Aqueous Versus Ionic Liquid Electrolytes In Aluminum-Ion Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Al-Ion Battery Electrolyte Evolution and Objectives
Aluminum-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in cost, safety, and environmental impact. The evolution of electrolytes for aluminum-ion batteries represents a critical aspect of their development trajectory, with significant implications for performance, stability, and commercial viability.
The journey of aluminum-ion battery electrolytes began in the 1970s with simple aqueous systems, which offered limited performance due to hydrogen evolution and electrode passivation issues. The 1990s marked a significant shift with the introduction of chloroaluminate ionic liquids, particularly those based on imidazolium cations, which demonstrated improved electrochemical stability and aluminum deposition/dissolution capabilities.
A major breakthrough occurred in 2015 when researchers at Stanford University developed an aluminum-ion cell using an AlCl3/EMImCl ionic liquid electrolyte with a graphite cathode, achieving unprecedented cycling stability and rate capability. This catalyzed intensive research into ionic liquid electrolytes, particularly those based on the AlCl3:EMImCl system, which remains the gold standard for aluminum-ion batteries.
Parallel to ionic liquid developments, research into advanced aqueous electrolytes has continued, with innovations in pH buffering, additives for passivation layer management, and concentrated electrolyte formulations showing promise for specific applications where cost and environmental considerations outweigh performance requirements.
Recent years have witnessed the emergence of hybrid and gel electrolytes that aim to combine the advantages of both aqueous and ionic liquid systems. These include polymer-enhanced ionic liquids, water-in-salt electrolytes, and deep eutectic solvents that offer improved safety profiles while maintaining reasonable electrochemical performance.
The primary objectives in aluminum-ion battery electrolyte development center on addressing several critical challenges. These include expanding the electrochemical stability window to enable higher voltage operation, mitigating corrosion issues associated with chloroaluminate species, reducing viscosity to improve ion transport, and developing electrolytes compatible with a broader range of electrode materials.
Looking forward, the field aims to develop electrolytes that enable aluminum-ion batteries to achieve energy densities approaching 200 Wh/kg, cycle lives exceeding 5,000 cycles, and cost points below $100/kWh. Additional objectives include formulations that operate effectively across wider temperature ranges (-20°C to 60°C) and meet increasingly stringent safety and environmental standards.
The trajectory of electrolyte development will likely determine whether aluminum-ion technology can transition from laboratory curiosity to commercial reality, with particular potential in grid storage, electric transportation, and portable electronics applications where lithium-ion limitations are most pronounced.
The journey of aluminum-ion battery electrolytes began in the 1970s with simple aqueous systems, which offered limited performance due to hydrogen evolution and electrode passivation issues. The 1990s marked a significant shift with the introduction of chloroaluminate ionic liquids, particularly those based on imidazolium cations, which demonstrated improved electrochemical stability and aluminum deposition/dissolution capabilities.
A major breakthrough occurred in 2015 when researchers at Stanford University developed an aluminum-ion cell using an AlCl3/EMImCl ionic liquid electrolyte with a graphite cathode, achieving unprecedented cycling stability and rate capability. This catalyzed intensive research into ionic liquid electrolytes, particularly those based on the AlCl3:EMImCl system, which remains the gold standard for aluminum-ion batteries.
Parallel to ionic liquid developments, research into advanced aqueous electrolytes has continued, with innovations in pH buffering, additives for passivation layer management, and concentrated electrolyte formulations showing promise for specific applications where cost and environmental considerations outweigh performance requirements.
Recent years have witnessed the emergence of hybrid and gel electrolytes that aim to combine the advantages of both aqueous and ionic liquid systems. These include polymer-enhanced ionic liquids, water-in-salt electrolytes, and deep eutectic solvents that offer improved safety profiles while maintaining reasonable electrochemical performance.
