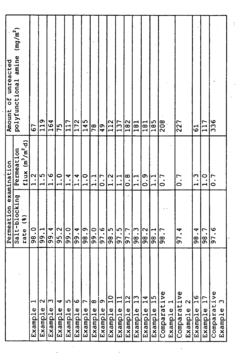
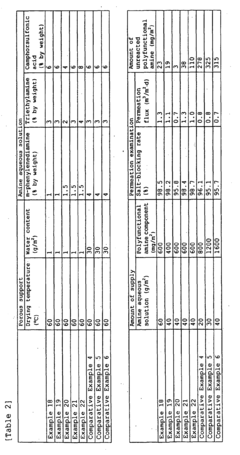
Amine Impregnation Methods for Porous Supports: Loadings, Distribution and Performance Effects
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Amine Impregnation Background and Objectives
Amine impregnation of porous supports has emerged as a crucial technology in various industrial applications, particularly in carbon capture and gas separation processes. This technique involves the incorporation of amine compounds into the pores of solid support materials, enhancing their capacity for selective gas adsorption and separation.
The development of amine impregnation methods can be traced back to the early 2000s when researchers began exploring ways to improve the efficiency of carbon dioxide capture from flue gases. Traditional aqueous amine solutions used in absorption columns faced challenges such as high energy consumption and equipment corrosion. The concept of immobilizing amines on solid supports offered a promising alternative, potentially reducing energy requirements and improving overall process economics.
Over the past two decades, significant advancements have been made in amine impregnation techniques, focusing on optimizing amine loadings, improving distribution within porous structures, and enhancing the overall performance of the resulting materials. The evolution of this technology has been driven by the urgent need for more efficient and cost-effective carbon capture solutions to address global climate change concerns.
The primary objectives of current research in amine impregnation methods are multifaceted. Firstly, there is a strong emphasis on maximizing amine loading while maintaining uniform distribution throughout the porous support. This goal aims to increase the overall adsorption capacity of the material without compromising its stability or regeneration potential.
Secondly, researchers are focused on understanding and optimizing the relationship between amine loading, distribution, and performance effects. This involves investigating how different impregnation techniques and process parameters influence the final material properties and their impact on gas separation efficiency.
Another critical objective is to develop amine-impregnated materials with enhanced stability and longer operational lifetimes. This includes addressing challenges such as amine leaching, thermal degradation, and oxidative stability, which are crucial for the practical implementation of these materials in industrial settings.
Furthermore, there is a growing interest in exploring novel amine compounds and support materials to expand the application range of amine-impregnated adsorbents. This includes investigating their potential use in separating other gas mixtures beyond carbon dioxide capture, such as natural gas purification or hydrogen production.
As the field continues to evolve, researchers are also focusing on scaling up amine impregnation processes for commercial production while maintaining consistent quality and performance. This objective is essential for bridging the gap between laboratory-scale developments and industrial-scale applications, ultimately paving the way for widespread adoption of this technology in various sectors.
The development of amine impregnation methods can be traced back to the early 2000s when researchers began exploring ways to improve the efficiency of carbon dioxide capture from flue gases. Traditional aqueous amine solutions used in absorption columns faced challenges such as high energy consumption and equipment corrosion. The concept of immobilizing amines on solid supports offered a promising alternative, potentially reducing energy requirements and improving overall process economics.
Over the past two decades, significant advancements have been made in amine impregnation techniques, focusing on optimizing amine loadings, improving distribution within porous structures, and enhancing the overall performance of the resulting materials. The evolution of this technology has been driven by the urgent need for more efficient and cost-effective carbon capture solutions to address global climate change concerns.
The primary objectives of current research in amine impregnation methods are multifaceted. Firstly, there is a strong emphasis on maximizing amine loading while maintaining uniform distribution throughout the porous support. This goal aims to increase the overall adsorption capacity of the material without compromising its stability or regeneration potential.
Secondly, researchers are focused on understanding and optimizing the relationship between amine loading, distribution, and performance effects. This involves investigating how different impregnation techniques and process parameters influence the final material properties and their impact on gas separation efficiency.
Another critical objective is to develop amine-impregnated materials with enhanced stability and longer operational lifetimes. This includes addressing challenges such as amine leaching, thermal degradation, and oxidative stability, which are crucial for the practical implementation of these materials in industrial settings.
