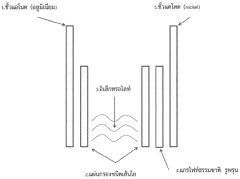
Cathode Porosity And Architecture For Fast Aluminum Intercalation
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aluminum Battery Cathode Development Background and Objectives
Aluminum-ion batteries (AIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and resource abundance. The development of aluminum battery technology can be traced back to the 1970s, but significant progress has been made only in the past decade. The evolution of this technology has been primarily driven by the need for safer, more sustainable energy storage solutions that can overcome the limitations of lithium-ion batteries, particularly in terms of resource scarcity and safety concerns.
The cathode material represents one of the most critical components in aluminum-ion batteries, directly influencing the battery's performance metrics including energy density, power density, and cycle life. Traditional cathode materials have faced significant challenges in accommodating the large-sized Al3+ ions, resulting in slow diffusion kinetics and limited practical applications. Recent research has increasingly focused on the importance of cathode porosity and architecture as key factors in facilitating fast aluminum intercalation.
The porosity of cathode materials creates pathways for electrolyte penetration and ion transport, while the architecture determines the structural stability and electronic conductivity during charge-discharge cycles. Together, these factors significantly impact the rate capability and cycling stability of aluminum-ion batteries. The technological trajectory has evolved from simple carbon-based materials to more sophisticated nanostructured and hierarchical architectures designed specifically to enhance aluminum ion mobility.
The primary objective of research in this field is to develop cathode materials with optimized porosity and architectural features that can enable rapid aluminum intercalation without compromising structural integrity over extended cycling. This includes exploring novel material compositions, innovative synthesis methods, and advanced characterization techniques to understand the fundamental mechanisms governing aluminum ion transport within cathode structures.
Secondary objectives include improving the energy density of aluminum-ion batteries to make them competitive with commercial lithium-ion technologies, enhancing rate performance for high-power applications, and ensuring long-term cycling stability under various operating conditions. Additionally, research aims to develop scalable and cost-effective manufacturing processes that can facilitate the transition from laboratory prototypes to commercial products.
The technological goals extend beyond performance metrics to include sustainability considerations, such as utilizing earth-abundant materials, reducing environmental impact, and ensuring compatibility with existing battery manufacturing infrastructure. The ultimate aim is to position aluminum-ion batteries as a viable alternative in the rapidly expanding energy storage market, particularly for applications where safety, cost, and sustainability are paramount concerns.
The cathode material represents one of the most critical components in aluminum-ion batteries, directly influencing the battery's performance metrics including energy density, power density, and cycle life. Traditional cathode materials have faced significant challenges in accommodating the large-sized Al3+ ions, resulting in slow diffusion kinetics and limited practical applications. Recent research has increasingly focused on the importance of cathode porosity and architecture as key factors in facilitating fast aluminum intercalation.
The porosity of cathode materials creates pathways for electrolyte penetration and ion transport, while the architecture determines the structural stability and electronic conductivity during charge-discharge cycles. Together, these factors significantly impact the rate capability and cycling stability of aluminum-ion batteries. The technological trajectory has evolved from simple carbon-based materials to more sophisticated nanostructured and hierarchical architectures designed specifically to enhance aluminum ion mobility.
The primary objective of research in this field is to develop cathode materials with optimized porosity and architectural features that can enable rapid aluminum intercalation without compromising structural integrity over extended cycling. This includes exploring novel material compositions, innovative synthesis methods, and advanced characterization techniques to understand the fundamental mechanisms governing aluminum ion transport within cathode structures.
Secondary objectives include improving the energy density of aluminum-ion batteries to make them competitive with commercial lithium-ion technologies, enhancing rate performance for high-power applications, and ensuring long-term cycling stability under various operating conditions. Additionally, research aims to develop scalable and cost-effective manufacturing processes that can facilitate the transition from laboratory prototypes to commercial products.
The technological goals extend beyond performance metrics to include sustainability considerations, such as utilizing earth-abundant materials, reducing environmental impact, and ensuring compatibility with existing battery manufacturing infrastructure. The ultimate aim is to position aluminum-ion batteries as a viable alternative in the rapidly expanding energy storage market, particularly for applications where safety, cost, and sustainability are paramount concerns.