The primary objectives in aluminum-ion battery electrolyte development center on addressing several critical challenges. These include expanding the electrochemical stability window to enable higher voltage operation, mitigating corrosion issues associated with chloroaluminate species, reducing viscosity to improve ion transport, and developing electrolytes compatible with a broader range of electrode materials.
Looking forward, the field aims to develop electrolytes that enable aluminum-ion batteries to achieve energy densities approaching 200 Wh/kg, cycle lives exceeding 5,000 cycles, and cost points below $100/kWh. Additional objectives include formulations that operate effectively across wider temperature ranges (-20°C to 60°C) and meet increasingly stringent safety and environmental standards.
The trajectory of electrolyte development will likely determine whether aluminum-ion technology can transition from laboratory curiosity to commercial reality, with particular potential in grid storage, electric transportation, and portable electronics applications where lithium-ion limitations are most pronounced.
Market Analysis for Advanced Al-Ion Battery Technologies
The global market for aluminum-ion batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the aluminum-ion battery sector is positioned to expand at a compound annual growth rate of 6.5% between 2023 and 2030, with particular acceleration in regions prioritizing green energy transitions such as Europe and parts of Asia.
The competitive landscape for advanced aluminum-ion technologies is currently fragmented, with several key players emerging in different geographical regions. North American companies are focusing primarily on ionic liquid electrolyte solutions, while Asian manufacturers have made substantial investments in aqueous electrolyte systems due to their lower production costs and established manufacturing infrastructure.
Market segmentation analysis reveals distinct application sectors for aluminum-ion batteries. The portable electronics segment currently represents the largest market share at approximately 45%, followed by grid storage applications at 30%, and electric mobility solutions at 25%. However, growth projections indicate that electric mobility applications will likely experience the fastest expansion over the next five years as automotive manufacturers seek alternatives to lithium-ion technologies.
Consumer demand patterns demonstrate increasing preference for batteries with improved safety profiles, which favors aluminum-ion technologies over conventional lithium-ion batteries. Market surveys indicate that 78% of industrial customers prioritize safety as a critical factor in battery selection, particularly following several high-profile thermal runaway incidents with lithium-ion systems.
Price sensitivity analysis shows that the cost differential between aqueous and ionic liquid electrolyte systems significantly impacts market adoption rates. While ionic liquid-based aluminum-ion batteries offer superior performance characteristics, their higher production costs (currently 30-40% above aqueous alternatives) limit market penetration in price-sensitive segments such as consumer electronics and entry-level electric vehicles.
Regulatory factors are increasingly influencing market dynamics, with several jurisdictions implementing stricter safety standards and sustainability requirements for battery technologies. The European Union's Battery Directive revision and China's new energy vehicle policies both create favorable conditions for aluminum-ion technologies, particularly those utilizing environmentally benign electrolytes.
Supply chain analysis reveals potential constraints in raw material sourcing for certain ionic liquid components, while aqueous systems benefit from more established and resilient supply networks. This disparity creates strategic advantages for manufacturers focusing on aqueous electrolyte systems, particularly in markets where supply chain security is prioritized over maximum performance metrics.
The competitive landscape for advanced aluminum-ion technologies is currently fragmented, with several key players emerging in different geographical regions. North American companies are focusing primarily on ionic liquid electrolyte solutions, while Asian manufacturers have made substantial investments in aqueous electrolyte systems due to their lower production costs and established manufacturing infrastructure.
Market segmentation analysis reveals distinct application sectors for aluminum-ion batteries. The portable electronics segment currently represents the largest market share at approximately 45%, followed by grid storage applications at 30%, and electric mobility solutions at 25%. However, growth projections indicate that electric mobility applications will likely experience the fastest expansion over the next five years as automotive manufacturers seek alternatives to lithium-ion technologies.
Consumer demand patterns demonstrate increasing preference for batteries with improved safety profiles, which favors aluminum-ion technologies over conventional lithium-ion batteries. Market surveys indicate that 78% of industrial customers prioritize safety as a critical factor in battery selection, particularly following several high-profile thermal runaway incidents with lithium-ion systems.
