Aluminum Anode Surface Chemistry And Corrosion Control
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aluminum Anode Technology Background and Objectives
Aluminum anodes have been a cornerstone technology in cathodic protection systems since the early 20th century, with significant advancements occurring post-1950s. Initially developed for marine applications to protect ship hulls and offshore structures, aluminum anodes have evolved to become critical components in various industries including oil and gas, water treatment, and infrastructure protection. The technology leverages aluminum's inherent electrochemical properties, particularly its position in the galvanic series, which makes it an excellent sacrificial material for protecting more noble metals.
The evolution of aluminum anode technology has been marked by continuous improvements in alloy composition. Early aluminum anodes suffered from rapid self-corrosion and passivation issues, limiting their effectiveness. The introduction of activator elements such as zinc, indium, and mercury in the 1960s and 1970s significantly enhanced performance by breaking down passive oxide films and ensuring consistent electrochemical activity. Recent environmental concerns have driven research toward mercury-free formulations, with indium-activated anodes becoming the industry standard.
Surface chemistry plays a pivotal role in aluminum anode performance. The natural formation of Al₂O₃ passive layers on aluminum surfaces presents a fundamental challenge, as these layers increase electrical resistance and reduce electrochemical efficiency. Understanding and controlling the interface reactions between the aluminum substrate, its oxide layer, and the electrolyte environment has become a central focus of research efforts. Advanced surface modification techniques, including chemical etching, mechanical activation, and novel coating technologies, have emerged to address these challenges.
The primary technical objectives in aluminum anode development currently focus on several key areas. First, enhancing electrochemical efficiency to maximize current output per unit mass of material consumed. Second, improving the consistency and reliability of performance across varying environmental conditions, particularly in high-temperature and high-pressure scenarios common in deep-sea applications. Third, developing environmentally sustainable formulations that maintain performance while eliminating toxic components. Fourth, extending service life to reduce maintenance requirements and total ownership costs for protected assets.
Recent technological trends indicate a shift toward smart cathodic protection systems incorporating aluminum anodes with integrated monitoring capabilities. These systems aim to provide real-time data on anode consumption rates, protection levels, and remaining service life. Additionally, research into nano-structured aluminum anodes and composite materials promises to deliver step-change improvements in performance metrics, potentially revolutionizing the field of cathodic protection.
The global push toward sustainable infrastructure and extended asset lifespans has elevated the importance of effective corrosion control technologies, positioning aluminum anode research as a strategic priority for industries dependent on metallic infrastructure in corrosive environments.
The evolution of aluminum anode technology has been marked by continuous improvements in alloy composition. Early aluminum anodes suffered from rapid self-corrosion and passivation issues, limiting their effectiveness. The introduction of activator elements such as zinc, indium, and mercury in the 1960s and 1970s significantly enhanced performance by breaking down passive oxide films and ensuring consistent electrochemical activity. Recent environmental concerns have driven research toward mercury-free formulations, with indium-activated anodes becoming the industry standard.
Surface chemistry plays a pivotal role in aluminum anode performance. The natural formation of Al₂O₃ passive layers on aluminum surfaces presents a fundamental challenge, as these layers increase electrical resistance and reduce electrochemical efficiency. Understanding and controlling the interface reactions between the aluminum substrate, its oxide layer, and the electrolyte environment has become a central focus of research efforts. Advanced surface modification techniques, including chemical etching, mechanical activation, and novel coating technologies, have emerged to address these challenges.
The primary technical objectives in aluminum anode development currently focus on several key areas. First, enhancing electrochemical efficiency to maximize current output per unit mass of material consumed. Second, improving the consistency and reliability of performance across varying environmental conditions, particularly in high-temperature and high-pressure scenarios common in deep-sea applications. Third, developing environmentally sustainable formulations that maintain performance while eliminating toxic components. Fourth, extending service life to reduce maintenance requirements and total ownership costs for protected assets.
Recent technological trends indicate a shift toward smart cathodic protection systems incorporating aluminum anodes with integrated monitoring capabilities. These systems aim to provide real-time data on anode consumption rates, protection levels, and remaining service life. Additionally, research into nano-structured aluminum anodes and composite materials promises to deliver step-change improvements in performance metrics, potentially revolutionizing the field of cathodic protection.