Furthermore, there is a growing interest in exploring novel amine compounds and support materials to expand the application range of amine-impregnated adsorbents. This includes investigating their potential use in separating other gas mixtures beyond carbon dioxide capture, such as natural gas purification or hydrogen production.
As the field continues to evolve, researchers are also focusing on scaling up amine impregnation processes for commercial production while maintaining consistent quality and performance. This objective is essential for bridging the gap between laboratory-scale developments and industrial-scale applications, ultimately paving the way for widespread adoption of this technology in various sectors.
Market Analysis for Amine-Functionalized Materials
The market for amine-functionalized materials has experienced significant growth in recent years, driven by their versatile applications across various industries. These materials, particularly those based on porous supports, have found extensive use in carbon capture and storage (CCS) technologies, gas separation processes, catalysis, and environmental remediation.
In the CCS sector, amine-functionalized materials have emerged as promising candidates for CO2 capture due to their high selectivity and capacity. The global carbon capture and storage market is projected to expand rapidly, with amine-based sorbents playing a crucial role. This growth is fueled by increasing environmental regulations and the urgent need to reduce greenhouse gas emissions across industries.
The gas separation industry has also shown keen interest in amine-functionalized materials. These materials offer improved performance in separating CO2 from other gases, making them valuable in natural gas purification, biogas upgrading, and industrial off-gas treatment. The demand for more efficient and cost-effective gas separation technologies continues to drive market growth in this sector.
Catalysis represents another significant market for amine-functionalized materials. Their unique properties make them effective catalysts or catalyst supports in various chemical processes, including fine chemical synthesis and pharmaceutical production. The growing emphasis on green chemistry and sustainable manufacturing practices has further boosted the demand for these materials in catalytic applications.
Environmental remediation is an emerging market for amine-functionalized materials. Their ability to adsorb heavy metals, organic pollutants, and other contaminants from water and soil has attracted attention in water treatment and soil decontamination applications. As environmental concerns intensify globally, this market segment is expected to witness substantial growth.
The market landscape for amine-functionalized materials is characterized by a mix of established chemical companies and innovative start-ups. Key players are investing heavily in research and development to enhance material performance, reduce production costs, and expand application areas. Collaborations between industry and academia are also driving innovation in this field.
Geographically, North America and Europe currently lead the market, owing to stringent environmental regulations and substantial investments in clean energy technologies. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, environmental concerns, and government initiatives to promote sustainable technologies.
In the CCS sector, amine-functionalized materials have emerged as promising candidates for CO2 capture due to their high selectivity and capacity. The global carbon capture and storage market is projected to expand rapidly, with amine-based sorbents playing a crucial role. This growth is fueled by increasing environmental regulations and the urgent need to reduce greenhouse gas emissions across industries.
The gas separation industry has also shown keen interest in amine-functionalized materials. These materials offer improved performance in separating CO2 from other gases, making them valuable in natural gas purification, biogas upgrading, and industrial off-gas treatment. The demand for more efficient and cost-effective gas separation technologies continues to drive market growth in this sector.
Catalysis represents another significant market for amine-functionalized materials. Their unique properties make them effective catalysts or catalyst supports in various chemical processes, including fine chemical synthesis and pharmaceutical production. The growing emphasis on green chemistry and sustainable manufacturing practices has further boosted the demand for these materials in catalytic applications.
Environmental remediation is an emerging market for amine-functionalized materials. Their ability to adsorb heavy metals, organic pollutants, and other contaminants from water and soil has attracted attention in water treatment and soil decontamination applications. As environmental concerns intensify globally, this market segment is expected to witness substantial growth.
The market landscape for amine-functionalized materials is characterized by a mix of established chemical companies and innovative start-ups. Key players are investing heavily in research and development to enhance material performance, reduce production costs, and expand application areas. Collaborations between industry and academia are also driving innovation in this field.
Geographically, North America and Europe currently lead the market, owing to stringent environmental regulations and substantial investments in clean energy technologies. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, environmental concerns, and government initiatives to promote sustainable technologies.
Current Challenges in Amine Impregnation Techniques
Amine impregnation techniques for porous supports face several significant challenges that hinder their widespread application and optimal performance. One of the primary issues is achieving uniform distribution of amines throughout the porous structure. The impregnation process often results in uneven amine loading, with higher concentrations near the surface and lower concentrations in the interior of the support. This non-uniform distribution can lead to reduced overall efficiency and underutilization of the support's internal surface area.
