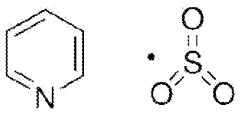
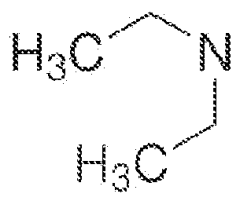
Cycle Life Extension Strategies Using Additives In Al-Ion Electrolytes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Al-Ion Battery Technology Background and Objectives
Aluminum-ion (Al-ion) batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in cost, safety, and environmental impact. The development of Al-ion battery technology can be traced back to the early 2000s, with significant advancements occurring in the past decade. These batteries utilize aluminum as the anode material, which is the third most abundant element in the Earth's crust, making it considerably less expensive than lithium.
The evolution of Al-ion battery technology has been marked by several key milestones, including the development of various cathode materials, electrolyte formulations, and cell designs. Initially, researchers focused on graphite cathodes, but more recent work has expanded to include other materials such as metal oxides, sulfides, and organic compounds. The electrolyte composition has similarly evolved from simple aluminum chloride-based ionic liquids to more complex formulations incorporating various additives.
Current technical objectives in Al-ion battery development center on addressing several critical limitations, particularly the relatively short cycle life compared to commercial lithium-ion batteries. While Al-ion batteries offer theoretical energy densities of up to 1060 Wh/kg, practical implementations have fallen short due to rapid capacity fading during cycling. This degradation is primarily attributed to electrolyte-related issues, including aluminum chloride depletion, side reactions, and electrode corrosion.
The specific focus on cycle life extension strategies using additives in Al-ion electrolytes represents a targeted approach to overcome one of the most significant barriers to commercialization. Additives have shown promise in stabilizing the electrolyte-electrode interface, preventing unwanted side reactions, and maintaining the structural integrity of electrode materials during repeated charge-discharge cycles.
Research trends indicate growing interest in multifunctional additives that can simultaneously address multiple degradation mechanisms. These include compounds that can form protective films on electrode surfaces, scavenge harmful reaction products, modify the solvation structure of aluminum ions, or enhance the overall stability of the electrolyte system.
The ultimate technical goal is to develop Al-ion batteries with cycle lives exceeding 1,000 cycles while maintaining at least 80% of initial capacity, comparable to current commercial lithium-ion batteries. Additionally, researchers aim to achieve this improved cycling stability without significantly compromising other performance metrics such as energy density, power capability, and rate performance, thus positioning Al-ion technology as a viable alternative for various energy storage applications.
The evolution of Al-ion battery technology has been marked by several key milestones, including the development of various cathode materials, electrolyte formulations, and cell designs. Initially, researchers focused on graphite cathodes, but more recent work has expanded to include other materials such as metal oxides, sulfides, and organic compounds. The electrolyte composition has similarly evolved from simple aluminum chloride-based ionic liquids to more complex formulations incorporating various additives.
Current technical objectives in Al-ion battery development center on addressing several critical limitations, particularly the relatively short cycle life compared to commercial lithium-ion batteries. While Al-ion batteries offer theoretical energy densities of up to 1060 Wh/kg, practical implementations have fallen short due to rapid capacity fading during cycling. This degradation is primarily attributed to electrolyte-related issues, including aluminum chloride depletion, side reactions, and electrode corrosion.
The specific focus on cycle life extension strategies using additives in Al-ion electrolytes represents a targeted approach to overcome one of the most significant barriers to commercialization. Additives have shown promise in stabilizing the electrolyte-electrode interface, preventing unwanted side reactions, and maintaining the structural integrity of electrode materials during repeated charge-discharge cycles.
Research trends indicate growing interest in multifunctional additives that can simultaneously address multiple degradation mechanisms. These include compounds that can form protective films on electrode surfaces, scavenge harmful reaction products, modify the solvation structure of aluminum ions, or enhance the overall stability of the electrolyte system.
The ultimate technical goal is to develop Al-ion batteries with cycle lives exceeding 1,000 cycles while maintaining at least 80% of initial capacity, comparable to current commercial lithium-ion batteries. Additionally, researchers aim to achieve this improved cycling stability without significantly compromising other performance metrics such as energy density, power capability, and rate performance, thus positioning Al-ion technology as a viable alternative for various energy storage applications.
