Strategies to Improve Alkyl Compound Purity
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Compound Purification Background and Objectives
Alkyl compounds play a crucial role in various industries, including pharmaceuticals, petrochemicals, and materials science. The purity of these compounds is of paramount importance, as even small impurities can significantly affect their properties and performance. Over the past few decades, the demand for high-purity alkyl compounds has grown exponentially, driven by advancements in technology and increasingly stringent quality requirements.
The evolution of alkyl compound purification techniques has been marked by continuous innovation and refinement. Early methods relied primarily on distillation and recrystallization, which were effective but often limited in their ability to remove closely related impurities. As analytical techniques improved, revealing the presence of trace contaminants, more sophisticated purification methods became necessary.
The development of chromatographic techniques, particularly high-performance liquid chromatography (HPLC) and gas chromatography (GC), revolutionized the field of alkyl compound purification. These methods allowed for the separation of compounds with very similar physical and chemical properties, greatly enhancing the achievable purity levels. Concurrently, advances in membrane technology and molecular sieves opened new avenues for purification, especially for larger-scale operations.
Recent years have seen a shift towards more sustainable and efficient purification processes. This trend is driven by environmental concerns and the need to reduce production costs. As a result, there is growing interest in developing green chemistry approaches to alkyl compound purification, including the use of supercritical fluids, ionic liquids, and biocatalytic methods.
The primary objective in the field of alkyl compound purification is to develop strategies that can consistently achieve higher levels of purity while minimizing energy consumption, reducing waste generation, and lowering production costs. This goal presents several technical challenges, including the removal of trace impurities, the separation of structurally similar compounds, and the scaling up of laboratory techniques to industrial production levels.
Another key objective is to enhance the versatility of purification methods to handle a wider range of alkyl compounds and potential impurities. This includes developing more robust and adaptable purification processes that can be quickly optimized for different compounds without significant retooling or process redesign.
As we look to the future, the field of alkyl compound purification is likely to see further integration of advanced technologies such as artificial intelligence for process optimization, in-line analytical techniques for real-time purity monitoring, and novel materials for more selective separations. These developments promise to push the boundaries of achievable purity levels and process efficiency, meeting the ever-increasing demands of various high-tech industries.
The evolution of alkyl compound purification techniques has been marked by continuous innovation and refinement. Early methods relied primarily on distillation and recrystallization, which were effective but often limited in their ability to remove closely related impurities. As analytical techniques improved, revealing the presence of trace contaminants, more sophisticated purification methods became necessary.
The development of chromatographic techniques, particularly high-performance liquid chromatography (HPLC) and gas chromatography (GC), revolutionized the field of alkyl compound purification. These methods allowed for the separation of compounds with very similar physical and chemical properties, greatly enhancing the achievable purity levels. Concurrently, advances in membrane technology and molecular sieves opened new avenues for purification, especially for larger-scale operations.
Recent years have seen a shift towards more sustainable and efficient purification processes. This trend is driven by environmental concerns and the need to reduce production costs. As a result, there is growing interest in developing green chemistry approaches to alkyl compound purification, including the use of supercritical fluids, ionic liquids, and biocatalytic methods.
The primary objective in the field of alkyl compound purification is to develop strategies that can consistently achieve higher levels of purity while minimizing energy consumption, reducing waste generation, and lowering production costs. This goal presents several technical challenges, including the removal of trace impurities, the separation of structurally similar compounds, and the scaling up of laboratory techniques to industrial production levels.
Another key objective is to enhance the versatility of purification methods to handle a wider range of alkyl compounds and potential impurities. This includes developing more robust and adaptable purification processes that can be quickly optimized for different compounds without significant retooling or process redesign.
As we look to the future, the field of alkyl compound purification is likely to see further integration of advanced technologies such as artificial intelligence for process optimization, in-line analytical techniques for real-time purity monitoring, and novel materials for more selective separations. These developments promise to push the boundaries of achievable purity levels and process efficiency, meeting the ever-increasing demands of various high-tech industries.
Market Demand for High-Purity Alkyl Compounds
The market demand for high-purity alkyl compounds has been steadily increasing across various industries, driven by the growing need for superior quality materials in manufacturing processes. The pharmaceutical sector, in particular, has emerged as a significant consumer of high-purity alkyl compounds, utilizing them as key intermediates in the synthesis of active pharmaceutical ingredients (APIs). This demand is further amplified by the stringent regulatory requirements for drug purity and safety.