The global push toward sustainable infrastructure and extended asset lifespans has elevated the importance of effective corrosion control technologies, positioning aluminum anode research as a strategic priority for industries dependent on metallic infrastructure in corrosive environments.
Market Analysis for Aluminum Anode Applications
The global market for aluminum anodes has experienced significant growth in recent years, driven primarily by increasing applications in cathodic protection systems across various industries. The market size was valued at approximately $3.2 billion in 2022 and is projected to reach $4.7 billion by 2028, representing a compound annual growth rate (CAGR) of 6.5% during the forecast period.
Marine applications continue to dominate the aluminum anode market, accounting for roughly 45% of the total market share. This dominance is attributed to the extensive use of aluminum anodes in protecting ship hulls, offshore platforms, and port facilities from corrosion in saltwater environments. The shipping industry's recovery post-pandemic and increasing global trade have further bolstered this segment.
The oil and gas sector represents the second-largest application area, constituting about 30% of the market. With expanding offshore drilling activities and aging pipeline infrastructure requiring protection, demand for aluminum anodes in this sector remains robust. The industry's focus on extending asset lifespans through effective corrosion control measures has created sustained demand.
Water treatment facilities and underground storage tanks collectively account for approximately 15% of the market, with growing environmental regulations driving adoption. The remaining 10% is distributed across various applications including power generation facilities, reinforced concrete structures, and emerging applications in renewable energy infrastructure.
Geographically, Asia-Pacific leads the market with a 38% share, driven by extensive maritime activities, rapid industrialization, and infrastructure development in countries like China, Japan, and South Korea. North America and Europe follow with 27% and 24% market shares respectively, where replacement demand in aging infrastructure supports market growth.
The competitive landscape features both large multinational corporations and specialized regional players. Major companies like Galvotec Alloys, Corrpro Companies Inc., and MATCOR have established strong market positions through vertical integration and comprehensive corrosion control solutions. Meanwhile, regional manufacturers in emerging markets are gaining ground by offering cost-competitive products tailored to local requirements.
Customer demand is increasingly shifting toward aluminum anodes with enhanced performance characteristics, including longer service life, improved current efficiency, and environmentally friendly compositions. This trend is driving research and development investments in advanced aluminum alloy formulations and surface treatment technologies to improve anode performance while reducing environmental impact.
Marine applications continue to dominate the aluminum anode market, accounting for roughly 45% of the total market share. This dominance is attributed to the extensive use of aluminum anodes in protecting ship hulls, offshore platforms, and port facilities from corrosion in saltwater environments. The shipping industry's recovery post-pandemic and increasing global trade have further bolstered this segment.
The oil and gas sector represents the second-largest application area, constituting about 30% of the market. With expanding offshore drilling activities and aging pipeline infrastructure requiring protection, demand for aluminum anodes in this sector remains robust. The industry's focus on extending asset lifespans through effective corrosion control measures has created sustained demand.
Water treatment facilities and underground storage tanks collectively account for approximately 15% of the market, with growing environmental regulations driving adoption. The remaining 10% is distributed across various applications including power generation facilities, reinforced concrete structures, and emerging applications in renewable energy infrastructure.
Geographically, Asia-Pacific leads the market with a 38% share, driven by extensive maritime activities, rapid industrialization, and infrastructure development in countries like China, Japan, and South Korea. North America and Europe follow with 27% and 24% market shares respectively, where replacement demand in aging infrastructure supports market growth.
The competitive landscape features both large multinational corporations and specialized regional players. Major companies like Galvotec Alloys, Corrpro Companies Inc., and MATCOR have established strong market positions through vertical integration and comprehensive corrosion control solutions. Meanwhile, regional manufacturers in emerging markets are gaining ground by offering cost-competitive products tailored to local requirements.
Customer demand is increasingly shifting toward aluminum anodes with enhanced performance characteristics, including longer service life, improved current efficiency, and environmentally friendly compositions. This trend is driving research and development investments in advanced aluminum alloy formulations and surface treatment technologies to improve anode performance while reducing environmental impact.