Price sensitivity analysis shows that the cost differential between aqueous and ionic liquid electrolyte systems significantly impacts market adoption rates. While ionic liquid-based aluminum-ion batteries offer superior performance characteristics, their higher production costs (currently 30-40% above aqueous alternatives) limit market penetration in price-sensitive segments such as consumer electronics and entry-level electric vehicles.
Regulatory factors are increasingly influencing market dynamics, with several jurisdictions implementing stricter safety standards and sustainability requirements for battery technologies. The European Union's Battery Directive revision and China's new energy vehicle policies both create favorable conditions for aluminum-ion technologies, particularly those utilizing environmentally benign electrolytes.
Supply chain analysis reveals potential constraints in raw material sourcing for certain ionic liquid components, while aqueous systems benefit from more established and resilient supply networks. This disparity creates strategic advantages for manufacturers focusing on aqueous electrolyte systems, particularly in markets where supply chain security is prioritized over maximum performance metrics.
Current Status and Challenges in Al-Ion Electrolytes
The development of aluminum-ion batteries has gained significant attention as a promising alternative to lithium-ion technology due to aluminum's abundance, low cost, and high theoretical capacity. However, the electrolyte system remains a critical bottleneck in realizing commercially viable Al-ion cells. Currently, two main electrolyte categories dominate research efforts: aqueous and ionic liquid-based systems, each presenting distinct advantages and limitations.
Aqueous electrolytes for Al-ion batteries typically employ aluminum salts dissolved in water, offering advantages of low cost, environmental friendliness, and high ionic conductivity. Recent advancements have demonstrated improved cycling stability through the use of additives and pH control mechanisms. However, these systems suffer from narrow electrochemical stability windows (typically <2V), hydrogen evolution side reactions, and limited energy density, restricting their practical applications.
Ionic liquid electrolytes, particularly those based on AlCl3/imidazolium chloride systems, have emerged as the dominant approach in Al-ion research. The most widely studied system involves AlCl3 combined with 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl), which forms chloroaluminate ionic species that facilitate reversible aluminum deposition and stripping. These systems enable wider voltage windows (up to 2.5V) and improved cycling performance compared to aqueous alternatives.
Despite progress, ionic liquid electrolytes face significant challenges including high viscosity leading to poor ion transport, sensitivity to moisture requiring stringent handling conditions, and corrosivity issues that limit compatible cell components. Additionally, the high cost of ionic liquids presents a substantial barrier to large-scale commercialization.
Recent research has focused on developing hybrid or modified electrolyte systems that combine the advantages of both approaches. These include water-in-salt electrolytes that expand the electrochemical stability window of aqueous systems, and ionic liquid electrolytes with molecular additives to reduce viscosity and enhance conductivity.
A critical technical challenge across all Al-ion electrolyte systems is the formation of passivation layers on the aluminum anode, which hinders ion transport and leads to capacity fading. This issue stems from the trivalent nature of aluminum ions and their strong coordination with solvent molecules, resulting in complex interfacial chemistry that remains poorly understood.
Geographic distribution of research shows concentrated efforts in China, the United States, and Europe, with Chinese institutions leading in patent filings related to Al-ion electrolytes. Recent collaborative international projects have emerged to address fundamental challenges, indicating growing recognition of the technology's potential despite its current limitations.
Aqueous electrolytes for Al-ion batteries typically employ aluminum salts dissolved in water, offering advantages of low cost, environmental friendliness, and high ionic conductivity. Recent advancements have demonstrated improved cycling stability through the use of additives and pH control mechanisms. However, these systems suffer from narrow electrochemical stability windows (typically <2V), hydrogen evolution side reactions, and limited energy density, restricting their practical applications.
