Binder Systems And Conductive Networks For Al-Ion Cathodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Al-Ion Battery Technology Background and Objectives
Aluminum-ion (Al-ion) batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in cost, safety, and environmental impact. Aluminum is the third most abundant element in the Earth's crust, making it significantly more accessible and economical than lithium. The evolution of Al-ion battery technology can be traced back to the early 2000s, with significant acceleration in research occurring over the past decade as limitations of lithium-ion technology became more apparent.
The technical trajectory of Al-ion batteries has been characterized by persistent challenges, particularly in cathode development. While aluminum anodes offer theoretical capacities of approximately 2980 mAh/g—nearly ten times that of conventional graphite anodes in lithium-ion batteries—cathode materials have struggled with issues of capacity, cycle life, and rate capability. These limitations have historically prevented Al-ion batteries from achieving commercial viability despite their theoretical advantages.
Binder systems and conductive networks represent critical components in the architecture of Al-ion cathodes. Traditional binder systems developed for lithium-ion batteries have proven inadequate for Al-ion applications due to the unique electrochemical environment created by aluminum electrolytes, which are typically more corrosive and operate through different intercalation mechanisms than their lithium counterparts.
The technical objectives for advancing binder systems and conductive networks for Al-ion cathodes include developing materials that can withstand the highly acidic environment of chloroaluminate electrolytes while maintaining structural integrity over extended cycling. Additionally, these materials must facilitate efficient electron transport throughout the cathode structure to maximize energy density and power capability.
Current research trends are focusing on novel polymer binders with enhanced chemical stability and mechanical properties, as well as advanced carbon-based conductive additives that can form robust networks within the cathode matrix. Graphene, carbon nanotubes, and their derivatives have shown particular promise in creating conductive pathways that remain stable during the insertion and extraction of complex aluminum-containing ions.
The anticipated technical milestones include achieving cathode materials with reversible capacities exceeding 100 mAh/g at practical current densities, cycle life of over 1000 cycles with minimal capacity degradation, and rate capabilities that enable rapid charging comparable to commercial lithium-ion systems. These advancements would position Al-ion technology as a viable alternative for grid storage applications and potentially for electric vehicles in the longer term.
As global energy demands continue to rise and sustainability concerns intensify, the development of effective binder systems and conductive networks for Al-ion cathodes represents a critical pathway toward next-generation energy storage solutions that are simultaneously high-performing, economical, and environmentally responsible.
The technical trajectory of Al-ion batteries has been characterized by persistent challenges, particularly in cathode development. While aluminum anodes offer theoretical capacities of approximately 2980 mAh/g—nearly ten times that of conventional graphite anodes in lithium-ion batteries—cathode materials have struggled with issues of capacity, cycle life, and rate capability. These limitations have historically prevented Al-ion batteries from achieving commercial viability despite their theoretical advantages.
Binder systems and conductive networks represent critical components in the architecture of Al-ion cathodes. Traditional binder systems developed for lithium-ion batteries have proven inadequate for Al-ion applications due to the unique electrochemical environment created by aluminum electrolytes, which are typically more corrosive and operate through different intercalation mechanisms than their lithium counterparts.
The technical objectives for advancing binder systems and conductive networks for Al-ion cathodes include developing materials that can withstand the highly acidic environment of chloroaluminate electrolytes while maintaining structural integrity over extended cycling. Additionally, these materials must facilitate efficient electron transport throughout the cathode structure to maximize energy density and power capability.
Current research trends are focusing on novel polymer binders with enhanced chemical stability and mechanical properties, as well as advanced carbon-based conductive additives that can form robust networks within the cathode matrix. Graphene, carbon nanotubes, and their derivatives have shown particular promise in creating conductive pathways that remain stable during the insertion and extraction of complex aluminum-containing ions.