Current Challenges in Aluminum Surface Chemistry
Despite significant advancements in aluminum anode technology, several critical challenges persist in aluminum surface chemistry that impede optimal performance in energy storage applications and corrosion protection systems. The primary obstacle remains the formation of passive oxide films on aluminum surfaces, which while providing natural corrosion resistance, simultaneously increases electrical resistance and reduces electrochemical activity. This paradoxical behavior creates a fundamental challenge for applications requiring both corrosion resistance and electrical conductivity.
The instability of aluminum in aqueous electrolytes presents another significant hurdle. When exposed to water-based solutions, aluminum undergoes parasitic hydrogen evolution reactions, resulting in energy efficiency losses and potential safety hazards in battery applications. This reaction accelerates in alkaline environments, limiting the use of aluminum anodes in certain electrolyte systems despite their theoretical advantages.
Surface contamination from manufacturing processes introduces variability in aluminum anode performance. Residual oils, machining fluids, and atmospheric contaminants can create inconsistent surface chemistry profiles, leading to unpredictable corrosion rates and electrochemical behavior. The industry lacks standardized surface preparation protocols that can ensure reproducible surface conditions across different manufacturing batches.
Galvanic coupling effects when aluminum interfaces with other metals in multi-material systems create localized corrosion cells that accelerate degradation. This challenge is particularly pronounced in applications where aluminum must interface with more noble metals, creating design constraints for engineers developing integrated systems.
Temperature sensitivity represents another significant challenge, as aluminum's surface chemistry and corrosion behavior change dramatically across different operating temperatures. The protective oxide layer's properties vary with temperature fluctuations, creating reliability concerns for applications experiencing thermal cycling.
Recent research has identified the critical role of microstructure in determining surface reactivity. Grain boundaries, intermetallic particles, and crystallographic orientation significantly influence local electrochemical behavior, yet controlling these microstructural features during manufacturing remains difficult. The heterogeneous nature of commercial aluminum alloys creates microscopic regions with vastly different electrochemical properties.
Additionally, environmental factors such as chloride ions, pH variations, and dissolved oxygen content dramatically alter aluminum surface chemistry, making it challenging to predict performance across diverse operating environments. This environmental sensitivity necessitates extensive testing under various conditions, increasing development costs and timelines for new applications.
The instability of aluminum in aqueous electrolytes presents another significant hurdle. When exposed to water-based solutions, aluminum undergoes parasitic hydrogen evolution reactions, resulting in energy efficiency losses and potential safety hazards in battery applications. This reaction accelerates in alkaline environments, limiting the use of aluminum anodes in certain electrolyte systems despite their theoretical advantages.
Surface contamination from manufacturing processes introduces variability in aluminum anode performance. Residual oils, machining fluids, and atmospheric contaminants can create inconsistent surface chemistry profiles, leading to unpredictable corrosion rates and electrochemical behavior. The industry lacks standardized surface preparation protocols that can ensure reproducible surface conditions across different manufacturing batches.
Galvanic coupling effects when aluminum interfaces with other metals in multi-material systems create localized corrosion cells that accelerate degradation. This challenge is particularly pronounced in applications where aluminum must interface with more noble metals, creating design constraints for engineers developing integrated systems.
Temperature sensitivity represents another significant challenge, as aluminum's surface chemistry and corrosion behavior change dramatically across different operating temperatures. The protective oxide layer's properties vary with temperature fluctuations, creating reliability concerns for applications experiencing thermal cycling.
Recent research has identified the critical role of microstructure in determining surface reactivity. Grain boundaries, intermetallic particles, and crystallographic orientation significantly influence local electrochemical behavior, yet controlling these microstructural features during manufacturing remains difficult. The heterogeneous nature of commercial aluminum alloys creates microscopic regions with vastly different electrochemical properties.
Additionally, environmental factors such as chloride ions, pH variations, and dissolved oxygen content dramatically alter aluminum surface chemistry, making it challenging to predict performance across diverse operating environments. This environmental sensitivity necessitates extensive testing under various conditions, increasing development costs and timelines for new applications.