In the electronics industry, the push for miniaturization and enhanced performance of semiconductor devices has led to a surge in demand for ultra-pure alkyl compounds. These compounds play a crucial role in the production of advanced materials for electronic components, such as high-performance polymers and specialty coatings. The automotive sector has also shown increased interest in high-purity alkyl compounds for the development of advanced lubricants and fuel additives, aiming to improve engine efficiency and reduce emissions.
The global market for high-purity alkyl compounds is projected to experience robust growth in the coming years. This growth is attributed to the expanding applications in emerging technologies, such as renewable energy systems and advanced materials for aerospace. The solar energy sector, for instance, requires high-purity alkyl compounds in the production of photovoltaic cells, contributing to the overall market expansion.
Geographically, Asia-Pacific has emerged as a key market for high-purity alkyl compounds, driven by the rapid industrialization and technological advancements in countries like China, Japan, and South Korea. North America and Europe continue to be significant consumers, primarily due to their well-established pharmaceutical and electronics industries.
The market dynamics are also influenced by the increasing focus on sustainability and environmental regulations. This has led to a growing demand for bio-based and environmentally friendly alkyl compounds, opening new avenues for market growth and innovation. Manufacturers are investing in research and development to meet these evolving market needs, focusing on developing sustainable production methods and exploring new applications for high-purity alkyl compounds.
As industries continue to prioritize quality and performance, the demand for high-purity alkyl compounds is expected to maintain its upward trajectory. This trend is likely to drive further investments in purification technologies and production capacity expansion. The market is also witnessing a shift towards customized solutions, with manufacturers offering tailored high-purity alkyl compounds to meet specific industry requirements, thereby adding value and expanding their market share.
In the electronics industry, the push for miniaturization and enhanced performance of semiconductor devices has led to a surge in demand for ultra-pure alkyl compounds. These compounds play a crucial role in the production of advanced materials for electronic components, such as high-performance polymers and specialty coatings. The automotive sector has also shown increased interest in high-purity alkyl compounds for the development of advanced lubricants and fuel additives, aiming to improve engine efficiency and reduce emissions.
The global market for high-purity alkyl compounds is projected to experience robust growth in the coming years. This growth is attributed to the expanding applications in emerging technologies, such as renewable energy systems and advanced materials for aerospace. The solar energy sector, for instance, requires high-purity alkyl compounds in the production of photovoltaic cells, contributing to the overall market expansion.
Geographically, Asia-Pacific has emerged as a key market for high-purity alkyl compounds, driven by the rapid industrialization and technological advancements in countries like China, Japan, and South Korea. North America and Europe continue to be significant consumers, primarily due to their well-established pharmaceutical and electronics industries.
The market dynamics are also influenced by the increasing focus on sustainability and environmental regulations. This has led to a growing demand for bio-based and environmentally friendly alkyl compounds, opening new avenues for market growth and innovation. Manufacturers are investing in research and development to meet these evolving market needs, focusing on developing sustainable production methods and exploring new applications for high-purity alkyl compounds.
As industries continue to prioritize quality and performance, the demand for high-purity alkyl compounds is expected to maintain its upward trajectory. This trend is likely to drive further investments in purification technologies and production capacity expansion. The market is also witnessing a shift towards customized solutions, with manufacturers offering tailored high-purity alkyl compounds to meet specific industry requirements, thereby adding value and expanding their market share.
Current Challenges in Alkyl Compound Purification
The purification of alkyl compounds presents several significant challenges in the current chemical industry landscape. One of the primary obstacles is the presence of structurally similar impurities, which often have physical and chemical properties closely resembling the target alkyl compounds. This similarity makes traditional separation techniques, such as distillation or crystallization, less effective and more energy-intensive.
Another major challenge lies in the thermal instability of many alkyl compounds. High temperatures often employed in conventional purification methods can lead to degradation or unwanted side reactions, compromising the purity and yield of the final product. This necessitates the development of low-temperature purification techniques, which can be technically complex and economically demanding.
The formation of azeotropes poses a substantial hurdle in alkyl compound purification. Many alkyl compounds form azeotropic mixtures with solvents or other impurities, making complete separation through simple distillation impossible. Breaking these azeotropes often requires specialized techniques like azeotropic distillation or the use of entrainers, adding complexity and cost to the purification process.
