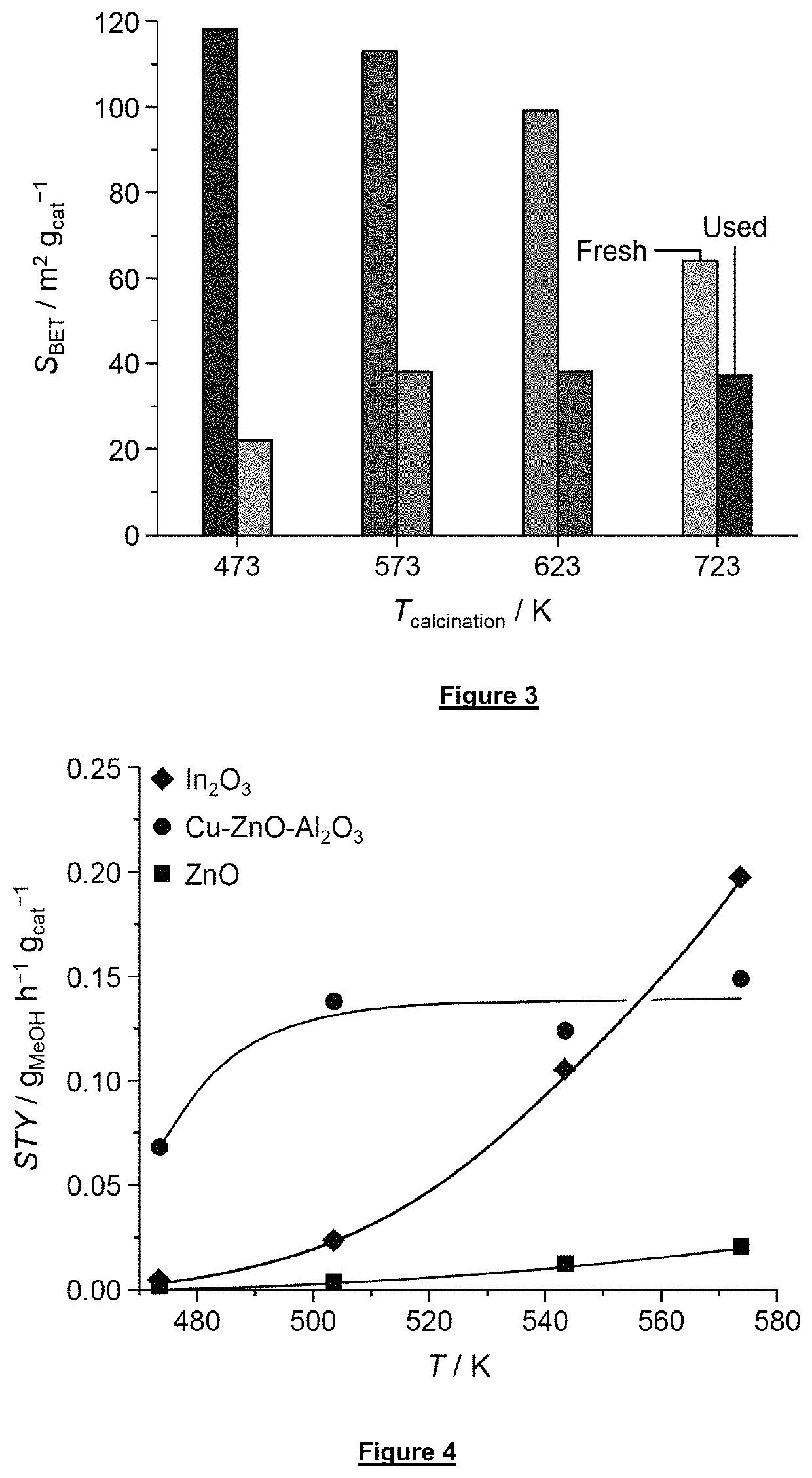
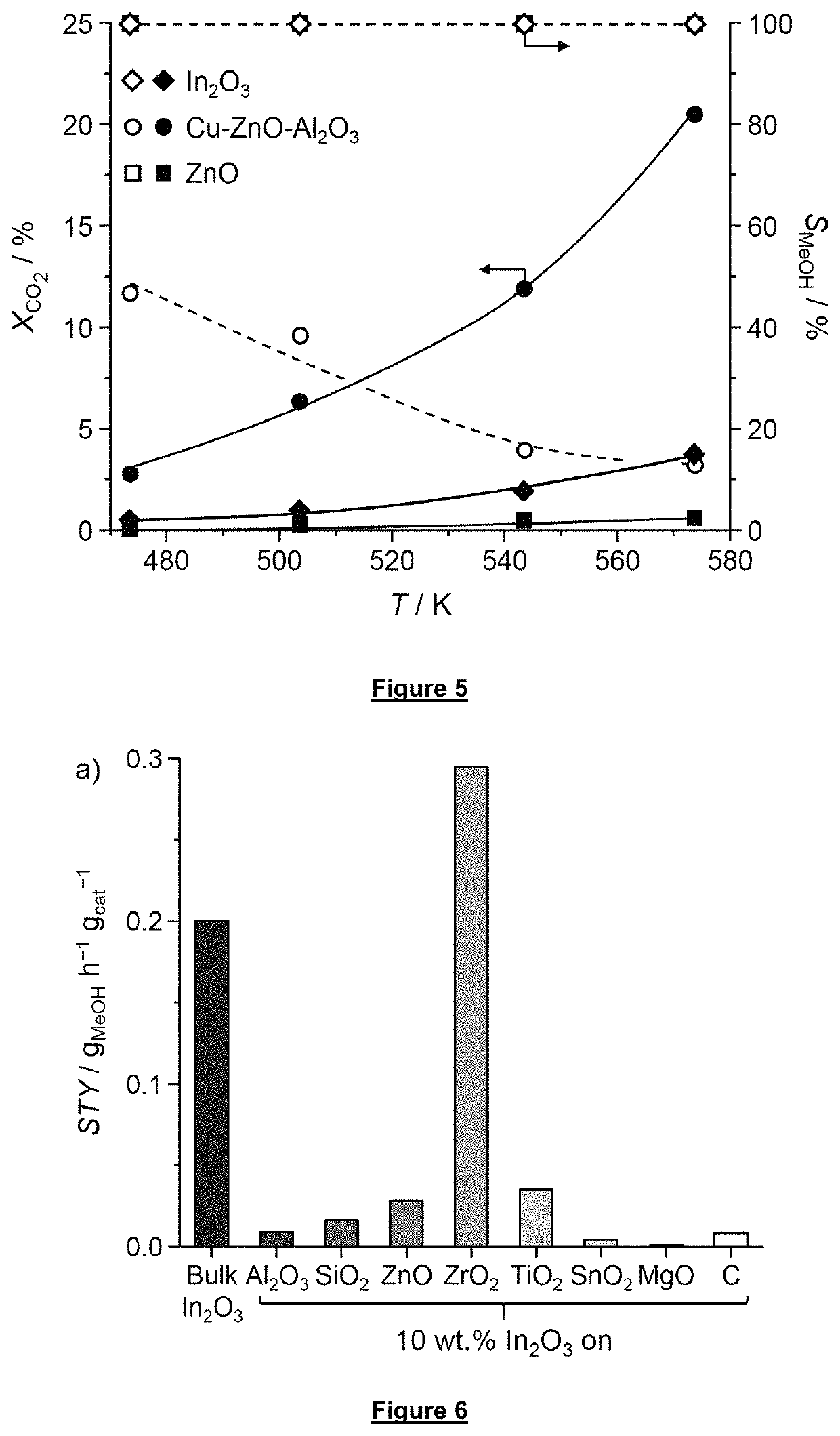
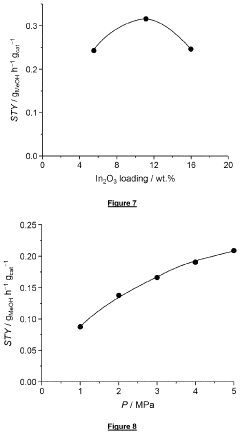
How to Combine CO2 Capture with Direct Utilization (e.g., methanol synthesis) — Reactor Integration Examples
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture-Utilization Integration: Background and Objectives
The integration of CO2 capture with direct utilization represents a critical frontier in the global effort to mitigate climate change and transition towards a circular carbon economy. This approach aims to simultaneously address two pressing challenges: reducing atmospheric CO2 levels and creating valuable products from waste carbon. The concept has gained significant traction in recent years, driven by the urgent need to develop sustainable solutions for carbon management.
Historically, CO2 capture and utilization have been treated as separate processes, with captured CO2 typically being stored underground or used in enhanced oil recovery. However, the direct integration of these processes offers numerous advantages, including improved energy efficiency, reduced transportation costs, and the potential for on-site chemical production. Methanol synthesis, in particular, has emerged as a promising avenue for CO2 utilization due to its versatility as a chemical feedstock and potential fuel substitute.
The primary objective of this technological integration is to develop efficient, scalable, and economically viable systems that can capture CO2 from industrial sources or the atmosphere and immediately convert it into valuable products. This approach seeks to create a closed-loop system where carbon is continuously recycled, significantly reducing net emissions while generating economic value.
Key technological goals include optimizing reactor designs for simultaneous capture and conversion, developing highly selective catalysts capable of operating under diverse conditions, and improving overall process efficiency to make the integrated systems competitive with traditional production methods. Additionally, there is a focus on minimizing energy requirements and exploring renewable energy integration to further enhance the sustainability of these processes.