Another challenge is controlling the amine loading level. While higher amine loadings generally increase CO2 adsorption capacity, they can also lead to pore blockage, reducing the accessibility of internal binding sites. Striking the right balance between amine content and maintaining porosity is crucial for optimizing performance. Additionally, the type of amine used and its molecular weight can significantly impact the impregnation process and subsequent performance, making it challenging to develop a one-size-fits-all approach.
The stability of impregnated amines poses another significant hurdle. Amine leaching during repeated adsorption-desorption cycles can result in decreased performance over time. This issue is particularly pronounced in the presence of moisture or at elevated temperatures, which are common conditions in many practical applications. Developing methods to anchor amines more securely to the support material without compromising their CO2 capture ability remains an ongoing challenge.
Scalability and cost-effectiveness of amine impregnation techniques also present significant obstacles. Many laboratory-scale methods are difficult to translate to industrial-scale production while maintaining consistent quality and performance. The cost of high-purity amines and the energy-intensive nature of some impregnation processes can make large-scale implementation economically challenging.
Furthermore, the environmental impact of amine-impregnated materials is a growing concern. The potential release of amines or their degradation products during use or disposal raises questions about long-term environmental sustainability. Developing greener impregnation methods and more environmentally friendly amine compounds is an area that requires further research and innovation.
Lastly, characterizing and predicting the performance of amine-impregnated materials remains challenging. The complex interplay between amine loading, distribution, support properties, and operating conditions makes it difficult to develop accurate models for optimizing and scaling up these materials. Improved analytical techniques and modeling approaches are needed to better understand and control the impregnation process and resulting material properties.
Another challenge is controlling the amine loading level. While higher amine loadings generally increase CO2 adsorption capacity, they can also lead to pore blockage, reducing the accessibility of internal binding sites. Striking the right balance between amine content and maintaining porosity is crucial for optimizing performance. Additionally, the type of amine used and its molecular weight can significantly impact the impregnation process and subsequent performance, making it challenging to develop a one-size-fits-all approach.
The stability of impregnated amines poses another significant hurdle. Amine leaching during repeated adsorption-desorption cycles can result in decreased performance over time. This issue is particularly pronounced in the presence of moisture or at elevated temperatures, which are common conditions in many practical applications. Developing methods to anchor amines more securely to the support material without compromising their CO2 capture ability remains an ongoing challenge.
Scalability and cost-effectiveness of amine impregnation techniques also present significant obstacles. Many laboratory-scale methods are difficult to translate to industrial-scale production while maintaining consistent quality and performance. The cost of high-purity amines and the energy-intensive nature of some impregnation processes can make large-scale implementation economically challenging.
Furthermore, the environmental impact of amine-impregnated materials is a growing concern. The potential release of amines or their degradation products during use or disposal raises questions about long-term environmental sustainability. Developing greener impregnation methods and more environmentally friendly amine compounds is an area that requires further research and innovation.
Lastly, characterizing and predicting the performance of amine-impregnated materials remains challenging. The complex interplay between amine loading, distribution, support properties, and operating conditions makes it difficult to develop accurate models for optimizing and scaling up these materials. Improved analytical techniques and modeling approaches are needed to better understand and control the impregnation process and resulting material properties.
Existing Amine Impregnation Protocols
01 Impregnation methods for amine loading
Various techniques are used to impregnate amines onto support materials. These methods include incipient wetness impregnation, solution impregnation, and vapor deposition. The choice of method affects the amine loading and distribution within the support structure, influencing the overall performance of the material.- Impregnation methods for amine loading: Various techniques are used to impregnate amines onto support materials. These methods include incipient wetness impregnation, solution impregnation, and vapor deposition. The choice of method affects the amine loading and distribution within the support structure, influencing the overall performance of the material.
- Amine distribution optimization: Optimizing amine distribution is crucial for maximizing the effectiveness of amine-impregnated materials. Techniques such as controlled pore filling, gradient impregnation, and surface modification are employed to achieve uniform or strategically non-uniform amine distribution. This optimization can lead to improved adsorption capacity and kinetics.
- Amine loading control and measurement: Precise control and measurement of amine loading are essential for consistent material performance. Methods such as gravimetric analysis, elemental analysis, and spectroscopic techniques are used to quantify amine loading. Advanced characterization techniques help in understanding the relationship between loading levels and material properties.