The anticipated technical milestones include achieving cathode materials with reversible capacities exceeding 100 mAh/g at practical current densities, cycle life of over 1000 cycles with minimal capacity degradation, and rate capabilities that enable rapid charging comparable to commercial lithium-ion systems. These advancements would position Al-ion technology as a viable alternative for grid storage applications and potentially for electric vehicles in the longer term.
As global energy demands continue to rise and sustainability concerns intensify, the development of effective binder systems and conductive networks for Al-ion cathodes represents a critical pathway toward next-generation energy storage solutions that are simultaneously high-performing, economical, and environmentally responsible.
Market Analysis for Al-Ion Battery Applications
The aluminum-ion battery market is experiencing significant growth potential as an alternative to traditional lithium-ion batteries. Current market projections indicate that the global aluminum battery market could reach $15 billion by 2030, with a compound annual growth rate of approximately 6.5% between 2023 and 2030. This growth is primarily driven by increasing demand for sustainable energy storage solutions with improved safety profiles and reduced environmental impact.
The market for Al-ion batteries spans multiple sectors, with electric vehicles representing the largest potential application segment. As automotive manufacturers seek alternatives to lithium-ion technology due to supply chain concerns and raw material costs, Al-ion batteries with optimized cathode systems present a compelling value proposition. The commercial vehicle segment particularly values the potential fast-charging capabilities and enhanced safety characteristics of aluminum-based systems.
Grid-scale energy storage represents another substantial market opportunity, estimated to grow at 8.2% annually through 2028. Utility companies are increasingly exploring aluminum-ion technology for stationary storage applications where energy density requirements are less stringent than in transportation, but cycle life and cost efficiency are paramount. The integration of renewable energy sources further amplifies demand for advanced storage solutions.
Consumer electronics constitutes a third significant market segment, with manufacturers seeking battery technologies offering improved safety and potentially lower costs. This sector values the non-flammable nature of aluminum-ion electrolytes and the theoretical possibility of higher power density compared to conventional lithium-ion systems.
Market adoption faces several challenges, including performance limitations of current cathode materials and binding systems. Industry analysis indicates that improvements in cathode conductivity networks could accelerate market penetration by addressing cycle stability issues that currently limit commercial viability. Market research suggests that achieving 1000+ stable cycles would position Al-ion technology to capture up to 5% of the energy storage market within five years of commercialization.
Regional market distribution shows Asia-Pacific leading development efforts, with China accounting for over 40% of patents related to aluminum-ion battery technology. North American and European markets demonstrate growing interest, particularly in specialized applications where the safety advantages of aluminum-ion chemistry outweigh current performance limitations.
The competitive landscape includes both established battery manufacturers exploring aluminum-ion technology as portfolio diversification and startups focused exclusively on advancing Al-ion solutions. Strategic partnerships between material science companies and battery manufacturers are emerging as a dominant market entry strategy, with several joint ventures announced in the past 24 months.
The market for Al-ion batteries spans multiple sectors, with electric vehicles representing the largest potential application segment. As automotive manufacturers seek alternatives to lithium-ion technology due to supply chain concerns and raw material costs, Al-ion batteries with optimized cathode systems present a compelling value proposition. The commercial vehicle segment particularly values the potential fast-charging capabilities and enhanced safety characteristics of aluminum-based systems.
Grid-scale energy storage represents another substantial market opportunity, estimated to grow at 8.2% annually through 2028. Utility companies are increasingly exploring aluminum-ion technology for stationary storage applications where energy density requirements are less stringent than in transportation, but cycle life and cost efficiency are paramount. The integration of renewable energy sources further amplifies demand for advanced storage solutions.
Consumer electronics constitutes a third significant market segment, with manufacturers seeking battery technologies offering improved safety and potentially lower costs. This sector values the non-flammable nature of aluminum-ion electrolytes and the theoretical possibility of higher power density compared to conventional lithium-ion systems.