The evolution of this field has been marked by significant advancements in materials science, process engineering, and catalysis. Recent years have seen a shift from proof-of-concept studies to pilot-scale demonstrations, with several companies and research institutions working on commercializing integrated CO2 capture and utilization technologies. The trajectory of development points towards increasingly sophisticated reactor designs that can handle the complexities of multi-step processes within a single unit.
As the field progresses, interdisciplinary collaboration becomes increasingly crucial. Chemists, engineers, and materials scientists must work together to overcome the inherent challenges of integrating capture and utilization processes. This includes addressing issues such as catalyst poisoning, managing heat transfer, and optimizing reaction conditions for both capture and conversion steps.
Historically, CO2 capture and utilization have been treated as separate processes, with captured CO2 typically being stored underground or used in enhanced oil recovery. However, the direct integration of these processes offers numerous advantages, including improved energy efficiency, reduced transportation costs, and the potential for on-site chemical production. Methanol synthesis, in particular, has emerged as a promising avenue for CO2 utilization due to its versatility as a chemical feedstock and potential fuel substitute.
The primary objective of this technological integration is to develop efficient, scalable, and economically viable systems that can capture CO2 from industrial sources or the atmosphere and immediately convert it into valuable products. This approach seeks to create a closed-loop system where carbon is continuously recycled, significantly reducing net emissions while generating economic value.
Key technological goals include optimizing reactor designs for simultaneous capture and conversion, developing highly selective catalysts capable of operating under diverse conditions, and improving overall process efficiency to make the integrated systems competitive with traditional production methods. Additionally, there is a focus on minimizing energy requirements and exploring renewable energy integration to further enhance the sustainability of these processes.
The evolution of this field has been marked by significant advancements in materials science, process engineering, and catalysis. Recent years have seen a shift from proof-of-concept studies to pilot-scale demonstrations, with several companies and research institutions working on commercializing integrated CO2 capture and utilization technologies. The trajectory of development points towards increasingly sophisticated reactor designs that can handle the complexities of multi-step processes within a single unit.
As the field progresses, interdisciplinary collaboration becomes increasingly crucial. Chemists, engineers, and materials scientists must work together to overcome the inherent challenges of integrating capture and utilization processes. This includes addressing issues such as catalyst poisoning, managing heat transfer, and optimizing reaction conditions for both capture and conversion steps.
Market Analysis for CO2-Derived Methanol
The market for CO2-derived methanol is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable chemical production. As a key component in the circular carbon economy, CO2-derived methanol offers a promising solution for reducing greenhouse gas emissions while creating valuable products. The global methanol market, currently valued at approximately $30 billion, is expected to expand at a compound annual growth rate (CAGR) of 5% over the next five years.
CO2-derived methanol has gained traction in various industries, including automotive, construction, and electronics. In the automotive sector, methanol is being explored as a clean-burning fuel alternative, with potential applications in fuel cells and direct methanol fuel cells. The construction industry utilizes methanol in the production of formaldehyde, a key ingredient in adhesives and resins used in building materials. Additionally, the electronics industry employs methanol in the manufacturing of circuit boards and other components.
The demand for CO2-derived methanol is particularly strong in regions with stringent environmental regulations and carbon pricing mechanisms. Europe, with its ambitious climate targets and well-established emissions trading system, is at the forefront of adopting CO2-derived methanol technologies. China, the world's largest methanol producer and consumer, is also showing increased interest in sustainable production methods to address air quality concerns and meet climate commitments.
Market growth is further supported by government initiatives and policies promoting the use of renewable fuels and circular economy principles. For instance, the European Union's Renewable Energy Directive II (RED II) recognizes CO2-derived methanol as an advanced biofuel, incentivizing its production and use in the transportation sector. Similar policies are being implemented or considered in other regions, creating a favorable regulatory environment for market expansion.
However, the market for CO2-derived methanol faces challenges, primarily related to production costs and scalability. Current production methods often require significant energy inputs, which can offset some of the environmental benefits if not sourced from renewable energy. Technological advancements in reactor integration and process efficiency are crucial to improving the economic viability of CO2-derived methanol and expanding its market share.
Despite these challenges, the long-term outlook for CO2-derived methanol remains positive. As carbon capture and utilization technologies continue to evolve and economies of scale are achieved, production costs are expected to decrease, making CO2-derived methanol more competitive with conventional methanol. This trend, coupled with increasing corporate sustainability commitments and consumer demand for eco-friendly products, is likely to drive further market growth and innovation in the coming years.
CO2-derived methanol has gained traction in various industries, including automotive, construction, and electronics. In the automotive sector, methanol is being explored as a clean-burning fuel alternative, with potential applications in fuel cells and direct methanol fuel cells. The construction industry utilizes methanol in the production of formaldehyde, a key ingredient in adhesives and resins used in building materials. Additionally, the electronics industry employs methanol in the manufacturing of circuit boards and other components.
