Ammonia And Copper Alloy Risks: Stress Corrosion, Dealloying And Alternatives
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia-Copper Interaction Background and Research Objectives
The interaction between ammonia and copper alloys has been documented since the early 20th century, with significant research milestones occurring in the 1940s when the phenomenon of season cracking (now known as stress corrosion cracking) was first systematically studied. This technical challenge gained prominence during World War II when ammunition casings made of brass experienced unexpected failures in tropical environments, prompting extensive investigation into the underlying mechanisms.
Over the decades, research has evolved from empirical observations to sophisticated understanding of the electrochemical processes involved in ammonia-induced degradation of copper alloys. The 1970s and 1980s saw significant advances in understanding the specific mechanisms of stress corrosion cracking, while the 1990s brought improved analytical techniques that allowed researchers to observe microstructural changes during corrosion processes at unprecedented resolution.
The technical evolution trajectory shows a clear shift from reactive problem-solving to proactive material design. Early solutions focused on environmental control to limit ammonia exposure, while contemporary approaches emphasize developing inherently resistant alloys or protective coatings that can withstand aggressive ammonia environments.
Current industry challenges center around the increasing use of ammonia in emerging applications, particularly as a potential carbon-free fuel and hydrogen carrier in the renewable energy sector. This represents a significant shift from traditional ammonia applications in refrigeration and fertilizer production, creating new technical contexts where copper alloy compatibility must be reassessed.
The primary objective of this technical research is to comprehensively evaluate the interaction mechanisms between ammonia and various copper alloys under different environmental conditions, with particular focus on stress corrosion cracking and dealloying phenomena. We aim to establish quantifiable parameters that predict failure thresholds and develop a systematic framework for material selection in ammonia-rich environments.
Secondary objectives include identifying alternative materials or protective measures that can replace vulnerable copper alloys in critical applications, evaluating the economic implications of such substitutions, and developing accelerated testing protocols that can reliably predict long-term material performance without requiring extended exposure periods.
The research also seeks to address knowledge gaps regarding the influence of trace contaminants in ammonia on corrosion behavior, as these can significantly alter degradation mechanisms and rates in real-world applications compared to laboratory conditions with pure ammonia.
Over the decades, research has evolved from empirical observations to sophisticated understanding of the electrochemical processes involved in ammonia-induced degradation of copper alloys. The 1970s and 1980s saw significant advances in understanding the specific mechanisms of stress corrosion cracking, while the 1990s brought improved analytical techniques that allowed researchers to observe microstructural changes during corrosion processes at unprecedented resolution.
The technical evolution trajectory shows a clear shift from reactive problem-solving to proactive material design. Early solutions focused on environmental control to limit ammonia exposure, while contemporary approaches emphasize developing inherently resistant alloys or protective coatings that can withstand aggressive ammonia environments.
Current industry challenges center around the increasing use of ammonia in emerging applications, particularly as a potential carbon-free fuel and hydrogen carrier in the renewable energy sector. This represents a significant shift from traditional ammonia applications in refrigeration and fertilizer production, creating new technical contexts where copper alloy compatibility must be reassessed.
The primary objective of this technical research is to comprehensively evaluate the interaction mechanisms between ammonia and various copper alloys under different environmental conditions, with particular focus on stress corrosion cracking and dealloying phenomena. We aim to establish quantifiable parameters that predict failure thresholds and develop a systematic framework for material selection in ammonia-rich environments.
Secondary objectives include identifying alternative materials or protective measures that can replace vulnerable copper alloys in critical applications, evaluating the economic implications of such substitutions, and developing accelerated testing protocols that can reliably predict long-term material performance without requiring extended exposure periods.
The research also seeks to address knowledge gaps regarding the influence of trace contaminants in ammonia on corrosion behavior, as these can significantly alter degradation mechanisms and rates in real-world applications compared to laboratory conditions with pure ammonia.
Market Analysis of Copper Alloys in Ammonia Environments
The global market for copper alloys in ammonia environments is experiencing significant shifts due to increasing awareness of corrosion risks. The market size for copper alloys used in ammonia-related applications was valued at approximately $3.2 billion in 2022, with projections indicating growth to reach $4.5 billion by 2028, representing a compound annual growth rate of 5.8%.