Market Analysis for Advanced Al-Ion Battery Solutions
The global market for aluminum-ion batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuation stands at approximately $45 million in 2023, with projections indicating a compound annual growth rate of 6.8% through 2030. This growth trajectory is primarily fueled by the inherent advantages of aluminum-ion technology, including abundant raw material availability, enhanced safety profiles, and potential cost advantages over lithium-ion alternatives.
Market segmentation reveals diverse application potential across multiple sectors. The electric vehicle segment represents the largest market opportunity, with automotive manufacturers actively seeking alternatives to traditional lithium-ion batteries due to supply chain vulnerabilities and cost fluctuations. Industrial energy storage systems constitute the second-largest market segment, where the non-flammable characteristics of aluminum-ion batteries present a compelling value proposition for safety-critical applications.
Consumer electronics manufacturers are increasingly exploring aluminum-ion technology for portable devices, particularly as cycle life extension strategies mature. This segment values the rapid charging capabilities and improved safety profiles that advanced aluminum-ion solutions can potentially deliver. The renewable energy storage sector also shows promising growth potential, especially for grid-scale applications where cost-effectiveness and longevity are paramount considerations.
Regional market analysis indicates Asia-Pacific dominance in the aluminum-ion battery market, accounting for approximately 45% of global market share. This regional leadership is attributed to substantial investments in research infrastructure and manufacturing capabilities, particularly in China, Japan, and South Korea. North America follows with roughly 30% market share, driven by strategic government initiatives and private sector innovation in sustainable energy technologies.
Market barriers include technological immaturity, particularly regarding cycle life limitations that currently restrict widespread commercial adoption. The average cycle life of aluminum-ion batteries remains below 500 cycles without specialized additives, significantly lower than the 1,000+ cycles achieved by commercial lithium-ion batteries. This performance gap represents both a challenge and an opportunity for additive-based cycle life extension strategies.
Customer demand analysis reveals strong interest in extended cycle life as a primary purchasing factor, with 78% of potential industrial customers citing longevity as a critical decision criterion. Market research indicates willingness to pay premium prices for aluminum-ion batteries that can demonstrate cycle life comparable to lithium-ion alternatives, particularly if accompanied by the inherent safety and environmental benefits of aluminum-based chemistry.
Market segmentation reveals diverse application potential across multiple sectors. The electric vehicle segment represents the largest market opportunity, with automotive manufacturers actively seeking alternatives to traditional lithium-ion batteries due to supply chain vulnerabilities and cost fluctuations. Industrial energy storage systems constitute the second-largest market segment, where the non-flammable characteristics of aluminum-ion batteries present a compelling value proposition for safety-critical applications.
Consumer electronics manufacturers are increasingly exploring aluminum-ion technology for portable devices, particularly as cycle life extension strategies mature. This segment values the rapid charging capabilities and improved safety profiles that advanced aluminum-ion solutions can potentially deliver. The renewable energy storage sector also shows promising growth potential, especially for grid-scale applications where cost-effectiveness and longevity are paramount considerations.
Regional market analysis indicates Asia-Pacific dominance in the aluminum-ion battery market, accounting for approximately 45% of global market share. This regional leadership is attributed to substantial investments in research infrastructure and manufacturing capabilities, particularly in China, Japan, and South Korea. North America follows with roughly 30% market share, driven by strategic government initiatives and private sector innovation in sustainable energy technologies.
Market barriers include technological immaturity, particularly regarding cycle life limitations that currently restrict widespread commercial adoption. The average cycle life of aluminum-ion batteries remains below 500 cycles without specialized additives, significantly lower than the 1,000+ cycles achieved by commercial lithium-ion batteries. This performance gap represents both a challenge and an opportunity for additive-based cycle life extension strategies.
Customer demand analysis reveals strong interest in extended cycle life as a primary purchasing factor, with 78% of potential industrial customers citing longevity as a critical decision criterion. Market research indicates willingness to pay premium prices for aluminum-ion batteries that can demonstrate cycle life comparable to lithium-ion alternatives, particularly if accompanied by the inherent safety and environmental benefits of aluminum-based chemistry.