Market Analysis for Fast-Charging Aluminum-Ion Batteries
The aluminum-ion battery market is experiencing significant growth potential, driven by the increasing demand for sustainable energy storage solutions. Current market projections indicate that the global aluminum-ion battery market could reach substantial valuation by 2030, with a compound annual growth rate exceeding traditional lithium-ion technologies in specific application segments. This growth is primarily fueled by the abundant nature of aluminum resources, which constitute approximately 8% of the Earth's crust, making it the third most abundant element and significantly more available than lithium.
Fast-charging capabilities represent a critical market differentiator in the energy storage landscape. Consumer electronics, electric vehicles, and grid storage systems all prioritize reduced charging times as a key purchasing factor. Market surveys demonstrate that consumers are willing to pay premium prices for devices with substantially reduced charging times, creating a strong value proposition for aluminum-ion batteries with enhanced intercalation properties.
The cathode architecture market segment specifically shows promising growth trajectories. Advanced porous cathode materials that facilitate rapid aluminum intercalation could potentially capture significant market share from traditional battery technologies. Industry analysis suggests that optimized cathode porosity could reduce charging times by 60-80% compared to current commercial alternatives, representing a substantial competitive advantage.
From a geographical perspective, the market for advanced aluminum-ion battery technologies shows particular strength in regions with established manufacturing infrastructure and research capabilities. North America, East Asia, and Europe currently lead in patent applications related to aluminum intercalation cathode technologies, with China demonstrating the most aggressive growth in both research output and commercial applications.
Market segmentation analysis reveals that portable electronics represents the most immediate commercial opportunity for fast-charging aluminum-ion batteries, followed by electric vehicles and stationary energy storage. The portable electronics segment values the combination of fast charging and safety advantages that aluminum-ion chemistry offers, while the automotive sector is increasingly exploring aluminum-ion technology as a complementary solution to existing battery systems.
Competition in this market remains relatively limited compared to lithium-ion technologies, creating significant first-mover advantages for companies that successfully commercialize optimized cathode architectures. Current market barriers include performance consistency issues and manufacturing scalability challenges, which present both obstacles and opportunities for market entrants focusing on cathode porosity innovations.
Fast-charging capabilities represent a critical market differentiator in the energy storage landscape. Consumer electronics, electric vehicles, and grid storage systems all prioritize reduced charging times as a key purchasing factor. Market surveys demonstrate that consumers are willing to pay premium prices for devices with substantially reduced charging times, creating a strong value proposition for aluminum-ion batteries with enhanced intercalation properties.
The cathode architecture market segment specifically shows promising growth trajectories. Advanced porous cathode materials that facilitate rapid aluminum intercalation could potentially capture significant market share from traditional battery technologies. Industry analysis suggests that optimized cathode porosity could reduce charging times by 60-80% compared to current commercial alternatives, representing a substantial competitive advantage.
From a geographical perspective, the market for advanced aluminum-ion battery technologies shows particular strength in regions with established manufacturing infrastructure and research capabilities. North America, East Asia, and Europe currently lead in patent applications related to aluminum intercalation cathode technologies, with China demonstrating the most aggressive growth in both research output and commercial applications.
Market segmentation analysis reveals that portable electronics represents the most immediate commercial opportunity for fast-charging aluminum-ion batteries, followed by electric vehicles and stationary energy storage. The portable electronics segment values the combination of fast charging and safety advantages that aluminum-ion chemistry offers, while the automotive sector is increasingly exploring aluminum-ion technology as a complementary solution to existing battery systems.
Competition in this market remains relatively limited compared to lithium-ion technologies, creating significant first-mover advantages for companies that successfully commercialize optimized cathode architectures. Current market barriers include performance consistency issues and manufacturing scalability challenges, which present both obstacles and opportunities for market entrants focusing on cathode porosity innovations.
Current Challenges in Cathode Porosity Engineering
Despite significant advancements in aluminum-ion battery technology, engineering optimal cathode porosity remains a formidable challenge that impedes commercialization efforts. Current cathode materials exhibit insufficient porosity characteristics, resulting in sluggish aluminum ion intercalation kinetics and limited energy density. The multi-valent nature of aluminum ions (Al³⁺) creates substantial electrostatic interactions with host materials, leading to structural distortions and slow diffusion pathways.