Environmental concerns and stringent regulations present another set of challenges. Traditional purification methods often involve the use of large volumes of organic solvents, which can be environmentally harmful and costly to dispose of. There is a growing need for greener purification technologies that minimize solvent use and reduce environmental impact.
Scale-up issues also plague the purification of alkyl compounds. Methods that work efficiently at laboratory scale often face significant hurdles when translated to industrial production. Factors such as heat transfer limitations, mixing inefficiencies, and increased formation of by-products can dramatically affect the purity and yield at larger scales.
The presence of trace impurities, even in small quantities, can significantly impact the performance and properties of alkyl compounds in their final applications. Achieving ultra-high purity levels, often required in industries like electronics or pharmaceuticals, remains a formidable challenge. Conventional purification methods may not be sufficient to remove these trace contaminants, necessitating the development of more sophisticated and targeted purification strategies.
Lastly, the economic viability of purification processes poses a constant challenge. Many advanced purification techniques, while effective, are prohibitively expensive for large-scale implementation. Striking a balance between purity requirements and economic feasibility is an ongoing struggle in the industry, driving the need for innovative, cost-effective purification solutions.
Another major challenge lies in the thermal instability of many alkyl compounds. High temperatures often employed in conventional purification methods can lead to degradation or unwanted side reactions, compromising the purity and yield of the final product. This necessitates the development of low-temperature purification techniques, which can be technically complex and economically demanding.
The formation of azeotropes poses a substantial hurdle in alkyl compound purification. Many alkyl compounds form azeotropic mixtures with solvents or other impurities, making complete separation through simple distillation impossible. Breaking these azeotropes often requires specialized techniques like azeotropic distillation or the use of entrainers, adding complexity and cost to the purification process.
Environmental concerns and stringent regulations present another set of challenges. Traditional purification methods often involve the use of large volumes of organic solvents, which can be environmentally harmful and costly to dispose of. There is a growing need for greener purification technologies that minimize solvent use and reduce environmental impact.
Scale-up issues also plague the purification of alkyl compounds. Methods that work efficiently at laboratory scale often face significant hurdles when translated to industrial production. Factors such as heat transfer limitations, mixing inefficiencies, and increased formation of by-products can dramatically affect the purity and yield at larger scales.
The presence of trace impurities, even in small quantities, can significantly impact the performance and properties of alkyl compounds in their final applications. Achieving ultra-high purity levels, often required in industries like electronics or pharmaceuticals, remains a formidable challenge. Conventional purification methods may not be sufficient to remove these trace contaminants, necessitating the development of more sophisticated and targeted purification strategies.
Lastly, the economic viability of purification processes poses a constant challenge. Many advanced purification techniques, while effective, are prohibitively expensive for large-scale implementation. Striking a balance between purity requirements and economic feasibility is an ongoing struggle in the industry, driving the need for innovative, cost-effective purification solutions.
Existing Purification Methods for Alkyl Compounds
01 Purification methods for alkyl compounds
Various purification techniques are employed to enhance the purity of alkyl compounds. These methods may include distillation, crystallization, and chromatography. The choice of purification method depends on the specific alkyl compound and the desired level of purity.- Purification methods for alkyl compounds: Various purification techniques are employed to enhance the purity of alkyl compounds. These methods may include distillation, crystallization, and chromatography. The choice of purification method depends on the specific alkyl compound and the desired level of purity.
- Synthesis of high-purity alkyl compounds: Strategies for synthesizing high-purity alkyl compounds often involve careful control of reaction conditions, use of high-quality starting materials, and optimization of reaction parameters. These approaches aim to minimize the formation of byproducts and impurities during the synthesis process.
- Analysis and characterization of alkyl compound purity: Analytical techniques such as gas chromatography, mass spectrometry, and NMR spectroscopy are used to assess the purity of alkyl compounds. These methods help identify and quantify impurities, ensuring that the compounds meet the required purity standards for various applications.
- Stabilization of high-purity alkyl compounds: Methods for maintaining the purity of alkyl compounds during storage and handling are crucial. This may involve the use of stabilizers, antioxidants, or specialized packaging to prevent degradation and maintain the desired level of purity over time.