The demand for CO2-derived methanol is particularly strong in regions with stringent environmental regulations and carbon pricing mechanisms. Europe, with its ambitious climate targets and well-established emissions trading system, is at the forefront of adopting CO2-derived methanol technologies. China, the world's largest methanol producer and consumer, is also showing increased interest in sustainable production methods to address air quality concerns and meet climate commitments.
Market growth is further supported by government initiatives and policies promoting the use of renewable fuels and circular economy principles. For instance, the European Union's Renewable Energy Directive II (RED II) recognizes CO2-derived methanol as an advanced biofuel, incentivizing its production and use in the transportation sector. Similar policies are being implemented or considered in other regions, creating a favorable regulatory environment for market expansion.
However, the market for CO2-derived methanol faces challenges, primarily related to production costs and scalability. Current production methods often require significant energy inputs, which can offset some of the environmental benefits if not sourced from renewable energy. Technological advancements in reactor integration and process efficiency are crucial to improving the economic viability of CO2-derived methanol and expanding its market share.
Despite these challenges, the long-term outlook for CO2-derived methanol remains positive. As carbon capture and utilization technologies continue to evolve and economies of scale are achieved, production costs are expected to decrease, making CO2-derived methanol more competitive with conventional methanol. This trend, coupled with increasing corporate sustainability commitments and consumer demand for eco-friendly products, is likely to drive further market growth and innovation in the coming years.
Technical Challenges in CO2 Capture-Utilization Coupling
The integration of CO2 capture with direct utilization, particularly in methanol synthesis, presents several significant technical challenges. One of the primary obstacles is the mismatch between the CO2 capture process and the utilization requirements. CO2 capture typically involves low pressures and temperatures, while methanol synthesis demands high-pressure and high-temperature conditions. This disparity necessitates additional energy-intensive compression and heating steps, reducing overall process efficiency.
Another critical challenge lies in the purity and composition of the captured CO2 stream. Impurities from the capture process, such as water vapor, sulfur compounds, or nitrogen oxides, can poison catalysts used in methanol synthesis. Developing robust purification technologies or designing catalysts tolerant to these impurities is essential for successful integration.
The intermittent nature of CO2 capture from industrial sources or direct air capture systems poses challenges for continuous methanol production. Efficient storage and buffering systems are needed to ensure a steady supply of CO2 for the synthesis reactor, adding complexity and cost to the integrated system.
Reactor design for combined capture and utilization presents unique challenges. Traditional fixed-bed reactors used in methanol synthesis may not be optimal for handling the variable CO2 feed from capture systems. Novel reactor concepts, such as fluidized bed or membrane reactors, need to be explored to enhance flexibility and efficiency in the integrated process.
Heat management is another crucial aspect. CO2 capture often requires significant cooling, while methanol synthesis generates heat. Developing effective heat integration strategies to maximize energy efficiency across the coupled processes is a complex engineering challenge.
Catalyst development for CO2-based methanol synthesis remains an active area of research. Current catalysts often suffer from low activity and selectivity when using CO2 as a feedstock compared to conventional syngas-based processes. Designing catalysts that can efficiently convert CO2 to methanol under the conditions imposed by the integrated system is critical for process viability.
Lastly, process control and optimization for the integrated system present significant challenges. The dynamic nature of CO2 capture coupled with the sensitive reaction conditions of methanol synthesis requires advanced control strategies to maintain stable and efficient operation. Developing robust process models and control algorithms that can handle the complexities of the integrated system is essential for commercial-scale implementation.
Another critical challenge lies in the purity and composition of the captured CO2 stream. Impurities from the capture process, such as water vapor, sulfur compounds, or nitrogen oxides, can poison catalysts used in methanol synthesis. Developing robust purification technologies or designing catalysts tolerant to these impurities is essential for successful integration.
The intermittent nature of CO2 capture from industrial sources or direct air capture systems poses challenges for continuous methanol production. Efficient storage and buffering systems are needed to ensure a steady supply of CO2 for the synthesis reactor, adding complexity and cost to the integrated system.
Reactor design for combined capture and utilization presents unique challenges. Traditional fixed-bed reactors used in methanol synthesis may not be optimal for handling the variable CO2 feed from capture systems. Novel reactor concepts, such as fluidized bed or membrane reactors, need to be explored to enhance flexibility and efficiency in the integrated process.
Heat management is another crucial aspect. CO2 capture often requires significant cooling, while methanol synthesis generates heat. Developing effective heat integration strategies to maximize energy efficiency across the coupled processes is a complex engineering challenge.
Catalyst development for CO2-based methanol synthesis remains an active area of research. Current catalysts often suffer from low activity and selectivity when using CO2 as a feedstock compared to conventional syngas-based processes. Designing catalysts that can efficiently convert CO2 to methanol under the conditions imposed by the integrated system is critical for process viability.