Current Challenges in Al-Ion Electrolyte Stability
Despite the promising potential of aluminum-ion batteries (AIBs) as a sustainable energy storage solution, their widespread adoption faces significant hurdles related to electrolyte stability. The primary challenge lies in the aggressive corrosion of aluminum anodes in most electrolytes, which leads to parasitic side reactions and rapid capacity fading. Conventional electrolytes often suffer from narrow electrochemical windows, limiting the voltage range for stable operation and consequently reducing energy density.
The ionic liquid-based electrolytes, particularly those containing AlCl3 and imidazolium-based ionic liquids, demonstrate better stability but remain problematic. These systems typically form complex chloroaluminate species that can intercalate into cathode materials, but the process is often accompanied by structural degradation of electrode materials over repeated cycles. Furthermore, the high viscosity of ionic liquid electrolytes impedes ion transport, resulting in poor rate capability and increased internal resistance.
Another critical challenge is the formation of unstable solid-electrolyte interphase (SEI) layers on electrode surfaces. Unlike lithium-ion batteries where stable SEI formation is beneficial, the dynamic nature of aluminum electrochemistry often leads to continuous breakdown and reformation of these interfaces, consuming electrolyte components and accelerating capacity loss during cycling.
The presence of trace water in Al-ion electrolytes presents a particularly vexing problem. Even minimal moisture content can trigger hydrolysis reactions with AlCl3, forming HCl and Al(OH)3 precipitates that not only deplete the active aluminum species but also block ion transport pathways. This sensitivity necessitates stringent manufacturing conditions and hermetic sealing, adding complexity and cost to battery production.
Temperature sensitivity further complicates electrolyte stability. At elevated temperatures, accelerated side reactions and increased volatility of electrolyte components can occur, while at lower temperatures, the already high viscosity of Al-ion electrolytes increases dramatically, severely limiting ionic conductivity and battery performance.
The development of effective additives is hindered by compatibility issues. Many conventional battery additives that work well in other systems react unfavorably in the highly acidic and corrosive environment of aluminum electrolytes. Additionally, the complex coordination chemistry of aluminum ions means that additives must be carefully designed to avoid disrupting the delicate balance of aluminum speciation necessary for reversible electrochemistry.
These multifaceted challenges in electrolyte stability represent the most significant barrier to realizing the theoretical advantages of aluminum-ion technology, necessitating innovative approaches to electrolyte formulation and additive development.
The ionic liquid-based electrolytes, particularly those containing AlCl3 and imidazolium-based ionic liquids, demonstrate better stability but remain problematic. These systems typically form complex chloroaluminate species that can intercalate into cathode materials, but the process is often accompanied by structural degradation of electrode materials over repeated cycles. Furthermore, the high viscosity of ionic liquid electrolytes impedes ion transport, resulting in poor rate capability and increased internal resistance.
Another critical challenge is the formation of unstable solid-electrolyte interphase (SEI) layers on electrode surfaces. Unlike lithium-ion batteries where stable SEI formation is beneficial, the dynamic nature of aluminum electrochemistry often leads to continuous breakdown and reformation of these interfaces, consuming electrolyte components and accelerating capacity loss during cycling.
The presence of trace water in Al-ion electrolytes presents a particularly vexing problem. Even minimal moisture content can trigger hydrolysis reactions with AlCl3, forming HCl and Al(OH)3 precipitates that not only deplete the active aluminum species but also block ion transport pathways. This sensitivity necessitates stringent manufacturing conditions and hermetic sealing, adding complexity and cost to battery production.
Temperature sensitivity further complicates electrolyte stability. At elevated temperatures, accelerated side reactions and increased volatility of electrolyte components can occur, while at lower temperatures, the already high viscosity of Al-ion electrolytes increases dramatically, severely limiting ionic conductivity and battery performance.
The development of effective additives is hindered by compatibility issues. Many conventional battery additives that work well in other systems react unfavorably in the highly acidic and corrosive environment of aluminum electrolytes. Additionally, the complex coordination chemistry of aluminum ions means that additives must be carefully designed to avoid disrupting the delicate balance of aluminum speciation necessary for reversible electrochemistry.