- Applications of high-purity alkyl compounds: High-purity alkyl compounds find applications in various industries, including pharmaceuticals, electronics, and fine chemicals. The level of purity required often depends on the specific application, with some uses demanding extremely high levels of purity to ensure optimal performance and reliability.
02 Synthesis of high-purity alkyl compounds
Strategies for synthesizing high-purity alkyl compounds involve careful control of reaction conditions, use of high-quality starting materials, and optimization of reaction parameters. These approaches aim to minimize the formation of byproducts and impurities during the synthesis process.Expand Specific Solutions03 Analysis and characterization of alkyl compound purity
Analytical techniques such as gas chromatography, mass spectrometry, and NMR spectroscopy are used to assess the purity of alkyl compounds. These methods help identify and quantify impurities, ensuring that the compounds meet the required purity standards for various applications.Expand Specific Solutions04 Stabilization of high-purity alkyl compounds
Methods for maintaining the purity of alkyl compounds during storage and handling are crucial. This may involve the use of stabilizers, antioxidants, or specific storage conditions to prevent degradation or contamination of the purified compounds over time.Expand Specific Solutions05 Applications of high-purity alkyl compounds
High-purity alkyl compounds find applications in various industries, including pharmaceuticals, electronics, and fine chemicals. The level of purity required depends on the specific application, with some uses demanding extremely high levels of purity to ensure optimal performance or product quality.Expand Specific Solutions
Key Players in Alkyl Compound Industry
The strategies to improve alkyl compound purity are in a mature stage of development, with a substantial market size driven by demand from various industries. The technology's maturity is evident from the involvement of established chemical companies like BASF, Shin-Etsu Chemical, and Albemarle Corporation. These industry leaders have extensive experience in developing and refining purification techniques. The competitive landscape is characterized by a mix of large multinational corporations and specialized chemical companies, such as CyDex Pharmaceuticals and JGC Catalysts & Chemicals, indicating a diverse range of approaches to alkyl compound purification. The presence of research institutes like Sinopec Research Institute of Petroleum Processing suggests ongoing efforts to further enhance purification methods, potentially leading to incremental improvements in the technology.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced strategies to improve alkyl compound purity. They employ a multi-stage distillation process combined with selective adsorption techniques. The process involves using high-efficiency packing materials in distillation columns to enhance separation efficiency[1]. Additionally, they utilize molecular sieves with tailored pore sizes to selectively remove impurities based on molecular dimensions[3]. Sinopec has also implemented advanced process control systems that use real-time monitoring and adjustment of operating parameters to maintain optimal purification conditions[5]. Their approach includes the use of reactive distillation, where reaction and separation occur simultaneously, improving overall process efficiency and product purity[2].
Strengths: Integrated approach combining multiple purification techniques, advanced process control for optimization, and energy-efficient operations. Weaknesses: High capital investment required for advanced equipment and potential complexity in process control.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative strategies for improving alkyl compound purity. Their approach includes the use of advanced catalytic systems for selective alkylation reactions, minimizing the formation of byproducts[1]. They have also implemented membrane-based separation technologies, utilizing specially designed polymer membranes that can selectively permeate desired alkyl compounds while rejecting impurities[3]. BASF's purification strategy incorporates continuous flow chemistry principles, allowing for precise control of reaction conditions and real-time purification[2]. Additionally, they have developed proprietary adsorbents with high selectivity for specific impurities commonly found in alkyl compounds[4]. The company also utilizes advanced analytical techniques, including in-line spectroscopy, to monitor and control product purity throughout the production process[5].
Strengths: Diverse portfolio of purification technologies, strong focus on process intensification, and integration of advanced analytics. Weaknesses: Potential high costs associated with proprietary technologies and need for specialized expertise in multiple purification methods.
Innovative Approaches in Alkyl Purification
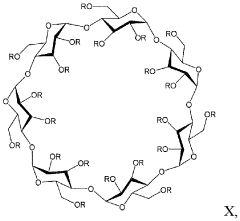
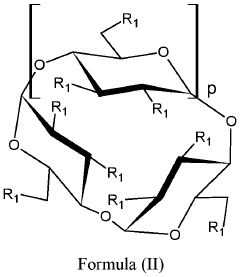
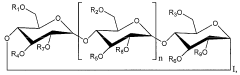
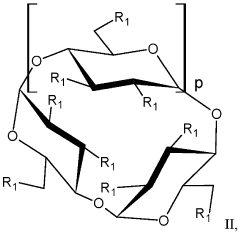
Alkylated cyclodextrin compositions and processes for preparing and using the same
PatentPendingIN202317027908A
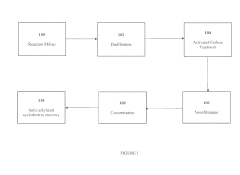
Innovation
- A process involving mixing cyclodextrin with an alkylating agent, followed by diafiltration through an ultrafiltration membrane, treatment with activated carbon, and subsequent nanofiltration through a nanofiltration membrane to achieve high purity alkylated cyclodextrin solutions, allowing for large-scale production with controlled impurity removal.
