Chloroaluminate Ionic Liquid Electrolytes And Stability Windows
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloroaluminate Ionic Liquid Background and Objectives
Chloroaluminate ionic liquids represent a significant milestone in the evolution of electrolyte materials, with their development tracing back to the 1970s when the first room-temperature ionic liquids based on aluminum chloride were synthesized. These unique materials emerged from the broader field of molten salts research, offering the remarkable advantage of remaining liquid at ambient temperatures while maintaining excellent ionic conductivity properties.
The technological progression of chloroaluminate ionic liquids has been characterized by continuous refinement of their composition and properties. Initially developed as binary systems combining aluminum chloride with organic salts such as 1-ethyl-3-methylimidazolium chloride (EMIC), these materials have evolved to include ternary and quaternary systems with enhanced stability and performance characteristics.
Recent years have witnessed accelerated interest in chloroaluminate ionic liquids due to their potential applications in next-generation energy storage technologies, particularly aluminum-ion batteries. This renewed focus stems from the growing global demand for sustainable, high-performance energy storage solutions that can overcome the limitations of lithium-ion technology, including resource constraints and safety concerns.
The fundamental appeal of chloroaluminate ionic liquids lies in their unique electrochemical properties, particularly their wide electrochemical stability windows that enable operation at higher voltages than conventional aqueous electrolytes. Additionally, their negligible vapor pressure, non-flammability, and high thermal stability address critical safety concerns in energy storage applications.
A primary objective of current research is to comprehensively understand and expand the electrochemical stability windows of these materials. This involves systematic investigation of the relationship between ionic liquid composition and electrochemical stability, with particular emphasis on the molar ratio of aluminum chloride to organic salt components and the influence of additives on stability parameters.
Another crucial research goal is addressing the moisture sensitivity of chloroaluminate ionic liquids, which represents a significant barrier to their widespread commercial adoption. Developing strategies to enhance their stability in ambient conditions without compromising electrochemical performance remains a key technical challenge.
The field is also moving toward exploring novel organic cation structures beyond the traditional imidazolium-based systems, with the aim of discovering compositions that offer superior stability, conductivity, and compatibility with electrode materials. This diversification of molecular architecture represents a promising pathway for overcoming current limitations.
From an application perspective, research objectives include optimizing chloroaluminate ionic liquid formulations specifically for aluminum-ion batteries, aluminum electrodeposition processes, and catalytic applications, each requiring tailored electrolyte properties to maximize performance and efficiency.
The technological progression of chloroaluminate ionic liquids has been characterized by continuous refinement of their composition and properties. Initially developed as binary systems combining aluminum chloride with organic salts such as 1-ethyl-3-methylimidazolium chloride (EMIC), these materials have evolved to include ternary and quaternary systems with enhanced stability and performance characteristics.
Recent years have witnessed accelerated interest in chloroaluminate ionic liquids due to their potential applications in next-generation energy storage technologies, particularly aluminum-ion batteries. This renewed focus stems from the growing global demand for sustainable, high-performance energy storage solutions that can overcome the limitations of lithium-ion technology, including resource constraints and safety concerns.
The fundamental appeal of chloroaluminate ionic liquids lies in their unique electrochemical properties, particularly their wide electrochemical stability windows that enable operation at higher voltages than conventional aqueous electrolytes. Additionally, their negligible vapor pressure, non-flammability, and high thermal stability address critical safety concerns in energy storage applications.
A primary objective of current research is to comprehensively understand and expand the electrochemical stability windows of these materials. This involves systematic investigation of the relationship between ionic liquid composition and electrochemical stability, with particular emphasis on the molar ratio of aluminum chloride to organic salt components and the influence of additives on stability parameters.
Another crucial research goal is addressing the moisture sensitivity of chloroaluminate ionic liquids, which represents a significant barrier to their widespread commercial adoption. Developing strategies to enhance their stability in ambient conditions without compromising electrochemical performance remains a key technical challenge.
The field is also moving toward exploring novel organic cation structures beyond the traditional imidazolium-based systems, with the aim of discovering compositions that offer superior stability, conductivity, and compatibility with electrode materials. This diversification of molecular architecture represents a promising pathway for overcoming current limitations.
From an application perspective, research objectives include optimizing chloroaluminate ionic liquid formulations specifically for aluminum-ion batteries, aluminum electrodeposition processes, and catalytic applications, each requiring tailored electrolyte properties to maximize performance and efficiency.