Lastly, process control and optimization for the integrated system present significant challenges. The dynamic nature of CO2 capture coupled with the sensitive reaction conditions of methanol synthesis requires advanced control strategies to maintain stable and efficient operation. Developing robust process models and control algorithms that can handle the complexities of the integrated system is essential for commercial-scale implementation.
Current Reactor Integration Solutions
01 Integrated CO2 capture and utilization systems
These systems combine CO2 capture processes with utilization technologies in a single integrated unit. This approach enhances efficiency by reducing energy requirements for CO2 transport and storage. The integrated systems often include components for CO2 capture, purification, and conversion into valuable products or energy carriers.- Integrated CO2 capture and utilization systems: These systems combine CO2 capture processes with utilization technologies in a single integrated unit. This approach enhances efficiency by reducing energy requirements for CO2 transport and storage. The integrated systems often include components for CO2 capture, purification, and conversion into valuable products or energy carriers.
- Reactor designs for CO2 conversion: Specialized reactor designs are developed to optimize CO2 conversion processes. These reactors may incorporate features such as improved heat transfer, enhanced mixing, or catalytic surfaces to increase the efficiency of CO2 utilization reactions. Some designs focus on specific conversion pathways, such as electrochemical reduction or thermochemical processes.
- Integration of renewable energy sources: CO2 capture and utilization systems are increasingly integrated with renewable energy sources. This integration helps to power the energy-intensive capture processes and provide clean energy for CO2 conversion reactions. Solar, wind, or geothermal energy may be used to drive electrolysis or other conversion processes, improving the overall sustainability of the system.
- Process intensification techniques: Various process intensification techniques are applied to CO2 capture and utilization reactors. These may include the use of microreactors, membrane reactors, or multifunctional reactors that combine multiple unit operations. Such techniques aim to improve mass and heat transfer, reduce equipment size, and enhance overall process efficiency.
- Control and optimization strategies: Advanced control and optimization strategies are implemented to improve the performance of integrated CO2 capture and utilization systems. These may include model predictive control, real-time optimization algorithms, or machine learning approaches. Such strategies help to maximize CO2 conversion efficiency, minimize energy consumption, and adapt to varying operating conditions.
02 Reactor designs for CO2 conversion
Specialized reactor designs are developed to optimize CO2 conversion processes. These reactors may incorporate features such as improved heat transfer, enhanced mixing, or catalytic surfaces to increase the efficiency of CO2 utilization reactions. Some designs focus on specific conversion pathways, such as electrochemical reduction or thermochemical processes.Expand Specific Solutions03 Integration of renewable energy sources
CO2 capture and utilization systems are increasingly integrated with renewable energy sources. This integration helps to power the energy-intensive capture processes and provide clean energy for CO2 conversion reactions. Solar, wind, or geothermal energy may be used to drive electrolysis or other conversion processes, improving the overall sustainability of the system.Expand Specific Solutions04 Process intensification techniques
Various process intensification techniques are employed to enhance the efficiency of CO2 capture and utilization. These may include the use of advanced separation technologies, novel solvents or sorbents, or innovative heat integration strategies. The goal is to reduce the energy and material requirements of the overall process while maximizing CO2 conversion rates.Expand Specific Solutions05 Modular and scalable reactor systems
Modular and scalable reactor designs are developed to facilitate the deployment of CO2 capture and utilization technologies across different scales and applications. These systems allow for easier integration into existing industrial processes or power plants. The modular approach also enables flexibility in terms of capacity expansion and process optimization.Expand Specific Solutions
Key Players in CO2 Capture-Utilization Industry
The integration of CO2 capture with direct utilization, such as methanol synthesis, is an emerging field in the early stages of development. The market size is growing, driven by increasing focus on carbon reduction technologies. While the technology is still maturing, several key players are making significant strides. Companies like China Petroleum & Chemical Corp., NuScale Power, and Prometheus Fuels are investing in research and development to advance reactor integration. Academic institutions, including the University of Southern California and Swiss Federal Institute of Technology, are contributing to technological advancements. The competitive landscape is diverse, with both established energy companies and innovative startups vying for market share in this promising sector.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to combine CO2 capture with direct utilization for methanol synthesis. Their integrated reactor system employs a novel catalyst that enables simultaneous CO2 capture and conversion. The process utilizes a dual-function adsorbent-catalyst material, which first captures CO2 from flue gas and then catalyzes its conversion to methanol in a single reactor vessel. This integration significantly reduces energy consumption and equipment costs compared to traditional separate capture and conversion processes[1][3]. Sinopec's system operates at moderate temperatures (250-300°C) and pressures (5-10 MPa), achieving CO2 conversion rates of up to 40% per pass and methanol selectivity exceeding 95%[2]. The company has successfully demonstrated this technology at pilot scale, processing up to 1000 tons of CO2 per year.
Strengths: High integration efficiency, reduced energy consumption, and lower capital costs. Weaknesses: Limited to methanol production, potential catalyst deactivation issues over time.