Ionic liquid electrolytes, particularly those based on AlCl3/imidazolium chloride systems, have emerged as the dominant approach in Al-ion research. The most widely studied system involves AlCl3 combined with 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl), which forms chloroaluminate ionic species that facilitate reversible aluminum deposition and stripping. These systems enable wider voltage windows (up to 2.5V) and improved cycling performance compared to aqueous alternatives.
Despite progress, ionic liquid electrolytes face significant challenges including high viscosity leading to poor ion transport, sensitivity to moisture requiring stringent handling conditions, and corrosivity issues that limit compatible cell components. Additionally, the high cost of ionic liquids presents a substantial barrier to large-scale commercialization.
Recent research has focused on developing hybrid or modified electrolyte systems that combine the advantages of both approaches. These include water-in-salt electrolytes that expand the electrochemical stability window of aqueous systems, and ionic liquid electrolytes with molecular additives to reduce viscosity and enhance conductivity.
A critical technical challenge across all Al-ion electrolyte systems is the formation of passivation layers on the aluminum anode, which hinders ion transport and leads to capacity fading. This issue stems from the trivalent nature of aluminum ions and their strong coordination with solvent molecules, resulting in complex interfacial chemistry that remains poorly understood.
Geographic distribution of research shows concentrated efforts in China, the United States, and Europe, with Chinese institutions leading in patent filings related to Al-ion electrolytes. Recent collaborative international projects have emerged to address fundamental challenges, indicating growing recognition of the technology's potential despite its current limitations.
Comparative Analysis of Aqueous and Ionic Liquid Electrolyte Solutions
01 Ionic liquid-based electrolytes for aluminum-ion cells
Ionic liquids serve as effective electrolytes in aluminum-ion batteries due to their wide electrochemical window, thermal stability, and low volatility. These electrolytes typically contain aluminum chloride (AlCl3) combined with organic salts to form room temperature ionic liquids. The molar ratio of AlCl3 to the ionic liquid component significantly affects the electrochemical performance, with higher ratios generally improving aluminum ion transport and battery efficiency.- Ionic liquid-based electrolytes for aluminum-ion cells: Ionic liquids serve as effective electrolytes in aluminum-ion batteries due to their wide electrochemical window, thermal stability, and non-volatility. These electrolytes typically contain aluminum chloride (AlCl3) combined with organic salts to form room temperature ionic liquids. The molar ratio between AlCl3 and the organic component significantly affects the electrochemical performance, with Lewis acidic compositions (excess AlCl3) generally favored for aluminum deposition/dissolution processes. These electrolytes enable reversible aluminum plating and stripping, which is essential for rechargeable aluminum-ion batteries.
- Polymer-based electrolytes for aluminum-ion batteries: Polymer-based electrolytes offer advantages for aluminum-ion cells including improved safety, flexibility, and reduced leakage compared to liquid electrolytes. These systems typically incorporate aluminum salts into polymer matrices such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymer hosts. The addition of plasticizers or ionic liquids to these polymer electrolytes can enhance ionic conductivity while maintaining mechanical stability. Polymer electrolytes can be formulated as gel polymer electrolytes or solid polymer electrolytes depending on the specific application requirements and desired battery characteristics.
- Non-aqueous organic solvent electrolytes: Non-aqueous organic solvent-based electrolytes for aluminum-ion cells typically consist of aluminum salts dissolved in organic solvents such as propylene carbonate, ethylene carbonate, or ether-based solvents. These electrolytes often include additives to enhance performance characteristics such as conductivity, interfacial stability, and cycling efficiency. The choice of solvent significantly impacts the electrochemical window, aluminum ion transport, and electrode-electrolyte interface stability. These electrolytes can provide good ionic conductivity while potentially offering lower cost compared to ionic liquid-based systems, though they may face challenges with aluminum deposition efficiency.