Alkylated cyclodextrin compositions and processes for preparing and using the same
PatentWO2021101842A1
Innovation
- A process involving the mixing of cyclodextrin with an alkylating agent, followed by separations to form a partially purified solution, and then treating activated carbon with this solution after thorough washing to produce a final purified alkylated cyclodextrin composition, ensuring low residual conductivity and reduced chloride and phosphate levels.
Environmental Impact of Purification Processes
The purification of alkyl compounds is a critical process in various industries, including pharmaceuticals, petrochemicals, and fine chemicals. However, these purification processes often come with significant environmental implications that need to be carefully considered and addressed. Traditional purification methods, such as distillation and crystallization, can be energy-intensive and generate substantial amounts of waste, contributing to environmental degradation.
One of the primary environmental concerns associated with alkyl compound purification is the high energy consumption required for processes like fractional distillation. This energy demand often translates to increased greenhouse gas emissions, particularly when fossil fuels are the primary energy source. Additionally, the use of organic solvents in extraction and recrystallization processes can lead to air and water pollution if not properly managed.
Waste generation is another significant environmental impact of purification processes. Byproducts, spent solvents, and other residues from purification steps can accumulate, requiring proper disposal or treatment. Improper handling of these wastes can result in soil and water contamination, potentially affecting ecosystems and human health.
Water usage is a critical factor to consider, especially in regions facing water scarcity. Many purification techniques require substantial amounts of water for cooling, washing, or as a process medium. The discharge of contaminated wastewater from these processes can also pose risks to aquatic environments if not adequately treated.
To mitigate these environmental impacts, the industry is increasingly focusing on developing and implementing more sustainable purification strategies. Green chemistry principles are being applied to design processes that minimize waste generation, reduce energy consumption, and utilize less harmful solvents. For instance, the use of supercritical fluid extraction, particularly with carbon dioxide, offers a more environmentally friendly alternative to traditional solvent-based extractions.
Membrane-based separation technologies are gaining traction as they often require less energy and fewer chemicals compared to conventional methods. These technologies can significantly reduce the environmental footprint of purification processes while maintaining or even improving product quality.
Biotechnological approaches, such as enzymatic purification methods, are also emerging as promising alternatives. These bio-based processes often operate under milder conditions, consume less energy, and produce fewer toxic byproducts compared to chemical-based purification methods.
As environmental regulations become more stringent globally, companies are increasingly investing in closed-loop systems and recycling technologies to minimize waste and maximize resource efficiency in their purification processes. This shift not only reduces environmental impact but also often leads to cost savings and improved process economics in the long run.
One of the primary environmental concerns associated with alkyl compound purification is the high energy consumption required for processes like fractional distillation. This energy demand often translates to increased greenhouse gas emissions, particularly when fossil fuels are the primary energy source. Additionally, the use of organic solvents in extraction and recrystallization processes can lead to air and water pollution if not properly managed.
Waste generation is another significant environmental impact of purification processes. Byproducts, spent solvents, and other residues from purification steps can accumulate, requiring proper disposal or treatment. Improper handling of these wastes can result in soil and water contamination, potentially affecting ecosystems and human health.
Water usage is a critical factor to consider, especially in regions facing water scarcity. Many purification techniques require substantial amounts of water for cooling, washing, or as a process medium. The discharge of contaminated wastewater from these processes can also pose risks to aquatic environments if not adequately treated.
To mitigate these environmental impacts, the industry is increasingly focusing on developing and implementing more sustainable purification strategies. Green chemistry principles are being applied to design processes that minimize waste generation, reduce energy consumption, and utilize less harmful solvents. For instance, the use of supercritical fluid extraction, particularly with carbon dioxide, offers a more environmentally friendly alternative to traditional solvent-based extractions.