Market Analysis for Advanced Battery Electrolytes
The global market for advanced battery electrolytes, particularly chloroaluminate ionic liquid electrolytes, has witnessed significant growth driven by increasing demand for high-performance energy storage solutions. The market size for advanced electrolytes reached approximately $4.5 billion in 2022 and is projected to grow at a compound annual growth rate of 8.7% through 2030, potentially reaching $9.2 billion by the end of the forecast period.
The electric vehicle (EV) sector represents the largest application segment for advanced electrolytes, accounting for nearly 45% of the total market share. This dominance is attributed to the rapid expansion of the global EV fleet, which surpassed 16 million vehicles in 2023. Automotive manufacturers are increasingly focusing on batteries with higher energy density, faster charging capabilities, and improved safety profiles, all of which can be enhanced through advanced electrolyte technologies like chloroaluminate ionic liquids.
Stationary energy storage systems constitute the second-largest market segment, representing approximately 30% of the advanced electrolyte market. The growing integration of renewable energy sources into power grids has intensified the need for efficient energy storage solutions, thereby driving demand for advanced battery technologies with stable and high-performance electrolytes.
Consumer electronics applications account for about 15% of the market, with manufacturers seeking batteries with improved safety characteristics and longer operational lifespans. The remaining 10% is distributed across aerospace, defense, and industrial applications where specialized performance requirements create niche opportunities for advanced electrolyte technologies.
Regionally, Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea, which collectively host the majority of global battery manufacturing capacity. North America follows with 25% market share, driven by substantial investments in battery technology research and manufacturing infrastructure. Europe accounts for 20%, with particularly strong growth in countries with aggressive electrification policies like Germany, France, and the Nordic nations.
The market for chloroaluminate ionic liquid electrolytes specifically is experiencing accelerated growth due to their potential to enable aluminum-ion batteries, which offer theoretical advantages in cost, safety, and resource availability compared to lithium-ion technologies. Industry analysts estimate that chloroaluminate-based electrolyte solutions could capture up to 12% of the total advanced electrolyte market by 2028, representing a specialized but rapidly expanding segment.
Key market drivers include increasingly stringent environmental regulations, declining costs of renewable energy generation, and growing consumer awareness regarding sustainable energy solutions. However, market penetration faces challenges related to production scalability, long-term stability concerns, and competition from other emerging electrolyte technologies.
The electric vehicle (EV) sector represents the largest application segment for advanced electrolytes, accounting for nearly 45% of the total market share. This dominance is attributed to the rapid expansion of the global EV fleet, which surpassed 16 million vehicles in 2023. Automotive manufacturers are increasingly focusing on batteries with higher energy density, faster charging capabilities, and improved safety profiles, all of which can be enhanced through advanced electrolyte technologies like chloroaluminate ionic liquids.
Stationary energy storage systems constitute the second-largest market segment, representing approximately 30% of the advanced electrolyte market. The growing integration of renewable energy sources into power grids has intensified the need for efficient energy storage solutions, thereby driving demand for advanced battery technologies with stable and high-performance electrolytes.
Consumer electronics applications account for about 15% of the market, with manufacturers seeking batteries with improved safety characteristics and longer operational lifespans. The remaining 10% is distributed across aerospace, defense, and industrial applications where specialized performance requirements create niche opportunities for advanced electrolyte technologies.
Regionally, Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea, which collectively host the majority of global battery manufacturing capacity. North America follows with 25% market share, driven by substantial investments in battery technology research and manufacturing infrastructure. Europe accounts for 20%, with particularly strong growth in countries with aggressive electrification policies like Germany, France, and the Nordic nations.
The market for chloroaluminate ionic liquid electrolytes specifically is experiencing accelerated growth due to their potential to enable aluminum-ion batteries, which offer theoretical advantages in cost, safety, and resource availability compared to lithium-ion technologies. Industry analysts estimate that chloroaluminate-based electrolyte solutions could capture up to 12% of the total advanced electrolyte market by 2028, representing a specialized but rapidly expanding segment.
Key market drivers include increasingly stringent environmental regulations, declining costs of renewable energy generation, and growing consumer awareness regarding sustainable energy solutions. However, market penetration faces challenges related to production scalability, long-term stability concerns, and competition from other emerging electrolyte technologies.