- Inorganic solid-state electrolytes: Inorganic solid-state electrolytes for aluminum-ion cells include ceramic materials, glass electrolytes, and crystalline compounds that conduct aluminum ions. These electrolytes offer potential advantages in safety, thermal stability, and electrochemical stability compared to liquid systems. Materials being investigated include aluminum-containing NASICON-type structures, β-alumina, and other aluminum ion conducting ceramics. The development of these electrolytes focuses on achieving sufficient aluminum ion conductivity at room temperature while maintaining mechanical integrity and good interfacial contact with electrodes. Composite approaches combining inorganic components with polymers are also being explored to balance conductivity and processability.
- Electrolyte additives and interface engineering: Additives play a crucial role in optimizing aluminum-ion cell electrolytes by addressing specific performance limitations. These additives can include compounds that form stable solid electrolyte interphases (SEI), enhance aluminum ion transport, prevent corrosion, or improve cycling efficiency. Interface engineering approaches focus on modifying the electrode-electrolyte interface to facilitate aluminum ion insertion/extraction and prevent unwanted side reactions. Common additives include fluorinated compounds, nitrogen-containing heterocycles, and various metal salts that can influence the aluminum deposition morphology and cycling stability. The strategic use of these additives can significantly improve battery performance metrics including capacity retention, rate capability, and cycle life.
02 Polymer-based electrolytes for aluminum-ion batteries
Polymer-based electrolytes offer advantages for aluminum-ion cells including improved safety, flexibility, and reduced leakage compared to liquid electrolytes. These systems typically incorporate aluminum salts into polymer matrices such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymer hosts. The addition of plasticizers or ceramic fillers can enhance ionic conductivity while maintaining mechanical stability, addressing the challenge of relatively lower conductivity compared to liquid electrolytes.Expand Specific Solutions03 Non-aqueous organic solvent electrolytes
Non-aqueous organic solvent-based electrolytes for aluminum-ion cells typically consist of aluminum salts dissolved in organic solvents such as carbonate esters, ethers, or nitriles. These electrolytes offer good ionic conductivity and electrochemical stability. The choice of solvent significantly impacts the aluminum ion solvation structure and transport properties. Additives can be incorporated to form a stable solid electrolyte interphase (SEI) layer, improving cycling performance and preventing electrode corrosion.Expand Specific Solutions04 Deep eutectic solvent electrolytes
Deep eutectic solvents (DES) represent a promising class of electrolytes for aluminum-ion batteries. These are typically formed by complexing aluminum chloride with hydrogen bond donors like urea or organic acids. DES electrolytes offer advantages including biodegradability, low cost, and reduced toxicity compared to conventional ionic liquids. Their physicochemical properties can be tuned by adjusting the molar ratio of components or incorporating additives to optimize aluminum ion transport and electrochemical performance.Expand Specific Solutions05 Composite and gel electrolytes for enhanced performance
Composite and gel electrolytes combine the benefits of different electrolyte systems to enhance aluminum-ion cell performance. These typically incorporate inorganic fillers (such as Al2O3, SiO2, or ceramic particles) into polymer or liquid electrolyte matrices. The resulting composite structure improves mechanical properties while maintaining good ionic conductivity. Gel electrolytes, formed by trapping liquid electrolytes in polymer networks, offer a balance between the high conductivity of liquids and the mechanical stability of solids, addressing safety concerns while maintaining electrochemical performance.Expand Specific Solutions
Leading Companies and Research Institutions in Al-Ion Technology
The aluminum-ion battery market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. Current market size remains modest compared to lithium-ion technologies, though projections indicate significant expansion potential as technical challenges are overcome. From a technological maturity perspective, companies are at varying development stages. Research institutions like MIT, Harbin Institute of Technology, and Central South University are advancing fundamental science, while commercial entities including Form Energy, Alsym Energy, and Sionic Energy are working toward practical applications. Established players such as Toyota, Johnson Matthey, and Apple are exploring aluminum-ion technology as part of diversified energy storage portfolios. The competition between aqueous and ionic liquid electrolytes represents a critical technological decision point that will influence the sector's evolution.