- Support material selection for amine impregnation: The choice of support material significantly influences amine loading and distribution. Porous materials like activated carbon, silica, and metal-organic frameworks are commonly used. The support's surface chemistry, pore structure, and stability are key factors in determining the effectiveness of amine impregnation and the resulting material's performance.
- Post-impregnation treatments for enhanced performance: Various post-impregnation treatments are employed to enhance the performance of amine-loaded materials. These include thermal treatments, chemical modifications, and surface functionalization. Such treatments can improve amine stability, increase accessibility, and optimize the material's overall adsorption properties.
02 Amine distribution optimization
Optimizing amine distribution is crucial for maximizing the effectiveness of the impregnated material. Techniques such as controlled drying, temperature modulation during impregnation, and use of specific solvents can help achieve uniform amine distribution throughout the support structure, enhancing the material's performance.Expand Specific Solutions03 Amine loading capacity and control
The amine loading capacity of support materials is influenced by factors such as surface area, pore size, and chemical compatibility. Controlling the amine loading is essential for achieving optimal performance while avoiding oversaturation. Methods for precise control of amine loading include adjusting impregnation solution concentration and multiple impregnation cycles.Expand Specific Solutions04 Characterization of amine-impregnated materials
Various analytical techniques are employed to characterize amine-impregnated materials, including thermogravimetric analysis, nitrogen adsorption-desorption isotherms, and spectroscopic methods. These techniques help determine amine loading, distribution, and the nature of amine-support interactions, which are crucial for optimizing material performance.Expand Specific Solutions05 Novel support materials for amine impregnation
Research focuses on developing novel support materials with enhanced properties for amine impregnation. These materials include hierarchical porous structures, functionalized surfaces, and composite materials. The goal is to improve amine loading capacity, distribution, and stability while maintaining or enhancing the material's overall performance.Expand Specific Solutions
Key Players in Amine Impregnation Research
The competitive landscape for amine impregnation methods in porous supports is characterized by a mature market with established players and ongoing innovation. The industry is in a growth phase, driven by increasing demand for carbon capture and gas separation technologies. Major companies like China Petroleum & Chemical Corp., Nitto Denko Corp., and Corning, Inc. are actively involved in research and development, focusing on improving loading capacities, distribution uniformity, and overall performance of amine-impregnated materials. The market size is substantial, with applications spanning petrochemicals, environmental technologies, and advanced materials. Technological advancements are continually pushing the boundaries of efficiency and cost-effectiveness, with academic institutions like Katholieke Universiteit Leuven and East China University of Science & Technology contributing significantly to the field's progress.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced amine impregnation methods for porous supports in carbon capture applications. Their approach involves a multi-step process: First, they use a vacuum-assisted impregnation technique to ensure uniform distribution of amines throughout the porous structure[1]. This is followed by a controlled drying process to optimize amine loading while maintaining porosity. Sinopec has also implemented a proprietary surface modification technique to enhance the amine-support interaction, resulting in improved CO2 adsorption capacity and stability[3]. Their research has shown that this method can achieve amine loadings of up to 60 wt% while maintaining a CO2 capture efficiency of over 90% in simulated flue gas conditions[5].
Strengths: High amine loading capacity, improved CO2 adsorption, and stability. Weaknesses: Potential for amine leaching during long-term operation, energy-intensive regeneration process.
Nitto Denko Corp.
Technical Solution: Nitto Denko Corp. has pioneered a novel amine impregnation method for porous supports using their expertise in membrane technology. Their approach utilizes a controlled solvent-based impregnation process, where amine solutions are introduced into porous supports under precise pressure and temperature conditions[2]. This method allows for fine-tuning of amine distribution within the pore structure. Nitto Denko has also developed a proprietary post-impregnation treatment that enhances the amine-support interaction, reducing amine leaching and improving long-term stability[4]. Their research has demonstrated that this technique can achieve uniform amine loadings of up to 50 wt% while maintaining over 85% of the original pore volume[6].
Strengths: Precise control over amine distribution, reduced amine leaching. Weaknesses: Potentially higher production costs, limited to certain types of porous supports.