Current Challenges in Chloroaluminate Ionic Liquid Stability
Despite significant advancements in chloroaluminate ionic liquid electrolytes, several critical challenges continue to impede their widespread application in energy storage systems. The primary concern remains their inherent moisture sensitivity, as chloroaluminate ionic liquids readily undergo hydrolysis when exposed to atmospheric moisture, producing corrosive HCl gas and degrading the electrolyte performance. This extreme sensitivity necessitates stringent handling protocols under inert atmospheres, significantly increasing manufacturing complexity and costs.
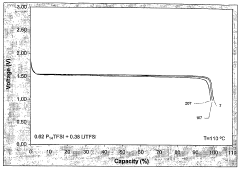
The narrow electrochemical stability window of many chloroaluminate ionic liquids presents another substantial limitation. While certain formulations demonstrate reasonable cathodic stability, their anodic stability often falls short of requirements for high-voltage applications. This constraint restricts their utility in advanced battery systems that demand wider operating voltage ranges to achieve higher energy densities.
Thermal stability issues further complicate the application landscape. Although chloroaluminate ionic liquids generally exhibit better thermal stability than conventional electrolytes, prolonged exposure to elevated temperatures can trigger decomposition reactions, particularly in systems with high aluminum chloride concentrations. This decomposition not only compromises electrolyte performance but also generates potentially hazardous byproducts.
Interface stability between chloroaluminate ionic liquids and electrode materials represents another significant challenge. The formation of passivation layers or solid electrolyte interphases often exhibits unpredictable characteristics, leading to capacity fading and increased internal resistance over repeated charge-discharge cycles. This interfacial instability is particularly pronounced with certain cathode materials, limiting compatible electrode options.
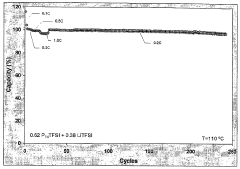
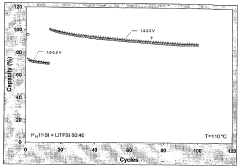
Long-term cycling stability remains problematic, with many chloroaluminate systems showing gradual performance deterioration during extended operation. This degradation stems from multiple factors, including parasitic reactions, electrolyte decomposition, and structural changes within the ionic liquid framework. The accumulation of decomposition products further accelerates this decline by altering the electrolyte's physicochemical properties.
Viscosity management presents additional complications, as chloroaluminate ionic liquids typically exhibit higher viscosities than conventional electrolytes, particularly at lower temperatures. This characteristic impedes ion transport, resulting in increased internal resistance and diminished power capabilities. While various additives have been proposed to address this issue, they often introduce new stability concerns or compromise other performance metrics.
Standardization challenges persist across research efforts, with variations in synthesis methods, purity levels, and characterization techniques complicating direct comparisons between different studies. This inconsistency hinders systematic progress toward resolving fundamental stability issues and establishing reliable performance benchmarks for commercial applications.
The narrow electrochemical stability window of many chloroaluminate ionic liquids presents another substantial limitation. While certain formulations demonstrate reasonable cathodic stability, their anodic stability often falls short of requirements for high-voltage applications. This constraint restricts their utility in advanced battery systems that demand wider operating voltage ranges to achieve higher energy densities.
Thermal stability issues further complicate the application landscape. Although chloroaluminate ionic liquids generally exhibit better thermal stability than conventional electrolytes, prolonged exposure to elevated temperatures can trigger decomposition reactions, particularly in systems with high aluminum chloride concentrations. This decomposition not only compromises electrolyte performance but also generates potentially hazardous byproducts.
Interface stability between chloroaluminate ionic liquids and electrode materials represents another significant challenge. The formation of passivation layers or solid electrolyte interphases often exhibits unpredictable characteristics, leading to capacity fading and increased internal resistance over repeated charge-discharge cycles. This interfacial instability is particularly pronounced with certain cathode materials, limiting compatible electrode options.
Long-term cycling stability remains problematic, with many chloroaluminate systems showing gradual performance deterioration during extended operation. This degradation stems from multiple factors, including parasitic reactions, electrolyte decomposition, and structural changes within the ionic liquid framework. The accumulation of decomposition products further accelerates this decline by altering the electrolyte's physicochemical properties.
