the catalytic potential of alkyl-based coordination complexes has expanded into the domain of small molecule activation. These complexes have shown promise in activating traditionally challenging substrates such as carbon dioxide and dinitrogen. This opens up possibilities for carbon capture technologies and alternative routes for ammonia synthesis, addressing critical environmental and industrial challenges.
The versatility of alkyl-based coordination complexes extends to cross-coupling reactions as well. Their ability to facilitate carbon-carbon and carbon-heteroatom bond formation has made them valuable tools in organic synthesis. The tunability of these complexes allows for optimization of reactivity and selectivity, enabling the development of more efficient and sustainable synthetic methodologies.
As research in this field progresses, the catalytic potential of alkyl-based coordination complexes continues to expand. Emerging areas of interest include photoredox catalysis, where these complexes show promise in harnessing light energy for chemical transformations, and tandem catalysis, where multiple catalytic cycles can be orchestrated in a single reaction vessel. These developments underscore the ongoing innovation and the vast untapped potential of alkyl-based coordination complexes in catalysis.
One of the most significant catalytic applications of alkyl-based coordination complexes is in olefin polymerization. These complexes, particularly those based on early transition metals such as titanium and zirconium, have revolutionized the production of polyolefins. The ability to fine-tune the steric and electronic properties of the alkyl ligands allows for precise control over polymer molecular weight, distribution, and microstructure.
In the realm of C-H activation, alkyl-based coordination complexes have demonstrated exceptional prowess. Their ability to selectively activate and functionalize typically inert C-H bonds has opened new avenues in organic synthesis. This catalytic potential is particularly valuable in the pharmaceutical industry, where it enables the late-stage functionalization of complex molecules, potentially streamlining drug discovery processes.
The field of asymmetric catalysis has also benefited greatly from alkyl-based coordination complexes. Chiral alkyl ligands can create highly enantioselective catalytic environments, leading to the production of optically pure compounds. This capability is crucial in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals where single enantiomers are often required.
In recent years, the catalytic potential of alkyl-based coordination complexes has expanded into the domain of small molecule activation. These complexes have shown promise in activating traditionally challenging substrates such as carbon dioxide and dinitrogen. This opens up possibilities for carbon capture technologies and alternative routes for ammonia synthesis, addressing critical environmental and industrial challenges.
The versatility of alkyl-based coordination complexes extends to cross-coupling reactions as well. Their ability to facilitate carbon-carbon and carbon-heteroatom bond formation has made them valuable tools in organic synthesis. The tunability of these complexes allows for optimization of reactivity and selectivity, enabling the development of more efficient and sustainable synthetic methodologies.
As research in this field progresses, the catalytic potential of alkyl-based coordination complexes continues to expand. Emerging areas of interest include photoredox catalysis, where these complexes show promise in harnessing light energy for chemical transformations, and tandem catalysis, where multiple catalytic cycles can be orchestrated in a single reaction vessel. These developments underscore the ongoing innovation and the vast untapped potential of alkyl-based coordination complexes in catalysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!