A primary obstacle lies in achieving the delicate balance between porosity and mechanical integrity. Highly porous cathode structures facilitate faster ion transport but often compromise structural stability during repeated charge-discharge cycles. Conversely, more compact structures offer enhanced mechanical properties but restrict ion mobility, resulting in poor rate capability and capacity utilization.
The non-uniform expansion during aluminum intercalation presents another significant challenge. Unlike lithium-ion systems, aluminum intercalation induces anisotropic volume changes that create internal stresses within cathode materials. These stresses can lead to microcracking, particle isolation, and eventual capacity fade. Current manufacturing techniques struggle to produce cathode architectures that can accommodate these volume changes while maintaining electrical connectivity.
Electrolyte penetration into cathode pores represents a persistent difficulty. The highly corrosive chloroaluminate electrolytes commonly used in aluminum-ion batteries can cause pore blockage through side reactions and precipitation of insoluble products. This phenomenon progressively reduces the effective porosity and active surface area available for intercalation reactions, resulting in capacity degradation over time.
The scalable manufacturing of precisely engineered porous cathodes remains elusive. Laboratory-scale techniques like freeze-casting and template-assisted synthesis can create well-defined porous structures but face significant challenges in scaling to industrial production volumes. Conventional manufacturing methods often yield inconsistent pore size distributions and suboptimal pore connectivity networks.
Temperature sensitivity further complicates cathode porosity engineering. Porous structures optimized for room temperature operation often exhibit dramatically different performance characteristics at elevated or reduced temperatures. This temperature-dependent behavior stems from changes in electrolyte viscosity, ion mobility, and interfacial resistance within the porous network.
Recent research has identified the critical role of pore surface chemistry in aluminum intercalation kinetics. The presence of specific functional groups can either facilitate or hinder aluminum ion transport at the electrolyte-cathode interface. However, precise control over surface functionalization within complex three-dimensional porous networks remains technically challenging with current manufacturing capabilities.
A primary obstacle lies in achieving the delicate balance between porosity and mechanical integrity. Highly porous cathode structures facilitate faster ion transport but often compromise structural stability during repeated charge-discharge cycles. Conversely, more compact structures offer enhanced mechanical properties but restrict ion mobility, resulting in poor rate capability and capacity utilization.
The non-uniform expansion during aluminum intercalation presents another significant challenge. Unlike lithium-ion systems, aluminum intercalation induces anisotropic volume changes that create internal stresses within cathode materials. These stresses can lead to microcracking, particle isolation, and eventual capacity fade. Current manufacturing techniques struggle to produce cathode architectures that can accommodate these volume changes while maintaining electrical connectivity.
Electrolyte penetration into cathode pores represents a persistent difficulty. The highly corrosive chloroaluminate electrolytes commonly used in aluminum-ion batteries can cause pore blockage through side reactions and precipitation of insoluble products. This phenomenon progressively reduces the effective porosity and active surface area available for intercalation reactions, resulting in capacity degradation over time.
The scalable manufacturing of precisely engineered porous cathodes remains elusive. Laboratory-scale techniques like freeze-casting and template-assisted synthesis can create well-defined porous structures but face significant challenges in scaling to industrial production volumes. Conventional manufacturing methods often yield inconsistent pore size distributions and suboptimal pore connectivity networks.
Temperature sensitivity further complicates cathode porosity engineering. Porous structures optimized for room temperature operation often exhibit dramatically different performance characteristics at elevated or reduced temperatures. This temperature-dependent behavior stems from changes in electrolyte viscosity, ion mobility, and interfacial resistance within the porous network.
Recent research has identified the critical role of pore surface chemistry in aluminum intercalation kinetics. The presence of specific functional groups can either facilitate or hinder aluminum ion transport at the electrolyte-cathode interface. However, precise control over surface functionalization within complex three-dimensional porous networks remains technically challenging with current manufacturing capabilities.