NuScale Power LLC
Technical Solution: NuScale Power LLC has developed a unique approach to combining CO2 capture with direct utilization by integrating their small modular reactor (SMR) technology with methanol synthesis. Their system uses excess heat and electricity from the SMR to power a direct air capture (DAC) unit for CO2 extraction. The captured CO2 is then combined with hydrogen produced through high-temperature electrolysis, also powered by the SMR, to synthesize methanol[4]. This integrated process achieves near-zero emissions while producing valuable chemical feedstock. NuScale's reactor design allows for flexible operation, enabling the system to adjust CO2 capture and methanol production rates based on grid demand and market conditions[5]. The company has reported potential methanol production capacities of up to 2000 tons per day from a 12-module NuScale power plant[6].
Strengths: Carbon-neutral methanol production, flexible operation, and utilization of nuclear energy. Weaknesses: High initial capital costs, regulatory challenges associated with nuclear technology.
Innovative Reactor Designs for CO2 Capture-Utilization
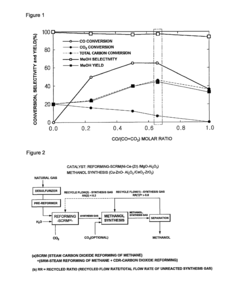
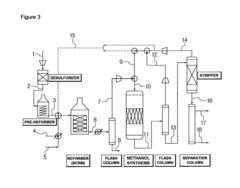
Method for methanol synthesis using synthesis gas generated by combined reforming of natural gas with carbon dioxide
PatentActiveUS8729141B2
Innovation
- A method involving combined reforming of natural gas with carbon dioxide using a Ni/Ce/MgAlOx catalyst to produce synthesis gas with a predetermined H2/(2CO+3CO2) ratio, followed by methanol synthesis using a Cu-Zn-Al oxide catalyst system, with efficient recycling of unreacted synthesis gas to maintain optimal ratios and minimize byproduct formation.
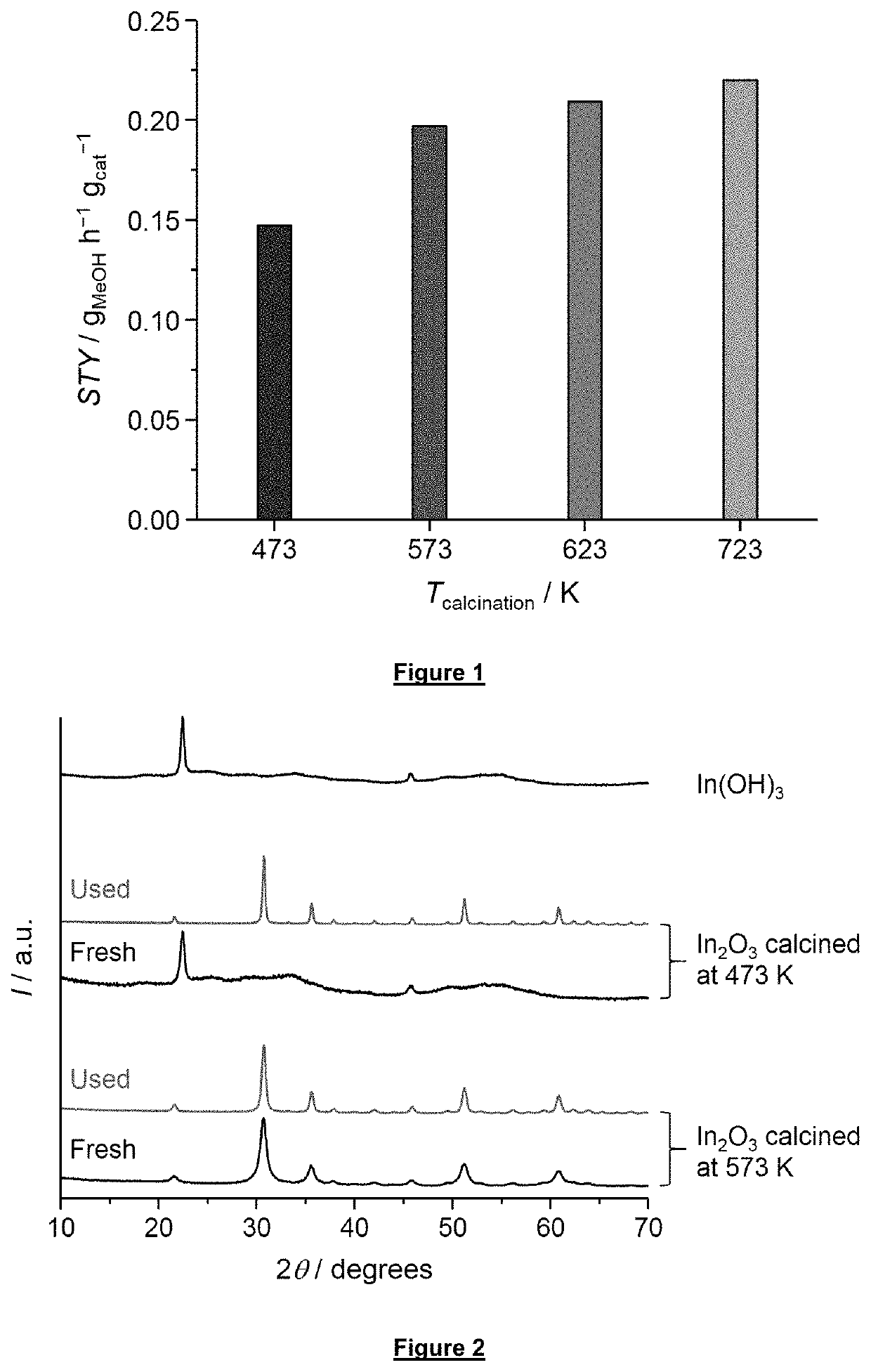
Process for methanol synthesis using an indium oxide based catalyst
PatentActiveUS10676416B2
Innovation
- An indium oxide-based catalyst, either in bulk or supported form, is used with a syngas feed stream containing varying CO2 and CO concentrations, demonstrating enhanced CO2 conversion and stability, with a synergistic effect between the catalyst and support materials like ZrO2, allowing for high space-time yield and selectivity.