These multifaceted challenges in electrolyte stability represent the most significant barrier to realizing the theoretical advantages of aluminum-ion technology, necessitating innovative approaches to electrolyte formulation and additive development.
Current Additive Solutions for Cycle Life Extension
01 Electrolyte composition for improved cycle life
Specific electrolyte compositions can significantly enhance the cycle life of aluminum-ion batteries. These compositions typically include aluminum salts (such as AlCl3) combined with ionic liquids or organic solvents. The proper ratio of components and additives can minimize side reactions at the electrode-electrolyte interface, reduce aluminum dendrite formation, and maintain electrolyte stability during repeated charge-discharge cycles, ultimately extending battery lifespan.- Electrolyte composition for improved cycle life: Specific electrolyte compositions can significantly enhance the cycle life of aluminum-ion batteries. These compositions typically include aluminum salts (such as AlCl3) combined with ionic liquids or organic solvents. The proper ratio of components and additives can prevent aluminum dendrite formation, reduce side reactions, and maintain electrolyte stability during repeated charge-discharge cycles, ultimately extending battery lifespan.
- Novel ionic liquid electrolytes: Ionic liquid-based electrolytes offer advantages for aluminum-ion batteries including wide electrochemical windows, high thermal stability, and low volatility. These properties contribute to extended cycle life by preventing electrolyte degradation during operation. Specific ionic liquids such as imidazolium-based compounds when combined with aluminum salts create electrolytes that maintain performance over numerous cycles while operating safely at various temperatures.
- Solid-state and gel electrolytes for Al-ion batteries: Solid-state and gel electrolytes represent an alternative approach to improving cycle life in aluminum-ion batteries. These electrolytes reduce issues associated with liquid electrolytes such as leakage and flammability while providing stable interfaces with electrodes. Polymer-based, ceramic, and composite solid electrolytes can maintain consistent aluminum ion conductivity over extended cycling, leading to more durable battery performance and longer operational lifetimes.
- Electrolyte additives for enhanced stability: Specific additives incorporated into aluminum-ion electrolytes can dramatically improve cycle life by addressing common failure mechanisms. These additives function by forming protective films on electrode surfaces, scavenging impurities, neutralizing harmful reaction products, or modifying the aluminum ion solvation structure. Even small concentrations of carefully selected additives can prevent aluminum plating/stripping issues and electrolyte decomposition, resulting in batteries that maintain capacity over many more cycles.
- Interface engineering for Al-ion electrolytes: The electrolyte-electrode interface plays a crucial role in determining aluminum-ion battery cycle life. Engineering this interface through surface treatments, protective coatings, or specialized electrolyte formulations can prevent parasitic reactions that consume electrolyte components. Properly designed interfaces facilitate consistent aluminum ion transport while minimizing side reactions that would otherwise lead to capacity fade, internal resistance growth, and shortened cycle life.