Viscosity management presents additional complications, as chloroaluminate ionic liquids typically exhibit higher viscosities than conventional electrolytes, particularly at lower temperatures. This characteristic impedes ion transport, resulting in increased internal resistance and diminished power capabilities. While various additives have been proposed to address this issue, they often introduce new stability concerns or compromise other performance metrics.
Standardization challenges persist across research efforts, with variations in synthesis methods, purity levels, and characterization techniques complicating direct comparisons between different studies. This inconsistency hinders systematic progress toward resolving fundamental stability issues and establishing reliable performance benchmarks for commercial applications.
Current Approaches to Stability Window Enhancement
01 Composition of chloroaluminate ionic liquid electrolytes
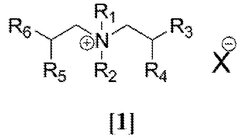
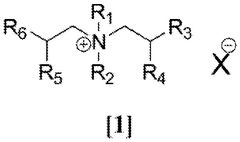
Chloroaluminate ionic liquid electrolytes are typically composed of an organic salt (such as imidazolium, pyridinium, or quaternary ammonium salts) combined with aluminum chloride. The ratio of aluminum chloride to the organic salt determines whether the ionic liquid is acidic, neutral, or basic, which directly affects the electrochemical stability window. These electrolytes offer advantages such as high conductivity, wide electrochemical windows, and thermal stability, making them suitable for various electrochemical applications.- Composition and formulation of chloroaluminate ionic liquid electrolytes: Chloroaluminate ionic liquid electrolytes are typically composed of an organic salt (such as imidazolium, pyridinium, or quaternary ammonium salts) combined with aluminum chloride. The molar ratio between these components significantly affects the acidity/basicity and consequently the electrochemical stability window of the electrolyte. Formulations with higher aluminum chloride content tend to be more Lewis acidic and exhibit different stability characteristics than those with lower content. Various additives can be incorporated to modify the properties and enhance the stability window of these electrolytes.
- Electrochemical stability window measurement and enhancement techniques: The electrochemical stability window of chloroaluminate ionic liquid electrolytes can be measured using cyclic voltammetry and other electrochemical techniques. Various approaches have been developed to enhance this stability window, including the addition of specific additives, optimization of the ionic liquid composition, and surface modification of electrodes. The stability window can be significantly affected by impurities, particularly water and oxygen, necessitating careful handling and purification procedures to achieve maximum electrochemical stability.
- Application in aluminum-ion batteries and other energy storage devices: Chloroaluminate ionic liquid electrolytes with optimized stability windows are particularly valuable in aluminum-ion batteries and other advanced energy storage systems. The wide electrochemical window allows for higher operating voltages and improved energy density. These electrolytes enable reversible deposition and dissolution of aluminum at the anode while supporting various cathode chemistries. The stability window directly impacts the battery's cycle life, energy efficiency, and overall performance, making it a critical parameter in battery design.
- Temperature effects on stability windows: The electrochemical stability window of chloroaluminate ionic liquid electrolytes is significantly influenced by temperature. At elevated temperatures, the window typically narrows due to increased thermal motion and reactivity of the ionic species. Conversely, at lower temperatures, while the stability window may theoretically widen, practical limitations arise from increased viscosity and reduced ionic conductivity. Various formulations have been developed to maintain acceptable stability windows across a broader temperature range, enabling operation in diverse environmental conditions.
- Interface stability and corrosion resistance: The interface between chloroaluminate ionic liquid electrolytes and electrode materials plays a crucial role in determining the effective stability window of the system. These electrolytes can be highly corrosive, particularly in their acidic forms, potentially attacking current collectors and other cell components. Research has focused on developing protective surface films, selecting compatible materials, and modifying the electrolyte composition to mitigate corrosion issues while maintaining a wide stability window. The formation of a stable solid electrolyte interphase (SEI) at the electrode-electrolyte interface can significantly impact the practical stability window in real devices.