Current Surface Treatment Solutions for Aluminum Anodes
01 Surface treatment methods for aluminum anodes
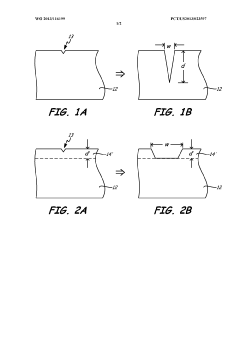
Various surface treatment methods can be applied to aluminum anodes to enhance their corrosion resistance and performance. These treatments include anodizing, chemical conversion coatings, and physical surface modifications that create protective oxide layers. These treatments alter the surface chemistry of aluminum to provide better resistance against corrosive environments while maintaining or improving the electrochemical properties necessary for anode functionality.- Surface treatments for aluminum anodes: Various surface treatments can be applied to aluminum anodes to enhance their corrosion resistance and performance. These treatments include chemical etching, anodizing, and the application of protective coatings. Such treatments modify the surface chemistry of aluminum anodes, creating a more stable oxide layer that protects against aggressive corrosion while maintaining the electrochemical activity necessary for anode functionality.
- Alloying elements to improve corrosion resistance: The addition of specific alloying elements to aluminum anodes can significantly improve their corrosion resistance and electrochemical performance. Elements such as zinc, indium, tin, and magnesium are commonly used to modify the surface chemistry and corrosion behavior of aluminum anodes. These alloying elements can help control the formation of protective oxide films, reduce pitting corrosion, and enhance the overall durability of aluminum anodes in various environments.
- Oxide film formation and stability: The formation and stability of oxide films on aluminum anode surfaces play a crucial role in their corrosion behavior. Research focuses on understanding the mechanisms of oxide film formation, factors affecting film stability, and methods to control film properties. Techniques such as controlled oxidation, chemical passivation, and electrochemical treatments can be used to develop more stable and protective oxide layers on aluminum anodes, thereby extending their service life and improving their performance.
- Environmental factors affecting aluminum anode corrosion: Various environmental factors significantly influence the corrosion behavior of aluminum anodes. These factors include pH, temperature, presence of aggressive ions (particularly chlorides), dissolved oxygen content, and flow conditions. Understanding how these environmental parameters affect the surface chemistry and corrosion mechanisms of aluminum anodes is essential for designing effective corrosion protection strategies and optimizing anode performance in specific applications.
- Novel composite and nanostructured aluminum anodes: Advanced research focuses on developing novel composite and nanostructured aluminum anodes with enhanced corrosion resistance and electrochemical performance. These innovative materials include aluminum-based composites reinforced with ceramic particles, carbon nanotubes, or other nanomaterials. Nanostructured aluminum anodes with controlled surface morphology and chemistry offer improved corrosion resistance, higher specific capacity, and better cycling stability compared to conventional aluminum anodes.
02 Aluminum alloy composition for corrosion resistance
The composition of aluminum alloys significantly affects their corrosion behavior when used as anodes. Specific alloying elements such as magnesium, zinc, indium, and gallium can be incorporated in precise proportions to enhance the electrochemical properties and corrosion resistance of aluminum anodes. These tailored compositions help control the formation of protective oxide films and influence the anodic dissolution behavior in various electrolytes.Expand Specific Solutions03 Protective coatings for aluminum anodes
Specialized coatings can be applied to aluminum anodes to provide enhanced protection against corrosion. These coatings include polymer-based layers, ceramic coatings, and composite materials that act as barriers against corrosive media while maintaining electrical conductivity where needed. The coatings modify the surface chemistry of aluminum anodes and can significantly extend their service life in aggressive environments.Expand Specific Solutions04 Electrochemical behavior of aluminum anodes in different environments
The electrochemical behavior of aluminum anodes varies significantly depending on the environment they are exposed to. Factors such as pH, temperature, presence of specific ions, and electrolyte composition affect the corrosion mechanisms and surface chemistry of aluminum anodes. Understanding these interactions is crucial for predicting anode performance and designing appropriate corrosion protection strategies for specific applications.Expand Specific Solutions05 Novel aluminum anode materials and structures
Innovative approaches to aluminum anode design include the development of novel material structures such as nanostructured surfaces, composite anodes, and functionally graded materials. These advanced materials feature engineered surface chemistry that provides superior corrosion resistance while maintaining or enhancing the electrochemical performance. Such innovations include porous structures, layered compositions, and surface-modified aluminum that offer improved performance in various applications including batteries, fuel cells, and cathodic protection systems.Expand Specific Solutions
Key Industry Players in Aluminum Anode Technology
The aluminum anode surface chemistry and corrosion control market is currently in a growth phase, with increasing applications in energy storage, automotive, and aerospace sectors. The global market size is estimated to exceed $5 billion, driven by demand for lightweight materials and sustainable energy solutions. Leading players include Henkel AG, BASF Corp, and Alcoa Inc., who are advancing corrosion-resistant coatings and treatments. Phinergy Ltd. is pioneering aluminum-air battery technology, while research institutions like Central South University and CSIR are developing next-generation surface treatments. Automotive manufacturers (Honda, Siemens) and aerospace companies (Safran, United Technologies) are driving innovation through application-specific requirements, creating a competitive landscape balanced between established chemical companies and emerging technology specialists.