Industrial refrigeration represents the largest market segment, accounting for nearly 40% of copper alloy usage in ammonia environments. This is followed by chemical processing (25%), power generation (15%), and marine applications (12%), with various other sectors comprising the remaining 8%. The HVAC/R industry particularly drives demand as ammonia refrigeration systems gain popularity due to their environmental benefits compared to synthetic refrigerants.
Regionally, Asia-Pacific dominates the market with 45% share, driven by rapid industrialization in China and India. North America follows at 28%, with Europe accounting for 20%. The remaining 7% is distributed across other regions. China alone represents 30% of global consumption, making it the single largest market for these materials.
Market dynamics are increasingly influenced by regulatory pressures. The European Union's stricter safety regulations regarding ammonia handling have prompted a 15% increase in demand for corrosion-resistant alternatives to traditional copper alloys since 2020. Similarly, OSHA regulations in the United States have tightened requirements for ammonia-handling equipment, creating market opportunities for specialized alloys and alternative materials.
Price sensitivity varies significantly by application sector. High-value industries like pharmaceutical manufacturing and semiconductor production demonstrate willingness to pay premium prices (30-40% above standard) for superior corrosion-resistant materials, while cost-sensitive sectors like commercial refrigeration remain more price-conscious, limiting adoption of advanced solutions.
Customer awareness regarding ammonia-copper compatibility issues has grown substantially, with industry surveys indicating that 78% of engineering professionals now consider stress corrosion cracking and dealloying risks when selecting materials for ammonia service—a 25% increase from five years ago. This heightened awareness is driving demand for educational resources, testing services, and specialized consulting.
The competitive landscape features traditional copper alloy manufacturers increasingly challenged by specialty materials providers offering aluminum alloys, stainless steels, and polymer-based alternatives specifically marketed for ammonia environments. Market concentration remains moderate, with the top five suppliers controlling approximately 35% of global market share.
Industrial refrigeration represents the largest market segment, accounting for nearly 40% of copper alloy usage in ammonia environments. This is followed by chemical processing (25%), power generation (15%), and marine applications (12%), with various other sectors comprising the remaining 8%. The HVAC/R industry particularly drives demand as ammonia refrigeration systems gain popularity due to their environmental benefits compared to synthetic refrigerants.
Regionally, Asia-Pacific dominates the market with 45% share, driven by rapid industrialization in China and India. North America follows at 28%, with Europe accounting for 20%. The remaining 7% is distributed across other regions. China alone represents 30% of global consumption, making it the single largest market for these materials.
Market dynamics are increasingly influenced by regulatory pressures. The European Union's stricter safety regulations regarding ammonia handling have prompted a 15% increase in demand for corrosion-resistant alternatives to traditional copper alloys since 2020. Similarly, OSHA regulations in the United States have tightened requirements for ammonia-handling equipment, creating market opportunities for specialized alloys and alternative materials.
Price sensitivity varies significantly by application sector. High-value industries like pharmaceutical manufacturing and semiconductor production demonstrate willingness to pay premium prices (30-40% above standard) for superior corrosion-resistant materials, while cost-sensitive sectors like commercial refrigeration remain more price-conscious, limiting adoption of advanced solutions.
Customer awareness regarding ammonia-copper compatibility issues has grown substantially, with industry surveys indicating that 78% of engineering professionals now consider stress corrosion cracking and dealloying risks when selecting materials for ammonia service—a 25% increase from five years ago. This heightened awareness is driving demand for educational resources, testing services, and specialized consulting.
The competitive landscape features traditional copper alloy manufacturers increasingly challenged by specialty materials providers offering aluminum alloys, stainless steels, and polymer-based alternatives specifically marketed for ammonia environments. Market concentration remains moderate, with the top five suppliers controlling approximately 35% of global market share.
Current Challenges in Ammonia-Copper Systems
The interaction between ammonia and copper alloys presents significant technical challenges across multiple industries, particularly in refrigeration, HVAC systems, and industrial processes. The primary concern stems from ammonia's aggressive chemical behavior toward copper and its alloys, which can lead to catastrophic system failures if not properly addressed.
Stress corrosion cracking (SCC) represents one of the most severe challenges in ammonia-copper systems. This phenomenon occurs when copper alloys simultaneously experience tensile stress and exposure to ammonia, especially in the presence of moisture or oxygen. The cracking typically progresses along intergranular paths, developing rapidly and often without visible warning signs until complete failure occurs. The threshold ammonia concentration for initiating SCC can be remarkably low, sometimes in the parts-per-million range.