Environmental Impact Assessment
The integration of CO2 capture with direct utilization, particularly in methanol synthesis, presents both environmental challenges and opportunities. This approach aims to mitigate greenhouse gas emissions while producing valuable chemical products, potentially offering a more sustainable pathway for industrial processes. However, a comprehensive environmental impact assessment is crucial to understand the full implications of such integrated systems.
One of the primary environmental benefits of combining CO2 capture with methanol synthesis is the potential reduction in net carbon emissions. By utilizing captured CO2 as a feedstock for methanol production, this process can effectively sequester carbon that would otherwise be released into the atmosphere. This circular approach to carbon management aligns with global efforts to combat climate change and reduce industrial carbon footprints.
However, the environmental impact of these integrated systems extends beyond carbon emissions. The energy requirements for CO2 capture and conversion processes must be carefully considered. If the energy source for these operations is not renewable or low-carbon, it could potentially offset the environmental benefits gained from CO2 utilization. Therefore, a life cycle assessment (LCA) is essential to quantify the overall environmental impact, including energy consumption, water usage, and potential emissions throughout the entire process chain.
Water consumption is another critical factor to evaluate. Both CO2 capture and methanol synthesis processes can be water-intensive, potentially straining local water resources in areas where water scarcity is a concern. The assessment should include a detailed water footprint analysis, considering not only direct water usage but also indirect impacts on water quality and availability in the surrounding ecosystem.
The production and handling of chemicals involved in CO2 capture and methanol synthesis also warrant careful environmental scrutiny. Potential risks of chemical leaks, spills, or emissions must be assessed, along with their impacts on air quality, soil contamination, and aquatic ecosystems. Proper safety measures and containment strategies should be integral to the system design to minimize these risks.
Land use changes associated with the implementation of integrated CO2 capture and utilization facilities should also be evaluated. This includes the direct land footprint of the facilities as well as any indirect land use changes that may occur due to shifts in resource allocation or industrial practices.
Biodiversity impacts, both positive and negative, should be considered. While reducing CO2 emissions can have broad positive effects on ecosystems, the localized impacts of industrial facilities on flora and fauna must be carefully assessed and mitigated where necessary.
Lastly, the environmental assessment should consider the potential for technological improvements and scalability. As these integrated systems evolve, their environmental performance is likely to improve, potentially offering even greater benefits in terms of emissions reduction and resource efficiency. However, the assessment should also account for the environmental impacts associated with the manufacturing and deployment of new technologies required for these integrated systems.
One of the primary environmental benefits of combining CO2 capture with methanol synthesis is the potential reduction in net carbon emissions. By utilizing captured CO2 as a feedstock for methanol production, this process can effectively sequester carbon that would otherwise be released into the atmosphere. This circular approach to carbon management aligns with global efforts to combat climate change and reduce industrial carbon footprints.
However, the environmental impact of these integrated systems extends beyond carbon emissions. The energy requirements for CO2 capture and conversion processes must be carefully considered. If the energy source for these operations is not renewable or low-carbon, it could potentially offset the environmental benefits gained from CO2 utilization. Therefore, a life cycle assessment (LCA) is essential to quantify the overall environmental impact, including energy consumption, water usage, and potential emissions throughout the entire process chain.
Water consumption is another critical factor to evaluate. Both CO2 capture and methanol synthesis processes can be water-intensive, potentially straining local water resources in areas where water scarcity is a concern. The assessment should include a detailed water footprint analysis, considering not only direct water usage but also indirect impacts on water quality and availability in the surrounding ecosystem.