BASF Corp.
Technical Solution: BASF has developed comprehensive aluminum surface treatment solutions through their CathoGuard® electrocoating technology specifically engineered for aluminum substrates. Their approach combines specialized pretreatment chemicals with advanced polymer coatings to create multi-layer protection systems. BASF's technology incorporates nano-ceramic conversion coatings that form covalent bonds with the aluminum substrate, providing exceptional adhesion and corrosion resistance. Their research has shown that these treatments can withstand over 2,500 hours in cyclic corrosion testing without significant degradation[5]. BASF has also pioneered water-based coating systems with self-healing capabilities through the incorporation of encapsulated corrosion inhibitors that release upon damage to the coating. Their solutions include specialized silane-based treatments that form nanoscale protective layers on aluminum surfaces, enhancing both corrosion resistance and paint adhesion properties.
Strengths: Comprehensive portfolio of solutions covering the entire aluminum protection process; strong R&D capabilities with continuous innovation in environmentally friendly technologies; global manufacturing and technical support network. Weaknesses: Some high-performance solutions may require multiple application steps; certain specialized treatments may have compatibility limitations with some manufacturing processes.
Beijing Institute of Aeronautical Materials
Technical Solution: The Beijing Institute of Aeronautical Materials (BIAM) has developed advanced aluminum surface protection technologies specifically for aerospace applications. Their research focuses on anodizing processes enhanced with rare earth elements that significantly improve corrosion resistance while maintaining fatigue properties. BIAM's technology incorporates nano-structured ceramic particles into anodic oxide layers, creating composite coatings with exceptional wear and corrosion resistance. Their studies have demonstrated that these modified anodic coatings can increase the corrosion potential of aluminum alloys by up to 300mV in chloride environments[9]. BIAM has also pioneered plasma electrolytic oxidation techniques that create super-hard ceramic-like surfaces on aluminum components, providing both corrosion and wear protection. Their multi-layer protection systems combine modified anodizing with specialized sealants containing corrosion inhibitors that provide active protection through controlled release mechanisms.
Strengths: Cutting-edge research specifically focused on high-performance aerospace applications; integration of nanotechnology with traditional surface treatment processes; solutions designed for extreme environmental conditions. Weaknesses: Some advanced technologies may require specialized equipment not widely available in general manufacturing; certain high-performance treatments may have higher processing costs than conventional methods.
Critical Patents in Aluminum Corrosion Prevention
Aluminum alloy surface treatment method and aluminum alloy thereby
PatentWO2024025330A1
Innovation
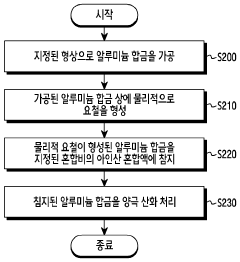
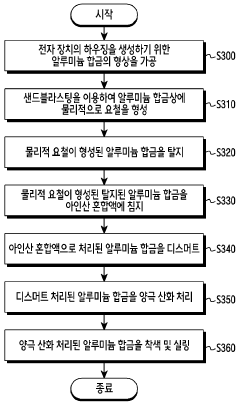
- A surface treatment method involving physical irregularities formed on aluminum alloys through sandblasting, followed by immersion in a phosphorous acid mixture containing phosphorous acid, sodium fluoride, and ammonium bifluoride, and subsequent anodizing, which creates a hydrophilic, corrosion-resistant, and durable anodizing layer.