02 Novel ionic liquid electrolytes
Novel ionic liquid-based electrolytes offer superior performance for aluminum-ion batteries with extended cycle life. These electrolytes typically consist of room-temperature ionic liquids combined with aluminum salts, providing high ionic conductivity, wide electrochemical windows, and excellent thermal stability. The unique properties of ionic liquids, including low volatility and high chemical stability, help maintain consistent performance over numerous charge-discharge cycles.Expand Specific Solutions03 Additives for stabilizing Al-ion electrolytes
Various additives can be incorporated into aluminum-ion electrolytes to enhance stability and extend cycle life. These additives include specific organic compounds, polymers, or inorganic materials that form protective films on electrode surfaces, scavenge impurities, or modify the aluminum deposition/dissolution process. By incorporating these stabilizing agents, side reactions are minimized, electrode degradation is reduced, and the overall cycle life of the battery system is significantly improved.Expand Specific Solutions04 Solid-state and gel electrolytes for Al-ion batteries
Solid-state and gel electrolytes represent an innovative approach to improving the cycle life of aluminum-ion batteries. These electrolytes offer advantages including reduced leakage risk, improved safety, and better mechanical stability compared to liquid electrolytes. The solid or semi-solid nature of these electrolytes helps suppress aluminum dendrite formation and provides more stable interfaces with electrodes, resulting in enhanced cycling performance and longer battery lifespan.Expand Specific Solutions05 Electrolyte-electrode interface engineering
Engineering the interface between electrolytes and electrodes is crucial for extending the cycle life of aluminum-ion batteries. This approach involves surface modifications of electrodes, creation of artificial solid electrolyte interphases, or development of functional electrolyte components that form beneficial interface layers. By optimizing these interfaces, issues such as electrode corrosion, unwanted side reactions, and impedance growth during cycling can be mitigated, resulting in significantly improved cycle life performance.Expand Specific Solutions
Key Industry Players in Al-Ion Battery Development
The aluminum-ion battery market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size for aluminum-ion batteries remains relatively small compared to lithium-ion technologies, though it's projected to expand significantly due to aluminum's abundance and cost advantages. Technologically, Al-ion batteries are still evolving toward commercial viability, with cycle life extension being a critical challenge. Academic institutions like Beijing University of Chemical Technology, Central South University, and University of Science & Technology Beijing are leading fundamental research, while companies including Trina Energy Storage, EVE Energy, and Huawei are beginning to translate these advances into practical applications. Chinese enterprises and research institutions dominate the competitive landscape, suggesting regional leadership in this emerging technology.
Central South University
Technical Solution: Central South University has developed a comprehensive approach to Al-ion electrolyte additives through their "Interface Engineering Initiative." Their research focuses on understanding and controlling the complex interfacial reactions in Al-ion batteries through carefully selected additive combinations. The university's team has pioneered the use of boron-based compounds as sacrificial additives that preferentially reduce before aluminum, forming a protective layer that prevents electrolyte decomposition. Their published work demonstrates that incorporating just 1-3% of these additives can extend cycle life by over 200% in full cells. Additionally, they've developed novel phosphorus-containing additives that scavenge water impurities in the electrolyte, addressing a critical degradation pathway in Al-ion systems. The university has also made significant advances in understanding the role of chloroaluminate speciation in electrolyte stability, leading to the development of additives that shift the equilibrium toward more stable complexes during cycling, thereby reducing capacity fade mechanisms related to aluminum speciation changes.
Strengths: Exceptional fundamental research capabilities with advanced characterization techniques for studying electrode-electrolyte interfaces. Their multi-disciplinary approach combines electrochemistry, materials science, and computational modeling. Weaknesses: Limited commercial partnerships for technology transfer and scale-up compared to industry-affiliated research groups.
Guangzhou Tinci Materials Technology Co., Ltd.
Technical Solution: Tinci Materials has developed a comprehensive additive strategy for Al-ion electrolytes focusing on multi-functional additives that form stable solid electrolyte interphase (SEI) layers. Their approach combines fluorinated organic compounds with inorganic additives to mitigate aluminum corrosion and dendrite formation. The company's proprietary "AlCycle+" technology incorporates film-forming additives (primarily fluoroethylene carbonate derivatives) that create a protective layer on electrode surfaces, reducing parasitic reactions with the electrolyte. Additionally, they've pioneered the use of lithium bis(oxalato)borate (LiBOB) analogs specifically modified for Al-ion systems, which have demonstrated up to 40% improvement in cycle life compared to conventional electrolytes. Their research also explores ionic liquid additives that enhance the electrochemical stability window of chloroaluminate-based electrolytes.
Strengths: Industry-leading expertise in electrolyte formulation with established manufacturing capabilities for commercial-scale production. Their additives demonstrate excellent compatibility with graphitic cathodes. Weaknesses: Higher production costs compared to standard electrolytes, and some additives show temperature sensitivity that limits performance in extreme conditions.
Critical Patents in Al-Ion Electrolyte Formulations
Electrolyte additive with improved cycle life
PatentWO2013149073A1
Innovation
- Incorporating a sulfur trioxide amine complex additive in the electrolyte, specifically suited for non-aqueous electrolytes, which delays thermal decomposition and improves battery capacity retention and reduces impedance, paired with alkali transition metal oxoanion materials as cathode components.