Innovations in Amine Loading and Distribution
Process for producing semipermeable composite membrane
PatentInactiveEP1806174B1
Innovation
- An amine impermeable treatment is applied to the porous support before forming a skin layer, preventing the permeation of polyfunctional amine components and omitting the need for a membrane washing treatment, thereby reducing the content of unreacted components and improving membrane efficiency.
Improved method for synthesis of polyamide composite membranes
PatentInactiveUS20170341036A1
Innovation
- The method involves replacing aqueous and organic solvents in the interfacial polymerization process with ionic liquids to form thin film composite membranes with a polyamide top layer, enhancing permeance and retention for nanofiltration applications.
Environmental Impact of Amine Impregnation Processes
The environmental impact of amine impregnation processes for porous supports is a critical consideration in the development and application of this technology. These processes, while effective for enhancing CO2 capture performance, can have significant environmental implications throughout their lifecycle.
One of the primary environmental concerns is the potential for amine emissions during the impregnation process and subsequent use of the impregnated materials. Volatile amines can be released into the atmosphere, contributing to air pollution and potentially affecting local air quality. These emissions may have adverse effects on human health and ecosystems if not properly controlled.
The production and disposal of amine-impregnated materials also raise environmental issues. The synthesis of amines often involves energy-intensive processes and the use of petrochemical feedstocks, contributing to greenhouse gas emissions and resource depletion. Additionally, the disposal of spent amine-impregnated supports may lead to soil and water contamination if not managed appropriately.
Water consumption and wastewater generation are other significant environmental aspects of amine impregnation processes. The preparation and washing steps can require substantial amounts of water, potentially straining local water resources. The resulting wastewater may contain amine residues and other contaminants, necessitating proper treatment before discharge to prevent water pollution.
Energy consumption during the impregnation process and subsequent regeneration cycles of the amine-impregnated materials also contributes to the overall environmental footprint. The energy requirements for heating, cooling, and maintaining process conditions can be substantial, especially when scaled up for industrial applications.
The choice of support materials and amines can influence the environmental impact. Some porous supports may be derived from more sustainable sources or have better recyclability, while certain amines may have lower volatility or toxicity profiles. Optimizing these selections can help mitigate environmental concerns.
Efforts to improve the environmental performance of amine impregnation processes include developing more efficient impregnation techniques, exploring greener amine alternatives, and implementing closed-loop systems to minimize emissions and waste. Advanced process control and monitoring systems can also help reduce environmental impacts by optimizing resource use and minimizing losses.
As regulations on environmental protection become more stringent, the industry is increasingly focusing on life cycle assessments and sustainable practices in amine impregnation technology. This holistic approach considers environmental impacts from raw material extraction to end-of-life disposal, driving innovations towards more environmentally friendly solutions.
One of the primary environmental concerns is the potential for amine emissions during the impregnation process and subsequent use of the impregnated materials. Volatile amines can be released into the atmosphere, contributing to air pollution and potentially affecting local air quality. These emissions may have adverse effects on human health and ecosystems if not properly controlled.
The production and disposal of amine-impregnated materials also raise environmental issues. The synthesis of amines often involves energy-intensive processes and the use of petrochemical feedstocks, contributing to greenhouse gas emissions and resource depletion. Additionally, the disposal of spent amine-impregnated supports may lead to soil and water contamination if not managed appropriately.
Water consumption and wastewater generation are other significant environmental aspects of amine impregnation processes. The preparation and washing steps can require substantial amounts of water, potentially straining local water resources. The resulting wastewater may contain amine residues and other contaminants, necessitating proper treatment before discharge to prevent water pollution.
Energy consumption during the impregnation process and subsequent regeneration cycles of the amine-impregnated materials also contributes to the overall environmental footprint. The energy requirements for heating, cooling, and maintaining process conditions can be substantial, especially when scaled up for industrial applications.
The choice of support materials and amines can influence the environmental impact. Some porous supports may be derived from more sustainable sources or have better recyclability, while certain amines may have lower volatility or toxicity profiles. Optimizing these selections can help mitigate environmental concerns.
Efforts to improve the environmental performance of amine impregnation processes include developing more efficient impregnation techniques, exploring greener amine alternatives, and implementing closed-loop systems to minimize emissions and waste. Advanced process control and monitoring systems can also help reduce environmental impacts by optimizing resource use and minimizing losses.
As regulations on environmental protection become more stringent, the industry is increasingly focusing on life cycle assessments and sustainable practices in amine impregnation technology. This holistic approach considers environmental impacts from raw material extraction to end-of-life disposal, driving innovations towards more environmentally friendly solutions.