Market adoption faces several challenges, including performance limitations of current cathode materials and binding systems. Industry analysis indicates that improvements in cathode conductivity networks could accelerate market penetration by addressing cycle stability issues that currently limit commercial viability. Market research suggests that achieving 1000+ stable cycles would position Al-ion technology to capture up to 5% of the energy storage market within five years of commercialization.
Regional market distribution shows Asia-Pacific leading development efforts, with China accounting for over 40% of patents related to aluminum-ion battery technology. North American and European markets demonstrate growing interest, particularly in specialized applications where the safety advantages of aluminum-ion chemistry outweigh current performance limitations.
The competitive landscape includes both established battery manufacturers exploring aluminum-ion technology as portfolio diversification and startups focused exclusively on advancing Al-ion solutions. Strategic partnerships between material science companies and battery manufacturers are emerging as a dominant market entry strategy, with several joint ventures announced in the past 24 months.
Current Challenges in Al-Ion Cathode Development
Despite significant advancements in aluminum-ion battery technology, cathode development remains a critical bottleneck limiting commercial viability. Current Al-ion cathodes face several interconnected challenges related to binder systems and conductive networks that must be addressed to enable practical applications.
The primary challenge lies in the severe volume expansion and contraction during Al3+ intercalation/deintercalation processes, which far exceeds what is observed in lithium-ion systems. This volumetric instability leads to mechanical degradation of the electrode structure, causing particle isolation and capacity fading over repeated cycling. Conventional binders used in lithium-ion batteries, such as polyvinylidene fluoride (PVDF), lack the elasticity and adhesion strength required to accommodate these extreme volume changes.
Another significant obstacle is the limited electronic conductivity of most cathode active materials. Unlike graphite anodes, many promising cathode materials (particularly transition metal oxides and sulfides) exhibit poor intrinsic conductivity. This necessitates the development of specialized conductive networks that can maintain electrical pathways throughout cycling while withstanding the mechanical stresses induced by volume changes.
The highly corrosive nature of aluminum electrolytes, typically containing AlCl3 in ionic liquids, presents additional challenges for binder stability. Many conventional polymer binders undergo degradation when exposed to these electrolytes, leading to premature electrode failure. This chemical incompatibility severely restricts the range of viable binder materials and necessitates the development of corrosion-resistant alternatives.
Interface stability between the cathode active material, binder, and conductive additives represents another critical challenge. The formation of resistive interfacial layers during cycling can impede Al3+ transport and increase cell impedance. Current understanding of these interfacial phenomena remains limited, hampering the rational design of optimized electrode architectures.
Manufacturing scalability also presents significant hurdles. Laboratory-scale electrode preparation methods often involve complex procedures or exotic materials that are difficult to translate to industrial production. The development of scalable, cost-effective manufacturing processes for Al-ion cathodes with optimized binder systems and conductive networks remains largely unexplored.
Finally, there is a fundamental knowledge gap regarding structure-property relationships in Al-ion cathode systems. The interplay between binder properties, conductive network architecture, and electrochemical performance is poorly understood, making rational design approaches challenging. This necessitates comprehensive fundamental studies to establish design principles for next-generation Al-ion cathodes.
The primary challenge lies in the severe volume expansion and contraction during Al3+ intercalation/deintercalation processes, which far exceeds what is observed in lithium-ion systems. This volumetric instability leads to mechanical degradation of the electrode structure, causing particle isolation and capacity fading over repeated cycling. Conventional binders used in lithium-ion batteries, such as polyvinylidene fluoride (PVDF), lack the elasticity and adhesion strength required to accommodate these extreme volume changes.
Another significant obstacle is the limited electronic conductivity of most cathode active materials. Unlike graphite anodes, many promising cathode materials (particularly transition metal oxides and sulfides) exhibit poor intrinsic conductivity. This necessitates the development of specialized conductive networks that can maintain electrical pathways throughout cycling while withstanding the mechanical stresses induced by volume changes.