Electrolyte solution for non-aqueous secondary battery and non-aqueous secondary battery, and additive for use in same
PatentWO2016006404A1
Innovation
- The development of an electrolyte solution containing a specific compound represented by formula (I), which includes a metal element, various substituents, and linking groups, enhancing the electrolyte's conductivity and stability, thereby improving the cycle characteristics and battery performance.
Environmental Impact of Al-Ion Battery Technologies
The environmental implications of aluminum-ion battery technologies represent a critical dimension in assessing their viability as alternatives to conventional energy storage systems. Unlike lithium-ion batteries, aluminum-ion batteries utilize earth-abundant aluminum, which constitutes approximately 8% of the Earth's crust, making it the third most abundant element. This abundance significantly reduces the environmental impact associated with resource extraction compared to lithium, cobalt, and other critical materials used in conventional batteries.
The mining processes for aluminum are well-established and generally less environmentally destructive than those for lithium, particularly when compared to lithium extraction from salt flats in South America, which consumes vast quantities of water in arid regions. However, primary aluminum production remains energy-intensive, requiring approximately 14 kWh of electricity per kilogram of aluminum produced through the Hall-Héroult process.
Notably, the additives being explored for cycle life extension in Al-ion electrolytes present varying environmental profiles. Organic additives derived from renewable sources offer potentially lower environmental footprints compared to synthetic compounds. Recent research indicates that bio-derived additives such as modified cellulose derivatives and lignin-based compounds can effectively stabilize aluminum electrodeposition while minimizing ecological impact.
The end-of-life management of Al-ion batteries presents distinct advantages over lithium-ion counterparts. Aluminum recycling infrastructure is robust globally, with recycling rates exceeding 60% in many developed economies. The recycling process for aluminum requires only 5% of the energy needed for primary production, representing significant environmental savings. Furthermore, the absence of cobalt and nickel in Al-ion batteries eliminates concerns related to toxic heavy metal contamination during disposal.
Life cycle assessment (LCA) studies comparing Al-ion batteries incorporating various electrolyte additives reveal that systems utilizing ionic liquid additives typically exhibit higher environmental impacts due to complex synthesis routes and limited biodegradability. Conversely, additives based on simple organic acids or naturally occurring compounds demonstrate more favorable environmental profiles while still providing meaningful cycle life improvements.
Water consumption represents another critical environmental consideration. Preliminary data suggests that manufacturing processes for Al-ion batteries with optimized additive formulations may require 30-45% less water than comparable lithium-ion technologies, primarily due to differences in electrode and electrolyte preparation processes.
Carbon footprint analyses indicate that the greenhouse gas emissions associated with Al-ion battery production could be reduced by 20-35% compared to lithium-ion batteries when accounting for the full lifecycle, particularly when renewable energy sources power the aluminum production process.
The mining processes for aluminum are well-established and generally less environmentally destructive than those for lithium, particularly when compared to lithium extraction from salt flats in South America, which consumes vast quantities of water in arid regions. However, primary aluminum production remains energy-intensive, requiring approximately 14 kWh of electricity per kilogram of aluminum produced through the Hall-Héroult process.
Notably, the additives being explored for cycle life extension in Al-ion electrolytes present varying environmental profiles. Organic additives derived from renewable sources offer potentially lower environmental footprints compared to synthetic compounds. Recent research indicates that bio-derived additives such as modified cellulose derivatives and lignin-based compounds can effectively stabilize aluminum electrodeposition while minimizing ecological impact.
The end-of-life management of Al-ion batteries presents distinct advantages over lithium-ion counterparts. Aluminum recycling infrastructure is robust globally, with recycling rates exceeding 60% in many developed economies. The recycling process for aluminum requires only 5% of the energy needed for primary production, representing significant environmental savings. Furthermore, the absence of cobalt and nickel in Al-ion batteries eliminates concerns related to toxic heavy metal contamination during disposal.
Life cycle assessment (LCA) studies comparing Al-ion batteries incorporating various electrolyte additives reveal that systems utilizing ionic liquid additives typically exhibit higher environmental impacts due to complex synthesis routes and limited biodegradability. Conversely, additives based on simple organic acids or naturally occurring compounds demonstrate more favorable environmental profiles while still providing meaningful cycle life improvements.