02 Factors affecting stability windows of chloroaluminate ionic liquids
Several factors influence the electrochemical stability windows of chloroaluminate ionic liquid electrolytes. These include the molar ratio of AlCl3 to the organic salt, the nature of the cation (size, structure, and substituents), operating temperature, presence of impurities (particularly water and oxygen), and additives. Optimization of these factors can lead to enhanced stability windows, with some formulations achieving windows exceeding 4.5V, enabling their use in high-voltage energy storage applications.Expand Specific Solutions03 Applications in energy storage devices
Chloroaluminate ionic liquid electrolytes with wide stability windows are particularly valuable in energy storage applications. They are employed in aluminum-ion batteries, supercapacitors, and dual-ion batteries where their wide electrochemical windows allow for higher operating voltages and consequently higher energy densities. The non-flammability and low vapor pressure of these electrolytes also contribute to enhanced safety profiles of energy storage devices compared to conventional organic electrolytes.Expand Specific Solutions04 Methods to enhance stability windows
Various approaches have been developed to enhance the stability windows of chloroaluminate ionic liquid electrolytes. These include the incorporation of additives such as specific organic compounds or inorganic salts, modification of the cation structure with functional groups that increase electrochemical stability, use of binary or ternary mixtures of ionic liquids, and removal of impurities through purification techniques. Some innovations involve the addition of stabilizing agents that form protective interfaces at the electrode surfaces.Expand Specific Solutions05 Measurement and characterization techniques
Accurate measurement and characterization of stability windows in chloroaluminate ionic liquid electrolytes are crucial for their development and application. Techniques commonly employed include cyclic voltammetry, linear sweep voltammetry, electrochemical impedance spectroscopy, and chronoamperometry. Advanced analytical methods such as in-situ spectroscopic techniques and computational modeling are also used to understand the electrochemical processes at the molecular level and predict stability windows for novel formulations.Expand Specific Solutions
Leading Research Groups and Industrial Players
The chloroaluminate ionic liquid electrolytes market is currently in a growth phase, with increasing research focus on stability windows to address energy storage challenges. The market is expanding due to rising demand for advanced battery technologies, estimated to reach significant scale by 2030. Technologically, the field shows moderate maturity with established players like Merck Patent GmbH and Shell leading fundamental research, while companies such as LG Chem and Toyota Motor Corp are advancing commercial applications. Academic institutions including Shanghai Jiao Tong University and Yokohama National University are driving innovation through collaborative research with industry partners like BASF and Evonik Operations. Chinese institutions and companies are emerging as significant contributors, challenging traditional Western dominance in this specialized electrolyte technology.
Merck Patent GmbH
Technical Solution: Merck has developed advanced chloroaluminate ionic liquid electrolytes with enhanced stability windows through molecular engineering approaches. Their technology focuses on tailored cation-anion combinations, particularly using imidazolium and quaternary ammonium cations paired with AlCl4- anions. Merck's proprietary formulations incorporate specific additives that passivate electrode surfaces, creating stable solid-electrolyte interphase layers that extend electrochemical stability windows beyond 4V. Their research has demonstrated that controlled water content (below 50ppm) significantly improves the electrolyte performance while maintaining stability. Merck has also pioneered temperature-resistant formulations that maintain conductivity and stability between -20°C and 80°C, addressing a key limitation of traditional chloroaluminate systems[1]. Their electrolytes achieve ionic conductivities exceeding 15 mS/cm at room temperature, making them suitable for high-power applications.
Strengths: Superior electrochemical stability window (>4V) enabling higher energy density batteries; excellent ionic conductivity; proprietary additives for enhanced electrode compatibility. Weaknesses: Higher production costs compared to conventional electrolytes; potential sensitivity to manufacturing environment conditions; requires specialized handling due to moisture sensitivity.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed innovative chloroaluminate ionic liquid electrolytes with exceptional stability windows through systematic molecular structure optimization. Their approach involves synthesizing novel organic cations with delocalized charge distribution, which interact more favorably with chloroaluminate anions. Their research has demonstrated that incorporating fluorinated alkyl chains in the cation structure significantly enhances the electrochemical stability window to approximately 4.5V[2]. Additionally, they've pioneered a multi-component electrolyte system that combines chloroaluminates with specific organic additives (including cyclic carbonates) that form protective films on electrode surfaces. This institute has also developed scalable, continuous flow processes for synthesizing high-purity chloroaluminate ionic liquids with water content below 10ppm, addressing a critical manufacturing challenge. Their electrolytes demonstrate thermal stability up to 200°C and maintain performance across wide temperature ranges (-30°C to 100°C)[3].