The highly corrosive nature of aluminum electrolytes, typically containing AlCl3 in ionic liquids, presents additional challenges for binder stability. Many conventional polymer binders undergo degradation when exposed to these electrolytes, leading to premature electrode failure. This chemical incompatibility severely restricts the range of viable binder materials and necessitates the development of corrosion-resistant alternatives.
Interface stability between the cathode active material, binder, and conductive additives represents another critical challenge. The formation of resistive interfacial layers during cycling can impede Al3+ transport and increase cell impedance. Current understanding of these interfacial phenomena remains limited, hampering the rational design of optimized electrode architectures.
Manufacturing scalability also presents significant hurdles. Laboratory-scale electrode preparation methods often involve complex procedures or exotic materials that are difficult to translate to industrial production. The development of scalable, cost-effective manufacturing processes for Al-ion cathodes with optimized binder systems and conductive networks remains largely unexplored.
Finally, there is a fundamental knowledge gap regarding structure-property relationships in Al-ion cathode systems. The interplay between binder properties, conductive network architecture, and electrochemical performance is poorly understood, making rational design approaches challenging. This necessitates comprehensive fundamental studies to establish design principles for next-generation Al-ion cathodes.
Current Binder and Conductive Network Solutions
01 Polymer binders for Al-ion cathodes
Various polymer binders are used in Al-ion battery cathodes to improve electrode stability and performance. These polymers help maintain structural integrity during charge-discharge cycles while facilitating ion transport. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that offer improved adhesion between active materials and current collectors. The selection of appropriate binders significantly impacts the electrochemical performance and cycle life of Al-ion batteries.- Polymer binders for Al-ion cathodes: Various polymer binders are used in Al-ion battery cathodes to improve electrode stability and performance. These polymers help maintain structural integrity during charge-discharge cycles while facilitating ion transport. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that offer improved adhesion between active materials and current collectors. The selection of appropriate binders significantly impacts electrode durability, cycling stability, and overall battery performance.
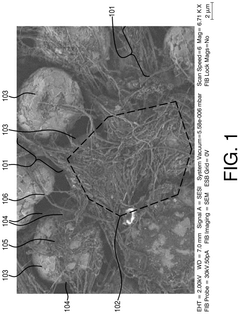
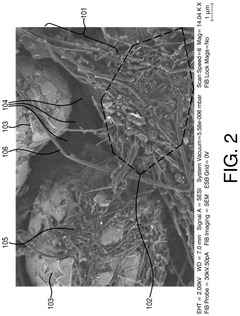
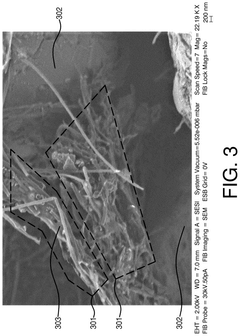
- Conductive additives for Al-ion cathode networks: Conductive additives are essential components in Al-ion cathodes to form effective electron transport networks. Carbon-based materials such as carbon black, graphene, carbon nanotubes, and conductive polymers are commonly used to enhance the electronic conductivity throughout the electrode. These additives create interconnected pathways that facilitate electron movement between active materials and current collectors, reducing internal resistance and improving rate capability. The type, amount, and distribution of conductive additives significantly influence the electrochemical performance of Al-ion batteries.
- Cathode active materials for Al-ion batteries: Various cathode active materials have been developed specifically for Al-ion batteries, including graphitic carbon, transition metal oxides, and Prussian blue analogs. These materials are designed to accommodate aluminum ion intercalation and deintercalation while maintaining structural stability. The selection of appropriate cathode materials affects the battery's voltage, capacity, and cycling performance. Research focuses on developing materials with high specific capacity, good rate capability, and long cycle life for Al-ion battery applications.