Membrane-based separation technologies are gaining traction as they often require less energy and fewer chemicals compared to conventional methods. These technologies can significantly reduce the environmental footprint of purification processes while maintaining or even improving product quality.
Biotechnological approaches, such as enzymatic purification methods, are also emerging as promising alternatives. These bio-based processes often operate under milder conditions, consume less energy, and produce fewer toxic byproducts compared to chemical-based purification methods.
As environmental regulations become more stringent globally, companies are increasingly investing in closed-loop systems and recycling technologies to minimize waste and maximize resource efficiency in their purification processes. This shift not only reduces environmental impact but also often leads to cost savings and improved process economics in the long run.
Quality Control and Analytical Methods
Quality control and analytical methods play a crucial role in improving the purity of alkyl compounds. These techniques ensure that the final product meets the required specifications and maintains consistency across batches. Gas chromatography (GC) is a widely used analytical method for determining the purity of alkyl compounds. It offers high sensitivity and excellent separation capabilities, allowing for the detection and quantification of impurities at low concentrations. GC can be coupled with mass spectrometry (GC-MS) to provide additional structural information about the impurities present in the sample.
High-performance liquid chromatography (HPLC) is another valuable technique for analyzing alkyl compounds. It is particularly useful for compounds that are thermally unstable or have high boiling points. HPLC can be used in both normal-phase and reverse-phase modes, depending on the polarity of the alkyl compounds and their impurities. The use of UV-Vis or refractive index detectors in HPLC allows for accurate quantification of the main compound and its impurities.
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for structural elucidation and purity assessment of alkyl compounds. 1H and 13C NMR can provide detailed information about the molecular structure and help identify impurities that may not be detected by chromatographic methods. Quantitative NMR (qNMR) can be used to determine the absolute purity of alkyl compounds with high accuracy.
Fourier-transform infrared spectroscopy (FTIR) is a complementary technique that can provide valuable information about the functional groups present in alkyl compounds and their impurities. It can be particularly useful for identifying and characterizing trace impurities that may not be easily detected by other methods.
To ensure the reliability of analytical results, it is essential to implement robust quality control measures. This includes the use of certified reference materials, regular instrument calibration, and participation in proficiency testing programs. The development and validation of analytical methods specific to each alkyl compound are crucial for accurate purity determination. Method validation should include parameters such as linearity, precision, accuracy, and limits of detection and quantification.
Statistical process control (SPC) techniques can be employed to monitor and improve the purity of alkyl compounds during production. Control charts and trend analysis can help identify process variations and potential sources of impurities. Implementing a quality management system, such as ISO 9001 or Good Manufacturing Practices (GMP), can further enhance the overall quality control process and ensure consistent product purity.
High-performance liquid chromatography (HPLC) is another valuable technique for analyzing alkyl compounds. It is particularly useful for compounds that are thermally unstable or have high boiling points. HPLC can be used in both normal-phase and reverse-phase modes, depending on the polarity of the alkyl compounds and their impurities. The use of UV-Vis or refractive index detectors in HPLC allows for accurate quantification of the main compound and its impurities.
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for structural elucidation and purity assessment of alkyl compounds. 1H and 13C NMR can provide detailed information about the molecular structure and help identify impurities that may not be detected by chromatographic methods. Quantitative NMR (qNMR) can be used to determine the absolute purity of alkyl compounds with high accuracy.
Fourier-transform infrared spectroscopy (FTIR) is a complementary technique that can provide valuable information about the functional groups present in alkyl compounds and their impurities. It can be particularly useful for identifying and characterizing trace impurities that may not be easily detected by other methods.
To ensure the reliability of analytical results, it is essential to implement robust quality control measures. This includes the use of certified reference materials, regular instrument calibration, and participation in proficiency testing programs. The development and validation of analytical methods specific to each alkyl compound are crucial for accurate purity determination. Method validation should include parameters such as linearity, precision, accuracy, and limits of detection and quantification.
Statistical process control (SPC) techniques can be employed to monitor and improve the purity of alkyl compounds during production. Control charts and trend analysis can help identify process variations and potential sources of impurities. Implementing a quality management system, such as ISO 9001 or Good Manufacturing Practices (GMP), can further enhance the overall quality control process and ensure consistent product purity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!