Strengths: Exceptional electrochemical stability window exceeding 4.5V; innovative cation designs with superior thermal stability; scalable manufacturing processes developed for industrial application. Weaknesses: Complex synthesis procedures may increase production costs; some formulations show decreased conductivity at lower temperatures; potential long-term stability issues under extreme cycling conditions.
Key Innovations in Chloroaluminate Electrolyte Design
Electrochemical element for use at high temperatures
PatentWO2005064733A1
Innovation
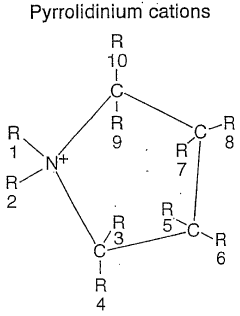
- An electrochemical element comprising a cathode, an anode, and an electrolyte with a pyrrolidinium-based ionic liquid, which includes an anion and a cation with a pyrrolidinium ring structure, paired with intercalation materials having an upper reversible-potential-limit of at most 4 V versus Li/Li+, enhancing ionic conductivity and stability for high-temperature operation.
Ionic liquids for energy storage systems
PatentWO2024141842A1
Innovation
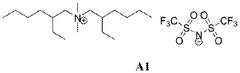
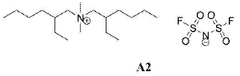
- Development of Bis(2-ethylhexyl)ammonium based ionic liquids with specific substituents that minimize Hoffmann degradation, offering high thermal stability above 500 °C and an electrochemical stability window greater than 5 V, suitable for use in energy storage systems and potentially other applications.
Environmental Impact and Safety Considerations
Chloroaluminate ionic liquid electrolytes present significant environmental and safety considerations that must be thoroughly evaluated before widespread industrial adoption. These electrolytes typically contain aluminum chloride (AlCl3), which is highly reactive with water and can generate hydrogen chloride gas upon exposure to moisture. This hygroscopic nature necessitates stringent handling protocols in manufacturing environments to prevent accidental exposure and subsequent environmental contamination.
The production processes for chloroaluminate ionic liquids often involve chlorinated precursors and metal halides that may pose environmental risks if released untreated. Lifecycle assessment studies indicate that the synthesis of these materials can have a higher carbon footprint compared to conventional electrolytes, primarily due to energy-intensive purification requirements and specialized containment needs during manufacturing.
Waste management represents another critical environmental concern. Used chloroaluminate electrolytes require specialized disposal procedures to prevent soil and groundwater contamination. Current recycling technologies for these materials remain limited, though recent advances in electrochemical recovery methods show promise for reducing end-of-life environmental impact. The development of closed-loop recycling systems is essential for improving the sustainability profile of these electrolytes.
From a safety perspective, chloroaluminate ionic liquids present several occupational hazards. Their corrosive nature can cause severe damage to skin, eyes, and respiratory systems upon direct contact. Laboratory and manufacturing facilities must implement comprehensive safety protocols, including appropriate personal protective equipment, specialized ventilation systems, and emergency response procedures for spills or accidental exposures.
The stability window limitations of these electrolytes also present safety considerations in energy storage applications. Operating outside the electrochemical stability window can lead to decomposition reactions that potentially generate toxic or flammable byproducts. This risk necessitates robust battery management systems with precise voltage control to prevent dangerous failure modes.
Recent regulatory developments have begun addressing these concerns, with several jurisdictions implementing specific guidelines for the handling, transport, and disposal of ionic liquid electrolytes. Compliance with these evolving regulations represents an additional consideration for commercial deployment. Industry stakeholders are increasingly collaborating with regulatory bodies to develop standards that balance innovation with environmental protection and worker safety.
The production processes for chloroaluminate ionic liquids often involve chlorinated precursors and metal halides that may pose environmental risks if released untreated. Lifecycle assessment studies indicate that the synthesis of these materials can have a higher carbon footprint compared to conventional electrolytes, primarily due to energy-intensive purification requirements and specialized containment needs during manufacturing.
Waste management represents another critical environmental concern. Used chloroaluminate electrolytes require specialized disposal procedures to prevent soil and groundwater contamination. Current recycling technologies for these materials remain limited, though recent advances in electrochemical recovery methods show promise for reducing end-of-life environmental impact. The development of closed-loop recycling systems is essential for improving the sustainability profile of these electrolytes.