- Electrode fabrication techniques for Al-ion batteries: Specialized fabrication techniques are employed to create high-performance Al-ion battery cathodes. These include slurry preparation methods, coating processes, calendering, and thermal treatments that optimize the distribution of active materials, binders, and conductive additives. Advanced techniques such as freeze-drying, electrospinning, and 3D printing are also being explored to create novel electrode architectures. The fabrication process significantly impacts electrode porosity, thickness uniformity, and interfacial properties, which in turn affect battery performance metrics.
- Electrolyte compatibility with Al-ion cathode systems: The compatibility between electrolytes and cathode components is crucial for Al-ion battery performance. Electrolyte formulations must be carefully designed to prevent unwanted side reactions with cathode materials, binders, and conductive additives. Ionic liquids, non-aqueous solutions, and deep eutectic solvents are being investigated as suitable electrolytes for Al-ion systems. The electrolyte composition affects aluminum ion transport, interfacial stability, and the formation of protective surface films on electrode materials, all of which influence battery cycling stability and efficiency.
02 Conductive additives for Al-ion cathode networks
Conductive additives are essential components in Al-ion cathodes to form effective electron transport networks. Carbon-based materials such as carbon black, graphene, carbon nanotubes, and conductive polymers are commonly used to enhance the electronic conductivity throughout the electrode. These additives create interconnected pathways that facilitate electron movement between active materials and current collectors, reducing internal resistance and improving rate capability. The type, amount, and distribution of conductive additives significantly influence the power density and overall performance of Al-ion batteries.Expand Specific Solutions03 Cathode active materials for Al-ion batteries
Various cathode active materials have been developed specifically for Al-ion batteries, including graphitic carbon, transition metal oxides, and organic compounds. These materials are designed to accommodate the intercalation and de-intercalation of Al ions while maintaining structural stability. The selection of appropriate cathode materials affects the voltage, capacity, and cycling stability of Al-ion batteries. Recent developments focus on materials with layered structures that can effectively host Al ions while providing high energy density and good reversibility.Expand Specific Solutions04 Electrode fabrication techniques for Al-ion batteries
Specialized electrode fabrication techniques are crucial for creating high-performance Al-ion cathodes. These methods include slurry preparation, coating processes, calendering, and thermal treatments that optimize the distribution of active materials, binders, and conductive additives. Advanced techniques such as freeze-drying, spray coating, and 3D printing are being explored to create electrode architectures with enhanced ion transport pathways and mechanical stability. The fabrication process significantly impacts the electrode's porosity, tortuosity, and interfacial properties, which in turn affect battery performance.Expand Specific Solutions05 Electrolyte compatibility with Al-ion cathode systems
The compatibility between electrolytes and cathode components is critical for Al-ion battery performance. Electrolyte formulations must be carefully designed to prevent degradation of binder systems and conductive networks while enabling efficient Al-ion transport. Ionic liquids, non-aqueous solvents with aluminum salts, and chloroaluminate-based electrolytes are commonly used in Al-ion batteries. The electrolyte composition affects the formation of solid-electrolyte interfaces, which influence the stability of the cathode structure and the reversibility of electrochemical reactions. Optimizing electrolyte-cathode interactions is essential for achieving long cycle life and high coulombic efficiency.Expand Specific Solutions
Leading Companies in Al-Ion Battery Research
The Al-ion cathode binder systems and conductive networks market is in an early growth phase, characterized by significant research activity but limited commercial deployment. Market size remains modest but is expanding rapidly due to increasing interest in aluminum-ion battery technology as a potential alternative to lithium-ion systems. Technologically, the field is still evolving, with academic institutions (University of California, University of Waterloo, Washington State University) leading fundamental research while industrial players (Robert Bosch, A123 Systems, Samsung Electronics) focus on practical applications and scalability. Companies like Kuraray and BASF are leveraging their expertise in polymer chemistry to develop specialized binder systems, while research organizations such as CEA and Chinese Academy of Science are advancing conductive network technologies. The ecosystem reflects a collaborative environment where material science innovations are gradually transitioning toward commercial viability.