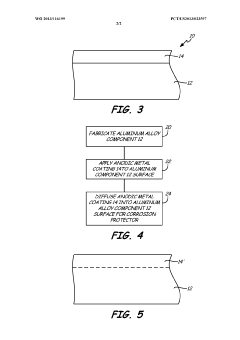
Surface implantation for corrosion protection of aluminum components
PatentWO2013116199A1
Innovation
- A surface region of aluminum alloy components is alloyed with anodic metals like zinc, beryllium, or magnesium through deposition methods followed by high-temperature diffusion anneal to enhance corrosion resistance, concentrating corrosion in the near-surface region and reducing stress concentration.
Environmental Impact of Aluminum Anode Technologies
The environmental implications of aluminum anode technologies extend far beyond their immediate applications in corrosion control systems. As aluminum anodes corrode sacrificially to protect other metals, they release aluminum ions and compounds into surrounding environments, creating potential ecological concerns that must be carefully evaluated.
Water systems receiving aluminum discharge from anodes may experience altered pH levels and increased aluminum concentrations. Research indicates that elevated aluminum levels in aquatic environments can adversely affect fish gill function and disrupt the reproductive cycles of various aquatic organisms. Studies have documented bioaccumulation of aluminum in certain species, potentially transferring these metals through food chains to higher trophic levels.
The manufacturing process of aluminum anodes also carries significant environmental considerations. Primary aluminum production is notably energy-intensive, with estimates suggesting that anode production contributes approximately 1.1 tons of CO2 equivalent per ton of aluminum produced. This carbon footprint becomes particularly relevant when evaluating the lifecycle environmental impact of aluminum anode systems compared to alternative corrosion protection methods.
Waste management presents another critical environmental challenge. Spent aluminum anodes contain residual metals and compounds that require proper disposal protocols. Improper handling can lead to soil contamination and groundwater pollution, particularly in marine environments where anodes are commonly deployed for ship hulls and offshore structures.
Recent technological innovations have focused on developing more environmentally sustainable aluminum anode formulations. These include aluminum alloys with reduced heavy metal content and improved efficiency ratios that minimize unnecessary material dissolution. Such advancements potentially reduce the environmental burden while maintaining effective corrosion protection performance.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of aluminum anode technologies. The European Union's Water Framework Directive and the United States Environmental Protection Agency have established guidelines for aluminum discharge limits in various water bodies. Compliance with these regulations drives research into environmentally optimized anode compositions and deployment strategies.
Life cycle assessment (LCA) studies comparing aluminum anodes with alternative corrosion protection systems reveal complex environmental trade-offs. While aluminum anodes may present certain environmental challenges during operation, their relatively simple installation requirements and lack of external power needs can result in lower overall environmental impacts compared to impressed current systems in specific applications.
Water systems receiving aluminum discharge from anodes may experience altered pH levels and increased aluminum concentrations. Research indicates that elevated aluminum levels in aquatic environments can adversely affect fish gill function and disrupt the reproductive cycles of various aquatic organisms. Studies have documented bioaccumulation of aluminum in certain species, potentially transferring these metals through food chains to higher trophic levels.
The manufacturing process of aluminum anodes also carries significant environmental considerations. Primary aluminum production is notably energy-intensive, with estimates suggesting that anode production contributes approximately 1.1 tons of CO2 equivalent per ton of aluminum produced. This carbon footprint becomes particularly relevant when evaluating the lifecycle environmental impact of aluminum anode systems compared to alternative corrosion protection methods.
Waste management presents another critical environmental challenge. Spent aluminum anodes contain residual metals and compounds that require proper disposal protocols. Improper handling can lead to soil contamination and groundwater pollution, particularly in marine environments where anodes are commonly deployed for ship hulls and offshore structures.
Recent technological innovations have focused on developing more environmentally sustainable aluminum anode formulations. These include aluminum alloys with reduced heavy metal content and improved efficiency ratios that minimize unnecessary material dissolution. Such advancements potentially reduce the environmental burden while maintaining effective corrosion protection performance.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of aluminum anode technologies. The European Union's Water Framework Directive and the United States Environmental Protection Agency have established guidelines for aluminum discharge limits in various water bodies. Compliance with these regulations drives research into environmentally optimized anode compositions and deployment strategies.
Life cycle assessment (LCA) studies comparing aluminum anodes with alternative corrosion protection systems reveal complex environmental trade-offs. While aluminum anodes may present certain environmental challenges during operation, their relatively simple installation requirements and lack of external power needs can result in lower overall environmental impacts compared to impressed current systems in specific applications.