The production and handling of chemicals involved in CO2 capture and methanol synthesis also warrant careful environmental scrutiny. Potential risks of chemical leaks, spills, or emissions must be assessed, along with their impacts on air quality, soil contamination, and aquatic ecosystems. Proper safety measures and containment strategies should be integral to the system design to minimize these risks.
Land use changes associated with the implementation of integrated CO2 capture and utilization facilities should also be evaluated. This includes the direct land footprint of the facilities as well as any indirect land use changes that may occur due to shifts in resource allocation or industrial practices.
Biodiversity impacts, both positive and negative, should be considered. While reducing CO2 emissions can have broad positive effects on ecosystems, the localized impacts of industrial facilities on flora and fauna must be carefully assessed and mitigated where necessary.
Lastly, the environmental assessment should consider the potential for technological improvements and scalability. As these integrated systems evolve, their environmental performance is likely to improve, potentially offering even greater benefits in terms of emissions reduction and resource efficiency. However, the assessment should also account for the environmental impacts associated with the manufacturing and deployment of new technologies required for these integrated systems.
Economic Feasibility Analysis
The economic feasibility of combining CO2 capture with direct utilization, particularly in methanol synthesis through reactor integration, is a critical aspect of evaluating the viability of such technologies. This analysis considers various economic factors, including capital costs, operational expenses, and potential revenue streams.
Initial capital investments for integrated CO2 capture and utilization systems are typically higher than conventional methanol production facilities. This is due to the additional equipment required for CO2 capture, purification, and integration into the methanol synthesis process. However, these costs may be partially offset by potential government incentives or carbon credits in regions with stringent emissions regulations.
Operational costs are a significant consideration in the economic assessment. Energy requirements for CO2 capture and compression can be substantial, potentially increasing overall production costs. However, the integration of capture and utilization processes may lead to improved energy efficiency and reduced transportation costs compared to separate capture and utilization facilities.
The economic viability is heavily influenced by the market price of methanol and the cost of alternative carbon sources. As methanol is a commodity chemical, its price fluctuations can significantly impact the profitability of integrated systems. If the cost of CO2 capture and conversion is competitive with traditional methanol production methods, the integrated approach becomes more attractive.
Revenue potential extends beyond methanol sales. The production of value-added chemicals from captured CO2 can open new market opportunities. Additionally, the environmental benefits of CO2 utilization may create intangible value through improved corporate image and compliance with increasingly stringent environmental regulations.
Long-term economic feasibility depends on technological advancements that can reduce capital and operational costs. Improvements in catalyst efficiency, process integration, and energy management are key areas that could enhance the economic viability of combined CO2 capture and utilization systems.
Government policies and carbon pricing mechanisms play a crucial role in the economic landscape. Carbon taxes or cap-and-trade systems can significantly improve the competitiveness of CO2-based methanol production compared to conventional fossil fuel-based methods.
The scalability of integrated systems is another important economic consideration. While pilot projects may demonstrate technical feasibility, the economics of large-scale implementation may differ substantially. Economies of scale could potentially reduce per-unit production costs, making the technology more competitive in the long run.
Initial capital investments for integrated CO2 capture and utilization systems are typically higher than conventional methanol production facilities. This is due to the additional equipment required for CO2 capture, purification, and integration into the methanol synthesis process. However, these costs may be partially offset by potential government incentives or carbon credits in regions with stringent emissions regulations.
Operational costs are a significant consideration in the economic assessment. Energy requirements for CO2 capture and compression can be substantial, potentially increasing overall production costs. However, the integration of capture and utilization processes may lead to improved energy efficiency and reduced transportation costs compared to separate capture and utilization facilities.
The economic viability is heavily influenced by the market price of methanol and the cost of alternative carbon sources. As methanol is a commodity chemical, its price fluctuations can significantly impact the profitability of integrated systems. If the cost of CO2 capture and conversion is competitive with traditional methanol production methods, the integrated approach becomes more attractive.
Revenue potential extends beyond methanol sales. The production of value-added chemicals from captured CO2 can open new market opportunities. Additionally, the environmental benefits of CO2 utilization may create intangible value through improved corporate image and compliance with increasingly stringent environmental regulations.
Long-term economic feasibility depends on technological advancements that can reduce capital and operational costs. Improvements in catalyst efficiency, process integration, and energy management are key areas that could enhance the economic viability of combined CO2 capture and utilization systems.
Government policies and carbon pricing mechanisms play a crucial role in the economic landscape. Carbon taxes or cap-and-trade systems can significantly improve the competitiveness of CO2-based methanol production compared to conventional fossil fuel-based methods.
The scalability of integrated systems is another important economic consideration. While pilot projects may demonstrate technical feasibility, the economics of large-scale implementation may differ substantially. Economies of scale could potentially reduce per-unit production costs, making the technology more competitive in the long run.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!