The Regents of the University of California
Technical Solution: The University of California has developed innovative binder systems for aluminum-ion battery cathodes that utilize a combination of water-soluble polymers and conductive additives. Their approach focuses on creating a robust 3D conductive network using carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) hybrid binders, which effectively accommodate the volume changes during Al-ion intercalation/deintercalation processes. The research team has demonstrated that these binder systems can maintain structural integrity over hundreds of cycles while facilitating efficient electron transport throughout the electrode. Their technology incorporates carbon nanotubes and graphene as conductive additives, which are dispersed homogeneously within the binder matrix to create percolation networks that enhance the overall conductivity of the cathode material. This approach has shown to reduce interfacial resistance and improve rate capability in Al-ion batteries.
Strengths: Superior cycling stability due to flexible polymer networks that accommodate volume changes; environmentally friendly water-based processing; excellent adhesion properties between active materials and current collectors. Weaknesses: Potential degradation of binder components in the highly corrosive AlCl3-based electrolytes commonly used in Al-ion batteries; higher cost associated with specialized conductive additives.
The University of Waterloo
Technical Solution: The University of Waterloo has pioneered a novel approach to Al-ion cathode binder systems through the development of self-healing polymer networks. Their technology utilizes functionalized polyacrylic acid (PAA) derivatives with pendant groups that form reversible bonds, allowing the binder matrix to repair microcracks that develop during cycling. This self-healing mechanism significantly extends electrode lifetime and maintains electrical connectivity throughout the cathode structure. The research team has integrated this binder system with a hierarchical conductive network comprising reduced graphene oxide (rGO) and carbon nanofibers, creating multiple electron transport pathways at different length scales. Their studies have demonstrated that this integrated approach can maintain over 85% capacity retention after 500 cycles in Al-ion cells, even at elevated current densities. The binder formulation also incorporates ionic liquids as plasticizers to enhance ion transport at the electrode-electrolyte interface, addressing one of the key limitations in Al-ion battery performance.
Strengths: Self-healing properties that maintain electrode integrity during cycling; hierarchical conductive network that provides multiple electron transport pathways; enhanced ionic conductivity at electrode-electrolyte interfaces. Weaknesses: Complex synthesis procedures that may challenge commercial scalability; potential compatibility issues with certain cathode active materials.
Key Patents in Al-Ion Cathode Materials
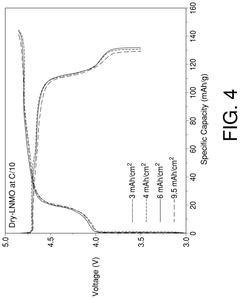
Cathodes for high voltage lithium-ion secondary battery and dry method for manufacture of same
PatentPendingUS20240379938A1
Innovation
- A dry binder fibrillation process is employed to fabricate cathodes using lithium transition metal oxide, fluoropolymer binder, and conductive carbon, forming a conducting structural web that enhances electronic conductivity and mechanical properties, thereby improving cycling stability and reducing parasitic reactions.
Supramolecular binders
PatentWO2024137606A2
Innovation
- The development of supramolecular binders that utilize chemical interactions between Lewis acid and Lewis base sites, allowing for the formation of a supramolecular polymer network without the need for covalent crosslinking, enabling the use of aqueous solvents and alternative electrolytes while maintaining mechanical integrity and energy density.
Environmental Impact of Al-Ion Battery Materials
The environmental impact of Al-ion battery materials represents a critical consideration in the development and deployment of aluminum-ion battery technology. Unlike lithium-ion batteries, which rely on scarce lithium resources with significant extraction impacts, aluminum is the most abundant metal in Earth's crust, comprising approximately 8% of its mass. This abundance translates to reduced environmental pressure from mining activities and lower habitat disruption compared to lithium extraction.