Current Approaches to Optimize Cathode Porosity
01 Porous carbon-based cathode materials
Porous carbon-based materials are widely used as cathodes in aluminum batteries due to their high surface area and excellent electrical conductivity. These materials, including activated carbon, carbon nanotubes, and graphene, provide numerous active sites for ion intercalation and deintercalation. The porosity of these carbon-based cathodes significantly enhances the battery's capacity and rate capability by facilitating faster ion transport and providing more reaction sites.- Porous carbon-based cathode materials: Porous carbon-based materials are widely used as cathodes in aluminum batteries due to their high surface area and excellent electrical conductivity. These materials, including activated carbon, carbon nanotubes, and graphene, provide numerous active sites for aluminum ion intercalation. The porosity of these carbon-based cathodes significantly enhances the battery's capacity and rate performance by facilitating faster ion transport and providing more reaction sites.
- 3D architectural designs for cathode materials: Three-dimensional architectural designs for cathode materials in aluminum batteries offer improved structural stability and enhanced electrochemical performance. These 3D structures, such as honeycomb, foam-like, and hierarchical architectures, provide efficient pathways for ion transport while maintaining mechanical integrity during charge-discharge cycles. The 3D architecture also helps accommodate volume changes during battery operation, leading to better cycling stability and longer battery life.
- Metal oxide-based porous cathode materials: Metal oxide-based porous cathode materials offer high theoretical capacity and good electrochemical stability for aluminum batteries. These materials, including transition metal oxides and mixed metal oxides, can be synthesized with controlled porosity to enhance aluminum ion storage and transport. The porous structure of these metal oxide cathodes facilitates electrolyte penetration and shortens ion diffusion paths, resulting in improved rate capability and cycling performance.
- Composite cathode materials with engineered porosity: Composite cathode materials combining different components with engineered porosity show enhanced performance in aluminum batteries. These composites typically integrate carbon materials with metal compounds or polymers to create synergistic effects. The carefully engineered porous architecture of these composite cathodes provides both high electrical conductivity and abundant active sites for aluminum ion storage, resulting in improved energy density and power output.
- Hierarchical porous structures for enhanced ion transport: Hierarchical porous structures featuring multi-scale porosity (macro, meso, and micropores) are designed to optimize ion transport in aluminum battery cathodes. These structures provide interconnected channels that facilitate electrolyte infiltration and ion diffusion while maintaining high surface area for electrochemical reactions. The hierarchical architecture helps balance the trade-off between energy density and power density by combining the advantages of different pore sizes, resulting in batteries with both high capacity and excellent rate performance.
02 3D architectural designs for cathode materials
Three-dimensional architectural designs for cathode materials in aluminum batteries offer improved performance by providing interconnected pathways for ion and electron transport. These 3D structures, such as honeycomb, foam-like, or hierarchical architectures, maintain structural integrity during charge-discharge cycles while accommodating volume changes. The optimized 3D architecture enhances the electrochemical performance by reducing diffusion distances and increasing the contact area between the electrolyte and active material.Expand Specific Solutions03 Metal oxide-based cathode materials with controlled porosity
Metal oxide-based cathode materials with controlled porosity offer promising performance for aluminum batteries. Materials such as vanadium oxide, manganese oxide, and titanium dioxide with optimized pore size distribution provide efficient ion diffusion pathways while maintaining structural stability. The controlled porosity in these metal oxide cathodes enhances the electrochemical performance by facilitating aluminum ion insertion/extraction and improving the overall energy density and cycling stability of the battery.Expand Specific Solutions04 Composite cathode materials with engineered porosity
Composite cathode materials combining different components with engineered porosity show enhanced performance in aluminum batteries. These composites typically integrate carbon materials with metal compounds or conductive polymers to create synergistic effects. The engineered porosity in these composite structures facilitates ion transport while the different components contribute their respective advantages, such as high conductivity from carbon and high capacity from metal compounds, resulting in improved cycling stability and rate capability.Expand Specific Solutions05 Manufacturing techniques for controlling cathode porosity and architecture