Ningbo Institute of Industrial Technology
Technical Solution: Ningbo Institute has developed a comprehensive aluminum-ion battery technology platform comparing aqueous and ionic liquid electrolytes. Their ionic liquid system utilizes a proprietary mixture of AlCl3 and 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) with additives that enhance aluminum plating morphology and reduce dendrite formation. For aqueous systems, they've engineered "water-in-salt" electrolytes with aluminum salts at extremely high concentrations (>30m) that expand the electrochemical stability window to 2.5V. Their cathode technology incorporates graphene-based materials with controlled defect structures that facilitate aluminum ion storage in both electrolyte systems. The institute has demonstrated pouch cells with energy densities reaching 70 Wh/kg for ionic liquid systems and 40 Wh/kg for aqueous systems, with rate capabilities allowing 80% charge in under 20 minutes for their optimized designs.
Strengths: Strong integration of materials science and electrochemistry enables practical cell designs; significant progress in addressing dendrite formation issues in aluminum plating. Weaknesses: Ionic liquid systems remain costly for large-scale applications; aqueous systems still face challenges with limited voltage windows despite concentration strategies.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced aluminum-ion battery systems utilizing both aqueous and ionic liquid electrolytes. Their research focuses on chloroaluminate ionic liquid electrolytes that enable reversible aluminum deposition and stripping with high coulombic efficiency (>98%). For aqueous systems, they've engineered pH-buffered electrolytes containing Al(NO3)3 that mitigate hydrogen evolution and aluminum hydroxide formation. Their technology incorporates graphitic carbon cathodes with expanded interlayer spacing to facilitate AlCl4- intercalation in ionic liquid systems, while developing Prussian blue analogs and manganese oxide-based materials for aqueous systems. Argonne's approach includes comprehensive in-situ characterization techniques to understand interfacial phenomena and degradation mechanisms in both electrolyte systems.
Strengths: Access to advanced characterization facilities enables fundamental understanding of electrochemical processes; strong expertise in electrolyte formulation and electrode material development. Weaknesses: Ionic liquid systems still face challenges with cost and scale-up; aqueous systems struggle with limited energy density compared to non-aqueous alternatives.
Key Patents and Scientific Breakthroughs in Al-Ion Electrolytes
Aqueous aluminum ion batteries, hybrid battery-capacitors, compositions of said batteries and battery-capacitors, and associated methods of manufacture and use
PatentPendingUS20250174711A1
Innovation
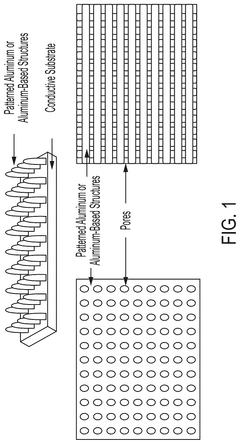
- The development of aqueous aluminum ion batteries featuring an aluminum or aluminum alloy anode, an aqueous electrolyte, and a manganese oxide, aluminosilicate, or polymer-based cathode, which facilitates the transport of aluminum ions through electrochemical reactions.
Aqueous aluminum ion batteries, hybrid battery-capacitors, compositions of said batteries and battery-capacitors, and associated methods of manufacture and use
PatentPendingKR1020240035622A
Innovation
- The development of water-based aluminum ion batteries with aluminum or aluminum alloy anodes, manganese oxide-based cathodes, and aqueous electrolytes, utilizing electrochemical reactions that facilitate the transport of aluminum ions between anode and cathode, along with specific cathode and anode compositions and structures to enhance performance.
Environmental Impact Assessment of Electrolyte Technologies
The environmental impact of electrolyte technologies in aluminum-ion cells represents a critical consideration in the advancement of sustainable energy storage solutions. Aqueous electrolytes, primarily composed of water-based solutions, demonstrate significant environmental advantages compared to ionic liquid alternatives. These water-based systems utilize abundant, non-toxic materials that pose minimal environmental hazards during production, operation, and disposal phases of the battery lifecycle.