From a safety perspective, chloroaluminate ionic liquids present several occupational hazards. Their corrosive nature can cause severe damage to skin, eyes, and respiratory systems upon direct contact. Laboratory and manufacturing facilities must implement comprehensive safety protocols, including appropriate personal protective equipment, specialized ventilation systems, and emergency response procedures for spills or accidental exposures.
The stability window limitations of these electrolytes also present safety considerations in energy storage applications. Operating outside the electrochemical stability window can lead to decomposition reactions that potentially generate toxic or flammable byproducts. This risk necessitates robust battery management systems with precise voltage control to prevent dangerous failure modes.
Recent regulatory developments have begun addressing these concerns, with several jurisdictions implementing specific guidelines for the handling, transport, and disposal of ionic liquid electrolytes. Compliance with these evolving regulations represents an additional consideration for commercial deployment. Industry stakeholders are increasingly collaborating with regulatory bodies to develop standards that balance innovation with environmental protection and worker safety.
Scalability and Manufacturing Challenges
The scaling of chloroaluminate ionic liquid electrolyte production from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Current manufacturing processes typically involve batch synthesis methods that are difficult to scale efficiently while maintaining consistent quality. The hygroscopic nature of these electrolytes requires stringent moisture control throughout the entire production chain, necessitating specialized equipment and controlled atmosphere environments that add substantial costs to manufacturing operations.
Raw material availability poses another critical constraint, particularly for high-purity aluminum chloride, which is essential for optimal electrochemical performance. The global supply chain for these specialty chemicals is limited, with few suppliers capable of meeting the purity requirements needed for advanced battery applications. This supply limitation creates potential bottlenecks for large-scale production and increases vulnerability to market fluctuations.
Process optimization remains underdeveloped for continuous flow manufacturing of chloroaluminate ionic liquids. The exothermic reactions involved in synthesis require precise temperature control systems that become increasingly complex at industrial scales. Current cooling technologies struggle to manage heat dissipation efficiently in large reactors, leading to potential quality inconsistencies and safety concerns.
Quality control presents additional challenges, as trace impurities can significantly impact the stability window and overall performance of the electrolyte. Developing robust, high-throughput analytical methods for real-time monitoring during production remains an active research area. The industry currently lacks standardized testing protocols specifically designed for chloroaluminate systems at production scales.
Cost factors further complicate scalability, with current production methods yielding electrolytes at prices approximately 5-10 times higher than conventional lithium-ion battery electrolytes. Economic viability requires either significant process innovations to reduce manufacturing costs or the development of applications where the performance advantages justify the premium price point.
Environmental and safety considerations add another layer of complexity. The reactive nature of chloroaluminate ionic liquids necessitates specialized handling protocols and waste management systems. Regulatory frameworks for large-scale production of these materials are still evolving in many jurisdictions, creating uncertainty for manufacturers planning significant capital investments in production facilities.
Raw material availability poses another critical constraint, particularly for high-purity aluminum chloride, which is essential for optimal electrochemical performance. The global supply chain for these specialty chemicals is limited, with few suppliers capable of meeting the purity requirements needed for advanced battery applications. This supply limitation creates potential bottlenecks for large-scale production and increases vulnerability to market fluctuations.
Process optimization remains underdeveloped for continuous flow manufacturing of chloroaluminate ionic liquids. The exothermic reactions involved in synthesis require precise temperature control systems that become increasingly complex at industrial scales. Current cooling technologies struggle to manage heat dissipation efficiently in large reactors, leading to potential quality inconsistencies and safety concerns.
Quality control presents additional challenges, as trace impurities can significantly impact the stability window and overall performance of the electrolyte. Developing robust, high-throughput analytical methods for real-time monitoring during production remains an active research area. The industry currently lacks standardized testing protocols specifically designed for chloroaluminate systems at production scales.
Cost factors further complicate scalability, with current production methods yielding electrolytes at prices approximately 5-10 times higher than conventional lithium-ion battery electrolytes. Economic viability requires either significant process innovations to reduce manufacturing costs or the development of applications where the performance advantages justify the premium price point.
Environmental and safety considerations add another layer of complexity. The reactive nature of chloroaluminate ionic liquids necessitates specialized handling protocols and waste management systems. Regulatory frameworks for large-scale production of these materials are still evolving in many jurisdictions, creating uncertainty for manufacturers planning significant capital investments in production facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!