Standardization and Testing Protocols for Corrosion Resistance
Standardized testing protocols are essential for evaluating and comparing the corrosion resistance of aluminum anodes across different applications and environments. The current landscape of testing methodologies reveals significant fragmentation, with various industries adopting distinct approaches based on their specific requirements. This inconsistency creates challenges in cross-industry comparisons and technology transfer.
The ASTM International has developed several standards specifically for aluminum corrosion testing, including ASTM G31 for immersion testing and ASTM G5 for potentiodynamic polarization measurements. These protocols provide baseline methodologies but often require adaptation for specific aluminum anode applications in batteries, fuel cells, or marine environments.
Electrochemical Impedance Spectroscopy (EIS) has emerged as a critical standardized technique for evaluating aluminum anode surface properties and corrosion behavior. The technique allows for non-destructive analysis of the electrode-electrolyte interface and provides valuable insights into corrosion mechanisms and kinetics. However, interpretation of EIS data requires sophisticated modeling and expertise, highlighting the need for more accessible standardized analysis frameworks.
Salt spray testing, governed by ASTM B117, remains a cornerstone for accelerated corrosion testing of aluminum components. Nevertheless, the correlation between accelerated testing results and real-world performance continues to be a significant challenge. Recent efforts have focused on developing more representative testing conditions that better simulate actual service environments, including cyclic corrosion tests that alternate between wet and dry conditions.
The development of in-situ monitoring techniques represents a promising frontier in standardization efforts. These methods enable real-time assessment of corrosion processes under operational conditions, providing more accurate data on performance degradation over time. Advanced techniques include in-situ Raman spectroscopy and synchrotron-based X-ray diffraction for monitoring surface film formation and breakdown.
International harmonization of testing standards remains an ongoing challenge. Organizations such as ISO, NACE International, and various national standards bodies have developed overlapping but distinct protocols. The International Aluminum Institute has initiated efforts to consolidate these approaches into a unified framework specifically for aluminum anode materials, though complete standardization remains elusive.
Digital twins and AI-based predictive models are increasingly being integrated into testing protocols, allowing for more sophisticated analysis of corrosion data and better prediction of long-term performance. These computational approaches help bridge the gap between accelerated testing and real-world service conditions, potentially reducing the time and cost associated with corrosion resistance validation.
The ASTM International has developed several standards specifically for aluminum corrosion testing, including ASTM G31 for immersion testing and ASTM G5 for potentiodynamic polarization measurements. These protocols provide baseline methodologies but often require adaptation for specific aluminum anode applications in batteries, fuel cells, or marine environments.
Electrochemical Impedance Spectroscopy (EIS) has emerged as a critical standardized technique for evaluating aluminum anode surface properties and corrosion behavior. The technique allows for non-destructive analysis of the electrode-electrolyte interface and provides valuable insights into corrosion mechanisms and kinetics. However, interpretation of EIS data requires sophisticated modeling and expertise, highlighting the need for more accessible standardized analysis frameworks.
Salt spray testing, governed by ASTM B117, remains a cornerstone for accelerated corrosion testing of aluminum components. Nevertheless, the correlation between accelerated testing results and real-world performance continues to be a significant challenge. Recent efforts have focused on developing more representative testing conditions that better simulate actual service environments, including cyclic corrosion tests that alternate between wet and dry conditions.
The development of in-situ monitoring techniques represents a promising frontier in standardization efforts. These methods enable real-time assessment of corrosion processes under operational conditions, providing more accurate data on performance degradation over time. Advanced techniques include in-situ Raman spectroscopy and synchrotron-based X-ray diffraction for monitoring surface film formation and breakdown.
International harmonization of testing standards remains an ongoing challenge. Organizations such as ISO, NACE International, and various national standards bodies have developed overlapping but distinct protocols. The International Aluminum Institute has initiated efforts to consolidate these approaches into a unified framework specifically for aluminum anode materials, though complete standardization remains elusive.
Digital twins and AI-based predictive models are increasingly being integrated into testing protocols, allowing for more sophisticated analysis of corrosion data and better prediction of long-term performance. These computational approaches help bridge the gap between accelerated testing and real-world service conditions, potentially reducing the time and cost associated with corrosion resistance validation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!