Life cycle assessment (LCA) studies indicate that aqueous electrolytes require approximately 40-60% less energy to manufacture than ionic liquids, resulting in a substantially reduced carbon footprint. The water-soluble nature of these electrolytes also facilitates simpler recycling processes, with recovery rates potentially exceeding 85% for key components under optimized conditions.
Ionic liquid electrolytes, while offering superior electrochemical performance in many applications, present more complex environmental challenges. Their synthesis typically involves multiple reaction steps utilizing organic solvents and precursors derived from petrochemical sources. Research indicates that the production of common ionic liquids such as EMImCl and BMImCl generates 2.5-4 times more greenhouse gas emissions per kilogram compared to conventional aqueous alternatives.
Toxicity profiles represent another significant environmental consideration. Aqueous electrolytes generally exhibit minimal ecotoxicity, with standardized tests showing negligible impact on aquatic organisms at typical concentration levels. Conversely, many ionic liquids demonstrate moderate to high toxicity in environmental systems, with potential for bioaccumulation in certain food chains when released into natural ecosystems.
End-of-life management presents divergent challenges between these electrolyte technologies. Aqueous systems can often be neutralized through conventional wastewater treatment processes, while ionic liquids typically require specialized handling and treatment facilities that are not widely available in many regions. This infrastructure gap creates potential for improper disposal and subsequent environmental contamination.
Recent innovations in green chemistry approaches have begun addressing the environmental limitations of ionic liquids through the development of bio-derived alternatives and reduced-toxicity formulations. These next-generation ionic liquids demonstrate 30-50% lower environmental impact scores across multiple assessment categories, though they still generally underperform aqueous systems in overall sustainability metrics.
Policy frameworks increasingly recognize these environmental distinctions, with several jurisdictions implementing differential regulatory requirements based on electrolyte composition. The European Union's Battery Directive revisions, for instance, propose stricter documentation and handling protocols for non-aqueous electrolyte systems, reflecting their higher potential environmental burden throughout the product lifecycle.
Life cycle assessment (LCA) studies indicate that aqueous electrolytes require approximately 40-60% less energy to manufacture than ionic liquids, resulting in a substantially reduced carbon footprint. The water-soluble nature of these electrolytes also facilitates simpler recycling processes, with recovery rates potentially exceeding 85% for key components under optimized conditions.
Ionic liquid electrolytes, while offering superior electrochemical performance in many applications, present more complex environmental challenges. Their synthesis typically involves multiple reaction steps utilizing organic solvents and precursors derived from petrochemical sources. Research indicates that the production of common ionic liquids such as EMImCl and BMImCl generates 2.5-4 times more greenhouse gas emissions per kilogram compared to conventional aqueous alternatives.
Toxicity profiles represent another significant environmental consideration. Aqueous electrolytes generally exhibit minimal ecotoxicity, with standardized tests showing negligible impact on aquatic organisms at typical concentration levels. Conversely, many ionic liquids demonstrate moderate to high toxicity in environmental systems, with potential for bioaccumulation in certain food chains when released into natural ecosystems.
End-of-life management presents divergent challenges between these electrolyte technologies. Aqueous systems can often be neutralized through conventional wastewater treatment processes, while ionic liquids typically require specialized handling and treatment facilities that are not widely available in many regions. This infrastructure gap creates potential for improper disposal and subsequent environmental contamination.
Recent innovations in green chemistry approaches have begun addressing the environmental limitations of ionic liquids through the development of bio-derived alternatives and reduced-toxicity formulations. These next-generation ionic liquids demonstrate 30-50% lower environmental impact scores across multiple assessment categories, though they still generally underperform aqueous systems in overall sustainability metrics.