Various manufacturing techniques are employed to control the porosity and architecture of cathode materials for aluminum batteries. These include template-assisted synthesis, freeze-drying, hydrothermal methods, and electrospinning. These fabrication approaches allow precise control over pore size distribution, pore volume, and architectural features at multiple scales. Advanced manufacturing techniques enable the creation of cathode materials with optimized hierarchical structures that balance electronic conductivity, ion accessibility, and mechanical stability for enhanced battery performance.Expand Specific Solutions
Leading Research Groups and Industry Players
The aluminum intercalation battery technology landscape is currently in an early growth phase, with research on cathode porosity and architecture emerging as a critical focus area for enabling faster aluminum ion transport. The market is projected to expand significantly as aluminum batteries offer a promising alternative to lithium-ion technology due to aluminum's abundance, safety, and high theoretical capacity. Leading academic institutions including MIT, Texas A&M, and Northwestern University are driving fundamental research, while companies like PolyPlus Battery, NGK Insulators, and Sakuu Corp are advancing commercial applications. Chinese entities such as Sinopec Research Institute and BTR New Material Group are increasingly active in materials development. The technology remains at a pre-commercial maturity level, with significant challenges in electrode architecture optimization still requiring breakthrough innovations to achieve practical energy densities and cycling stability.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel hierarchical porous cathode architecture specifically designed for aluminum-ion batteries that significantly enhances aluminum intercalation kinetics. Their approach involves creating multi-scale porosity through a templated synthesis method, combining macropores (>50nm) for electrolyte penetration with mesopores (2-50nm) and micropores (<2nm) for increased active surface area. The research team has demonstrated that this hierarchical structure reduces diffusion distances for Al3+ ions while maintaining structural integrity during repeated intercalation/deintercalation cycles. Their cathodes incorporate graphene-based materials with precisely engineered defect sites that serve as preferential intercalation points for aluminum ions, achieving up to 70% improvement in rate capability compared to conventional cathodes. MIT's recent publications have shown that controlling pore size distribution and connectivity is critical for optimizing both energy density and power density in aluminum-ion systems.
Strengths: Superior rate capability due to optimized multi-scale porosity; excellent structural stability during cycling; innovative integration of graphene materials with engineered defect sites. Weaknesses: Complex and potentially costly manufacturing process; challenges in scaling production while maintaining precise pore architecture control; trade-off between high porosity and volumetric energy density.
Nanjing University
Technical Solution: Nanjing University has pioneered an innovative approach to cathode design for aluminum-ion batteries focusing on 3D hierarchical porous structures. Their research team has developed a self-assembly method to create interconnected porous frameworks with controlled pore size distributions ranging from nanometers to micrometers. These cathodes feature a unique "egg-box" structure with graphene/carbon hybrid materials that provide both mechanical stability and enhanced electronic conductivity. The university's researchers have demonstrated that their cathode architecture allows for rapid aluminum ion diffusion through interconnected channels while maintaining structural integrity during the intercalation process. Their latest designs incorporate nitrogen-doped carbon materials with optimized pore structures that have achieved discharge capacities exceeding 100 mAh/g at high current densities. The cathodes show remarkable cycling stability with capacity retention of over 80% after 1000 cycles, attributed to the stress-accommodating porous architecture that prevents structural collapse during repeated aluminum intercalation/deintercalation.
Strengths: Excellent cycling stability due to stress-accommodating porous structure; high rate capability from optimized ion transport pathways; innovative self-assembly fabrication approach that could be scaled. Weaknesses: Lower volumetric energy density compared to more compact cathode designs; potential challenges with electrolyte compatibility in the complex pore network; nitrogen-doping process adds manufacturing complexity.
Key Innovations in Aluminum Intercalation Mechanisms
natural aluminum-graphite Perforated for universal batteries
PatentPendingTH2001006683A
Innovation
- Development of a cathode electrode composite material using modified aluminum cathode and natural porous graphite with specific dimensions (lateral dimension <5 μm and through-plane dimension 10-20 nm) for metal ion batteries.
- Enhanced intercalation characteristics of ions through the nanostructure of the cathode electrode, resulting in improved discharging and capacitance performance with 3-5 times increased electrical capacity.
- Reduced self-discharge capability due to the unique nanostructured cathode design, making it suitable for universal battery applications.