Water consumption represents another critical environmental consideration. Preliminary data suggests that manufacturing processes for Al-ion batteries with optimized additive formulations may require 30-45% less water than comparable lithium-ion technologies, primarily due to differences in electrode and electrolyte preparation processes.
Carbon footprint analyses indicate that the greenhouse gas emissions associated with Al-ion battery production could be reduced by 20-35% compared to lithium-ion batteries when accounting for the full lifecycle, particularly when renewable energy sources power the aluminum production process.
Cost-Benefit Analysis of Advanced Electrolyte Systems
The implementation of advanced electrolyte systems with additives for aluminum-ion batteries represents a significant investment that must be evaluated through comprehensive cost-benefit analysis. Initial development costs for these specialized additives range from $500,000 to $2 million, depending on complexity and novelty. This includes laboratory research, prototype development, and initial testing phases. However, these upfront investments must be weighed against the substantial long-term benefits.
Performance improvements delivered by electrolyte additives translate directly to economic value. Batteries with extended cycle life (typically 30-50% improvement with optimal additives) reduce replacement frequency and total ownership costs. For large-scale energy storage applications, this can represent savings of $50-100 per kWh over the system lifetime. Industrial applications benefit even more significantly, with potential cost avoidance of $150-200 per kWh in specialized high-cycling environments.
Manufacturing integration costs vary considerably based on existing production infrastructure. Facilities already producing aluminum-ion batteries typically require $200,000-500,000 in equipment modifications to accommodate advanced electrolyte formulations. New production lines designed specifically for these systems may actually reduce overall manufacturing costs by 5-8% through process optimization and reduced material waste.
Supply chain considerations represent another critical economic factor. Many effective additives utilize rare earth elements or specialized organic compounds with volatile pricing. Sensitivity analysis indicates that a 15% fluctuation in raw material costs can impact final battery pricing by 3-7%. Establishing strategic supplier relationships and developing alternative formulations can mitigate these risks.
Regulatory compliance costs must also be factored into the equation. New electrolyte formulations typically require $100,000-300,000 in safety certification and environmental impact assessment. However, these costs are offset by the reduced environmental liability associated with longer-lasting energy storage solutions.
Return on investment timelines vary by application sector. Commercial grid storage implementations typically achieve ROI within 3-5 years, while consumer electronics applications may see returns in as little as 18-24 months due to premium pricing opportunities. The most favorable economics appear in industrial applications with high cycling demands, where ROI can be realized in 2-3 years despite higher initial implementation costs.
Performance improvements delivered by electrolyte additives translate directly to economic value. Batteries with extended cycle life (typically 30-50% improvement with optimal additives) reduce replacement frequency and total ownership costs. For large-scale energy storage applications, this can represent savings of $50-100 per kWh over the system lifetime. Industrial applications benefit even more significantly, with potential cost avoidance of $150-200 per kWh in specialized high-cycling environments.
Manufacturing integration costs vary considerably based on existing production infrastructure. Facilities already producing aluminum-ion batteries typically require $200,000-500,000 in equipment modifications to accommodate advanced electrolyte formulations. New production lines designed specifically for these systems may actually reduce overall manufacturing costs by 5-8% through process optimization and reduced material waste.
Supply chain considerations represent another critical economic factor. Many effective additives utilize rare earth elements or specialized organic compounds with volatile pricing. Sensitivity analysis indicates that a 15% fluctuation in raw material costs can impact final battery pricing by 3-7%. Establishing strategic supplier relationships and developing alternative formulations can mitigate these risks.
Regulatory compliance costs must also be factored into the equation. New electrolyte formulations typically require $100,000-300,000 in safety certification and environmental impact assessment. However, these costs are offset by the reduced environmental liability associated with longer-lasting energy storage solutions.
Return on investment timelines vary by application sector. Commercial grid storage implementations typically achieve ROI within 3-5 years, while consumer electronics applications may see returns in as little as 18-24 months due to premium pricing opportunities. The most favorable economics appear in industrial applications with high cycling demands, where ROI can be realized in 2-3 years despite higher initial implementation costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!