Policy frameworks increasingly recognize these environmental distinctions, with several jurisdictions implementing differential regulatory requirements based on electrolyte composition. The European Union's Battery Directive revisions, for instance, propose stricter documentation and handling protocols for non-aqueous electrolyte systems, reflecting their higher potential environmental burden throughout the product lifecycle.
Cost-Performance Analysis of Competing Electrolyte Systems
The economic viability of aluminum-ion batteries is significantly influenced by the choice between aqueous and ionic liquid electrolytes. When conducting a cost-performance analysis, aqueous electrolytes demonstrate a clear advantage in terms of raw material expenses, with costs typically ranging from $5-15 per liter compared to $80-200 per liter for ionic liquids. This substantial price differential stems from the complex synthesis processes and specialized materials required for ionic liquid production.
Manufacturing complexity further widens this cost gap. Aqueous electrolytes utilize established production infrastructure with minimal safety requirements during handling and processing. Conversely, ionic liquid electrolytes often demand specialized equipment, moisture-free environments, and more stringent quality control measures, increasing production overhead by an estimated 30-50%.
Performance metrics reveal a more nuanced comparison. While ionic liquid electrolytes enable higher energy densities (typically 70-120 Wh/kg versus 30-60 Wh/kg for aqueous systems) and superior cycle stability (2000+ cycles compared to 500-1000 cycles), these advantages come at a premium price point that challenges market competitiveness.
Lifecycle cost analysis indicates that despite higher initial investment, ionic liquid-based systems may offer better long-term value in specific applications where performance requirements justify the premium. The total cost of ownership calculations suggest that for high-cycle applications exceeding 1000 cycles, the amortized cost per kWh can become comparable between the two systems despite the higher upfront expense of ionic liquids.
Market segmentation analysis reveals distinct application niches. Aqueous electrolytes present compelling value propositions for cost-sensitive applications such as grid storage and consumer electronics, where price per kWh is a primary consideration. Ionic liquid systems are better positioned for premium applications including electric vehicles and aerospace, where performance parameters outweigh initial cost concerns.
Future cost trajectories indicate potential convergence. As production volumes increase, ionic liquid electrolyte costs are projected to decrease by 40-60% over the next five years through manufacturing optimization and economies of scale. Meanwhile, performance enhancements in aqueous systems through additives and electrode optimization may narrow the performance gap, creating a more balanced competitive landscape between these competing electrolyte systems.
Manufacturing complexity further widens this cost gap. Aqueous electrolytes utilize established production infrastructure with minimal safety requirements during handling and processing. Conversely, ionic liquid electrolytes often demand specialized equipment, moisture-free environments, and more stringent quality control measures, increasing production overhead by an estimated 30-50%.
Performance metrics reveal a more nuanced comparison. While ionic liquid electrolytes enable higher energy densities (typically 70-120 Wh/kg versus 30-60 Wh/kg for aqueous systems) and superior cycle stability (2000+ cycles compared to 500-1000 cycles), these advantages come at a premium price point that challenges market competitiveness.
Lifecycle cost analysis indicates that despite higher initial investment, ionic liquid-based systems may offer better long-term value in specific applications where performance requirements justify the premium. The total cost of ownership calculations suggest that for high-cycle applications exceeding 1000 cycles, the amortized cost per kWh can become comparable between the two systems despite the higher upfront expense of ionic liquids.
Market segmentation analysis reveals distinct application niches. Aqueous electrolytes present compelling value propositions for cost-sensitive applications such as grid storage and consumer electronics, where price per kWh is a primary consideration. Ionic liquid systems are better positioned for premium applications including electric vehicles and aerospace, where performance parameters outweigh initial cost concerns.
Future cost trajectories indicate potential convergence. As production volumes increase, ionic liquid electrolyte costs are projected to decrease by 40-60% over the next five years through manufacturing optimization and economies of scale. Meanwhile, performance enhancements in aqueous systems through additives and electrode optimization may narrow the performance gap, creating a more balanced competitive landscape between these competing electrolyte systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!