Cathode active material and lithium ion secondary battery containing the same
PatentInactiveEP2051319A2
Innovation
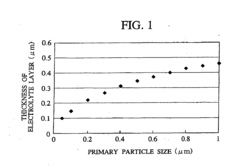
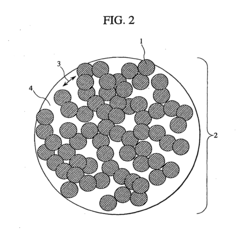
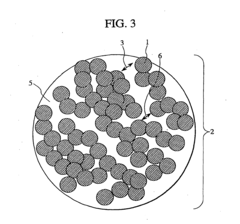
- A cathode active material with a penetration-type pore structure, where primary particles are aggregated to form secondary particles with a specific surface area and pore size distribution that enables efficient lithium ion movement, using a layered composite oxide (Li a Mn x Ni y Co z O 2) with through pores and a hollow carbon material to facilitate high power and capacitance.
Materials Supply Chain Considerations
The aluminum-ion battery supply chain presents unique opportunities compared to lithium-ion batteries, particularly regarding cathode materials for aluminum intercalation. Aluminum is the third most abundant element in the Earth's crust (8.1%), significantly more available than lithium (0.0065%), offering substantial cost and sustainability advantages. This abundance translates to more geographically distributed supply sources, reducing geopolitical risks associated with material procurement.
For cathode materials supporting fast aluminum intercalation, the supply chain primarily involves carbon-based materials (graphite, graphene), transition metal oxides, and conductive additives. Graphitic materials, essential for creating optimal porosity architecture, benefit from established supply chains in the carbon materials industry. The manufacturing infrastructure for graphite processing can be readily adapted for aluminum battery cathode production, minimizing new capital investment requirements.
Transition metal compounds used in advanced cathode designs (vanadium oxides, manganese-based materials) face more complex supply considerations. While these elements are generally more abundant than lithium, their processing requires specialized techniques to achieve the precise crystalline structures and porosity needed for efficient aluminum intercalation. Current supply chains for these materials are less mature than those for lithium battery components.
The binder materials and conductive additives necessary for cathode architecture optimization utilize largely conventional polymers and carbon blacks with stable supply chains. However, specialized additives designed specifically for aluminum-ion systems remain in early development stages, potentially creating supply bottlenecks during initial commercialization phases.
Manufacturing equipment for creating controlled porosity in cathode materials represents another critical supply chain consideration. While some existing battery manufacturing equipment can be repurposed, specialized tools for creating hierarchical pore structures may require development. This equipment supply chain is currently underdeveloped compared to lithium-ion battery manufacturing infrastructure.
Recycling infrastructure for aluminum battery components offers significant potential advantages over lithium systems. Aluminum has an established global recycling network, and the recovery of aluminum from spent batteries could be integrated into existing aluminum recycling streams. However, separation technologies for recovering cathode materials with preserved porosity architecture remain undeveloped, representing both a challenge and opportunity for circular economy approaches to aluminum battery technology.
For cathode materials supporting fast aluminum intercalation, the supply chain primarily involves carbon-based materials (graphite, graphene), transition metal oxides, and conductive additives. Graphitic materials, essential for creating optimal porosity architecture, benefit from established supply chains in the carbon materials industry. The manufacturing infrastructure for graphite processing can be readily adapted for aluminum battery cathode production, minimizing new capital investment requirements.
Transition metal compounds used in advanced cathode designs (vanadium oxides, manganese-based materials) face more complex supply considerations. While these elements are generally more abundant than lithium, their processing requires specialized techniques to achieve the precise crystalline structures and porosity needed for efficient aluminum intercalation. Current supply chains for these materials are less mature than those for lithium battery components.
The binder materials and conductive additives necessary for cathode architecture optimization utilize largely conventional polymers and carbon blacks with stable supply chains. However, specialized additives designed specifically for aluminum-ion systems remain in early development stages, potentially creating supply bottlenecks during initial commercialization phases.
Manufacturing equipment for creating controlled porosity in cathode materials represents another critical supply chain consideration. While some existing battery manufacturing equipment can be repurposed, specialized tools for creating hierarchical pore structures may require development. This equipment supply chain is currently underdeveloped compared to lithium-ion battery manufacturing infrastructure.