Characterization Techniques for Impregnated Supports
Characterization techniques play a crucial role in understanding the properties and performance of amine-impregnated porous supports. These methods provide valuable insights into the loading, distribution, and interaction of amines within the support structure, which directly influence the overall performance of the material.
Spectroscopic techniques, such as Fourier Transform Infrared (FTIR) spectroscopy and Raman spectroscopy, are widely employed to identify and quantify the amine species present in the impregnated supports. These methods can detect the characteristic vibrational modes of amine groups, providing information on their chemical environment and interactions with the support surface.
X-ray Photoelectron Spectroscopy (XPS) is another powerful tool for surface analysis, offering detailed information about the elemental composition and chemical states of the impregnated amines. This technique can reveal the nature of amine-support interactions and help identify any changes in the oxidation state of the support material upon impregnation.
Microscopy techniques, such as Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM), are essential for visualizing the morphology and distribution of amine species within the porous structure. These methods can provide high-resolution images of the support surface and cross-sections, revealing the uniformity of amine coating and any potential pore blockage.
Nitrogen adsorption-desorption isotherms are commonly used to assess the textural properties of impregnated supports, including specific surface area, pore volume, and pore size distribution. Changes in these parameters before and after impregnation can indicate the extent of amine loading and its impact on the support's porosity.
Thermogravimetric Analysis (TGA) is a valuable technique for determining the thermal stability and decomposition behavior of impregnated amines. This method can provide information on the amine loading amount and the strength of amine-support interactions by analyzing weight loss patterns during controlled heating.
Solid-state Nuclear Magnetic Resonance (NMR) spectroscopy offers insights into the local chemical environment of impregnated amines and their interactions with the support material. This technique can reveal information about the mobility and conformation of amine species within the porous structure.
Dynamic vapor sorption (DVS) experiments can be used to study the CO2 adsorption capacity and kinetics of amine-impregnated supports. This method provides valuable data on the material's performance under various conditions, including different temperatures and CO2 partial pressures.
By combining these characterization techniques, researchers can gain a comprehensive understanding of the amine impregnation process, its effects on support properties, and the resulting performance in applications such as CO2 capture. This knowledge is essential for optimizing impregnation methods and developing more efficient amine-functionalized materials for various industrial applications.
Spectroscopic techniques, such as Fourier Transform Infrared (FTIR) spectroscopy and Raman spectroscopy, are widely employed to identify and quantify the amine species present in the impregnated supports. These methods can detect the characteristic vibrational modes of amine groups, providing information on their chemical environment and interactions with the support surface.
X-ray Photoelectron Spectroscopy (XPS) is another powerful tool for surface analysis, offering detailed information about the elemental composition and chemical states of the impregnated amines. This technique can reveal the nature of amine-support interactions and help identify any changes in the oxidation state of the support material upon impregnation.
Microscopy techniques, such as Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM), are essential for visualizing the morphology and distribution of amine species within the porous structure. These methods can provide high-resolution images of the support surface and cross-sections, revealing the uniformity of amine coating and any potential pore blockage.
Nitrogen adsorption-desorption isotherms are commonly used to assess the textural properties of impregnated supports, including specific surface area, pore volume, and pore size distribution. Changes in these parameters before and after impregnation can indicate the extent of amine loading and its impact on the support's porosity.
Thermogravimetric Analysis (TGA) is a valuable technique for determining the thermal stability and decomposition behavior of impregnated amines. This method can provide information on the amine loading amount and the strength of amine-support interactions by analyzing weight loss patterns during controlled heating.
Solid-state Nuclear Magnetic Resonance (NMR) spectroscopy offers insights into the local chemical environment of impregnated amines and their interactions with the support material. This technique can reveal information about the mobility and conformation of amine species within the porous structure.
Dynamic vapor sorption (DVS) experiments can be used to study the CO2 adsorption capacity and kinetics of amine-impregnated supports. This method provides valuable data on the material's performance under various conditions, including different temperatures and CO2 partial pressures.
By combining these characterization techniques, researchers can gain a comprehensive understanding of the amine impregnation process, its effects on support properties, and the resulting performance in applications such as CO2 capture. This knowledge is essential for optimizing impregnation methods and developing more efficient amine-functionalized materials for various industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!