The binder systems and conductive networks used in Al-ion cathodes present their own environmental considerations. Traditional polymer binders like PVDF (polyvinylidene fluoride) require toxic solvents such as NMP (N-methyl-2-pyrrolidone) during manufacturing, posing environmental and health risks. However, emerging water-based binder systems for Al-ion cathodes significantly reduce these impacts by eliminating the need for harmful organic solvents, resulting in lower VOC emissions and reduced worker exposure to hazardous substances.
Carbon-based conductive additives commonly used in Al-ion cathodes, including carbon black and graphene, have varying environmental footprints. While carbon black production generates considerable CO2 emissions, graphene manufacturing can be energy-intensive. Recent advances in green synthesis methods for conductive networks, including biomass-derived carbon materials, offer promising pathways to reduce the environmental burden of these components.
The chloroaluminate ionic liquid electrolytes typical in Al-ion batteries present both advantages and challenges from an environmental perspective. These electrolytes are non-flammable, enhancing safety compared to conventional lithium-ion battery electrolytes. However, they often contain components that may be environmentally persistent and potentially toxic if released. Research into more environmentally benign electrolyte formulations remains an active area of investigation.
End-of-life considerations for Al-ion batteries show significant promise. The recyclability of aluminum is exceptional, with approximately 75% of all aluminum ever produced still in use today. This circular economy potential extends to the cathode materials, where the recovery of transition metals and graphitic materials is becoming increasingly feasible through hydrometallurgical processes, reducing waste and resource consumption.
Life cycle assessments of Al-ion battery systems indicate potentially lower global warming potential and reduced resource depletion compared to lithium-ion technologies, particularly when considering the full cradle-to-grave impacts. However, these advantages depend heavily on the specific binder systems and conductive networks employed, highlighting the importance of environmentally conscious materials selection in cathode design.
The binder systems and conductive networks used in Al-ion cathodes present their own environmental considerations. Traditional polymer binders like PVDF (polyvinylidene fluoride) require toxic solvents such as NMP (N-methyl-2-pyrrolidone) during manufacturing, posing environmental and health risks. However, emerging water-based binder systems for Al-ion cathodes significantly reduce these impacts by eliminating the need for harmful organic solvents, resulting in lower VOC emissions and reduced worker exposure to hazardous substances.
Carbon-based conductive additives commonly used in Al-ion cathodes, including carbon black and graphene, have varying environmental footprints. While carbon black production generates considerable CO2 emissions, graphene manufacturing can be energy-intensive. Recent advances in green synthesis methods for conductive networks, including biomass-derived carbon materials, offer promising pathways to reduce the environmental burden of these components.
The chloroaluminate ionic liquid electrolytes typical in Al-ion batteries present both advantages and challenges from an environmental perspective. These electrolytes are non-flammable, enhancing safety compared to conventional lithium-ion battery electrolytes. However, they often contain components that may be environmentally persistent and potentially toxic if released. Research into more environmentally benign electrolyte formulations remains an active area of investigation.
End-of-life considerations for Al-ion batteries show significant promise. The recyclability of aluminum is exceptional, with approximately 75% of all aluminum ever produced still in use today. This circular economy potential extends to the cathode materials, where the recovery of transition metals and graphitic materials is becoming increasingly feasible through hydrometallurgical processes, reducing waste and resource consumption.
Life cycle assessments of Al-ion battery systems indicate potentially lower global warming potential and reduced resource depletion compared to lithium-ion technologies, particularly when considering the full cradle-to-grave impacts. However, these advantages depend heavily on the specific binder systems and conductive networks employed, highlighting the importance of environmentally conscious materials selection in cathode design.
Manufacturing Scalability of Al-Ion Cathodes
The scalability of manufacturing processes for Al-ion cathodes represents a critical challenge in transitioning this promising technology from laboratory settings to commercial production. Current manufacturing approaches for Al-ion battery cathodes face significant limitations when considered for industrial-scale implementation. The primary bottleneck lies in the integration of binder systems and conductive networks that can maintain performance while being amenable to mass production techniques.