Recycling infrastructure for aluminum battery components offers significant potential advantages over lithium systems. Aluminum has an established global recycling network, and the recovery of aluminum from spent batteries could be integrated into existing aluminum recycling streams. However, separation technologies for recovering cathode materials with preserved porosity architecture remain undeveloped, representing both a challenge and opportunity for circular economy approaches to aluminum battery technology.
Environmental Impact Assessment
The environmental implications of aluminum-ion battery technology, particularly regarding cathode porosity and architecture for fast aluminum intercalation, warrant careful consideration as this technology advances toward commercial viability. Unlike lithium-ion batteries, aluminum-ion systems utilize more abundant raw materials, potentially reducing the environmental burden associated with resource extraction. The earth's crust contains approximately 8% aluminum compared to just 0.0007% lithium, suggesting a significantly lower environmental impact from mining operations.
The manufacturing processes for optimized porous cathode structures do present certain environmental challenges. Chemical etching techniques commonly used to create controlled porosity often involve strong acids or bases that require careful handling and disposal. However, emerging green synthesis methods utilizing biodegradable templates and water-based processing show promise for reducing the environmental footprint of cathode production.
Energy consumption during manufacturing represents another significant environmental consideration. Creating precisely engineered porous architectures typically requires energy-intensive processes such as high-temperature calcination or vacuum deposition. These processes contribute to indirect carbon emissions unless powered by renewable energy sources. Comparative lifecycle assessments indicate that the energy payback period for aluminum-ion batteries may be shorter than lithium-ion counterparts due to the lower embodied energy in aluminum materials.
End-of-life management presents both challenges and opportunities. The recyclability of aluminum is exceptional, with recovery rates exceeding 90% in many industrial applications. This suggests that cathode materials could be effectively reclaimed and reprocessed, creating a circular economy advantage over other battery chemistries. However, the complex composite nature of engineered porous cathodes may complicate separation processes, necessitating the development of specialized recycling protocols.
Water usage and potential contamination represent additional environmental concerns. The synthesis of nanostructured porous cathodes often requires substantial water volumes for washing and purification steps. Closed-loop water recycling systems and dry processing techniques are being investigated to mitigate these impacts. Furthermore, the risk of aluminum leaching into groundwater from improper disposal is significantly lower than for batteries containing heavy metals, though proper end-of-life management remains essential.
Overall, the environmental profile of advanced aluminum intercalation cathodes appears favorable compared to current commercial battery technologies, particularly when considering resource abundance and recyclability. However, continued research into green synthesis methods and energy-efficient manufacturing processes is necessary to fully realize the environmental benefits of this promising technology.
The manufacturing processes for optimized porous cathode structures do present certain environmental challenges. Chemical etching techniques commonly used to create controlled porosity often involve strong acids or bases that require careful handling and disposal. However, emerging green synthesis methods utilizing biodegradable templates and water-based processing show promise for reducing the environmental footprint of cathode production.
Energy consumption during manufacturing represents another significant environmental consideration. Creating precisely engineered porous architectures typically requires energy-intensive processes such as high-temperature calcination or vacuum deposition. These processes contribute to indirect carbon emissions unless powered by renewable energy sources. Comparative lifecycle assessments indicate that the energy payback period for aluminum-ion batteries may be shorter than lithium-ion counterparts due to the lower embodied energy in aluminum materials.
End-of-life management presents both challenges and opportunities. The recyclability of aluminum is exceptional, with recovery rates exceeding 90% in many industrial applications. This suggests that cathode materials could be effectively reclaimed and reprocessed, creating a circular economy advantage over other battery chemistries. However, the complex composite nature of engineered porous cathodes may complicate separation processes, necessitating the development of specialized recycling protocols.
Water usage and potential contamination represent additional environmental concerns. The synthesis of nanostructured porous cathodes often requires substantial water volumes for washing and purification steps. Closed-loop water recycling systems and dry processing techniques are being investigated to mitigate these impacts. Furthermore, the risk of aluminum leaching into groundwater from improper disposal is significantly lower than for batteries containing heavy metals, though proper end-of-life management remains essential.
Overall, the environmental profile of advanced aluminum intercalation cathodes appears favorable compared to current commercial battery technologies, particularly when considering resource abundance and recyclability. However, continued research into green synthesis methods and energy-efficient manufacturing processes is necessary to fully realize the environmental benefits of this promising technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!