Traditional slurry-based electrode manufacturing processes, widely used in lithium-ion battery production, require substantial adaptation for Al-ion systems due to the unique electrochemical properties and structural requirements of Al-ion cathodes. The binder systems that perform adequately in small-scale laboratory demonstrations often exhibit compromised adhesion and mechanical stability when scaled to larger electrode dimensions and higher production speeds.
Conductive additives such as carbon black and graphene, which are essential for creating efficient electron transport networks within the cathode structure, present mixing and dispersion challenges at industrial scales. The homogeneity achieved in laboratory-scale mixing cannot be easily replicated in large batch processes, leading to performance inconsistencies across the manufactured cathodes.
Roll-to-roll manufacturing compatibility represents another significant hurdle. The rheological properties of Al-ion cathode slurries containing appropriate binders and conductive networks must be precisely controlled to enable consistent coating on current collectors at high speeds. Current formulations often exhibit either excessive viscosity that impedes coating uniformity or insufficient cohesion that compromises structural integrity during drying and calendering steps.
The drying processes for Al-ion cathode materials require careful optimization to prevent cracking and delamination while maintaining the desired porosity and conductive network integrity. Conventional drying parameters established for lithium-ion manufacturing lines prove inadequate for Al-ion systems, necessitating the development of specialized protocols that can be implemented in existing production infrastructure.
Post-processing steps such as calendering and slitting also present unique challenges for Al-ion cathodes. The mechanical properties imparted by current binder systems often result in either excessive brittleness or insufficient compression response, compromising the electrochemical performance and physical durability of the final electrodes when processed using standard equipment parameters.
Addressing these manufacturing scalability challenges requires interdisciplinary approaches combining materials science innovations with process engineering adaptations. Development of specialized binder systems that maintain adhesion and flexibility while enabling high-speed processing will be crucial for successful commercialization of Al-ion battery technology.
Traditional slurry-based electrode manufacturing processes, widely used in lithium-ion battery production, require substantial adaptation for Al-ion systems due to the unique electrochemical properties and structural requirements of Al-ion cathodes. The binder systems that perform adequately in small-scale laboratory demonstrations often exhibit compromised adhesion and mechanical stability when scaled to larger electrode dimensions and higher production speeds.
Conductive additives such as carbon black and graphene, which are essential for creating efficient electron transport networks within the cathode structure, present mixing and dispersion challenges at industrial scales. The homogeneity achieved in laboratory-scale mixing cannot be easily replicated in large batch processes, leading to performance inconsistencies across the manufactured cathodes.
Roll-to-roll manufacturing compatibility represents another significant hurdle. The rheological properties of Al-ion cathode slurries containing appropriate binders and conductive networks must be precisely controlled to enable consistent coating on current collectors at high speeds. Current formulations often exhibit either excessive viscosity that impedes coating uniformity or insufficient cohesion that compromises structural integrity during drying and calendering steps.
The drying processes for Al-ion cathode materials require careful optimization to prevent cracking and delamination while maintaining the desired porosity and conductive network integrity. Conventional drying parameters established for lithium-ion manufacturing lines prove inadequate for Al-ion systems, necessitating the development of specialized protocols that can be implemented in existing production infrastructure.
Post-processing steps such as calendering and slitting also present unique challenges for Al-ion cathodes. The mechanical properties imparted by current binder systems often result in either excessive brittleness or insufficient compression response, compromising the electrochemical performance and physical durability of the final electrodes when processed using standard equipment parameters.
Addressing these manufacturing scalability challenges requires interdisciplinary approaches combining materials science innovations with process engineering adaptations. Development of specialized binder systems that maintain adhesion and flexibility while enabling high-speed processing will be crucial for successful commercialization of Al-ion battery technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!