Dealloying, particularly dezincification in brass components, constitutes another critical challenge. When brass encounters ammonia, selective leaching of zinc occurs, leaving behind a porous, copper-rich structure with significantly compromised mechanical properties. This process can reduce tensile strength by up to 70% in severely affected components while simultaneously increasing brittleness.
The formation of copper-ammonia complexes further complicates these systems. Ammonia readily forms coordination compounds with copper ions, facilitating copper dissolution even at room temperature. These complexes, recognizable by their characteristic deep blue coloration, accelerate the corrosion process through a self-catalyzing mechanism that becomes increasingly problematic as system temperatures rise.
Environmental factors significantly influence the severity of these challenges. Moisture content dramatically accelerates ammonia-induced corrosion, while temperature fluctuations can create condensation cycles that concentrate ammonia in specific system areas. Oxygen presence similarly exacerbates corrosion rates through the formation of more aggressive copper-ammonia-oxygen complexes.
Industry standards and regulations have evolved to address these challenges, with most refrigeration codes now explicitly prohibiting copper and copper alloys in ammonia systems. However, legacy systems and retrofit applications continue to present significant risks, particularly in aging infrastructure where material compatibility was not fully considered during original design.
Detection and monitoring present additional challenges, as ammonia-induced damage often progresses internally before becoming visually apparent. Non-destructive testing methods like ultrasonic inspection and eddy current testing show promise but require specialized equipment and expertise that may not be readily available in all maintenance settings.
Stress corrosion cracking (SCC) represents one of the most severe challenges in ammonia-copper systems. This phenomenon occurs when copper alloys simultaneously experience tensile stress and exposure to ammonia, especially in the presence of moisture or oxygen. The cracking typically progresses along intergranular paths, developing rapidly and often without visible warning signs until complete failure occurs. The threshold ammonia concentration for initiating SCC can be remarkably low, sometimes in the parts-per-million range.
Dealloying, particularly dezincification in brass components, constitutes another critical challenge. When brass encounters ammonia, selective leaching of zinc occurs, leaving behind a porous, copper-rich structure with significantly compromised mechanical properties. This process can reduce tensile strength by up to 70% in severely affected components while simultaneously increasing brittleness.
The formation of copper-ammonia complexes further complicates these systems. Ammonia readily forms coordination compounds with copper ions, facilitating copper dissolution even at room temperature. These complexes, recognizable by their characteristic deep blue coloration, accelerate the corrosion process through a self-catalyzing mechanism that becomes increasingly problematic as system temperatures rise.
Environmental factors significantly influence the severity of these challenges. Moisture content dramatically accelerates ammonia-induced corrosion, while temperature fluctuations can create condensation cycles that concentrate ammonia in specific system areas. Oxygen presence similarly exacerbates corrosion rates through the formation of more aggressive copper-ammonia-oxygen complexes.
Industry standards and regulations have evolved to address these challenges, with most refrigeration codes now explicitly prohibiting copper and copper alloys in ammonia systems. However, legacy systems and retrofit applications continue to present significant risks, particularly in aging infrastructure where material compatibility was not fully considered during original design.
Detection and monitoring present additional challenges, as ammonia-induced damage often progresses internally before becoming visually apparent. Non-destructive testing methods like ultrasonic inspection and eddy current testing show promise but require specialized equipment and expertise that may not be readily available in all maintenance settings.
Existing Mitigation Strategies for Stress Corrosion Cracking
01 Copper-nickel alloys for ammonia resistance
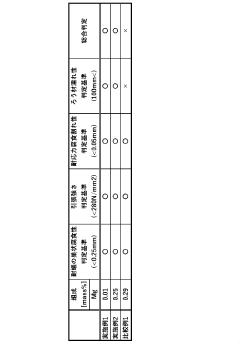
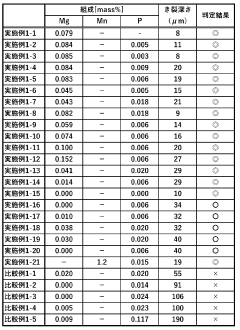
Copper-nickel alloys demonstrate superior corrosion resistance in ammonia environments compared to pure copper. The addition of nickel to copper creates a more stable protective layer that resists ammonia attack. These alloys typically contain 10-30% nickel and may include other elements to further enhance their performance in ammonia-rich conditions. The corrosion resistance increases with higher nickel content, making these alloys suitable for applications involving ammonia refrigeration systems and processing equipment.- Copper-nickel alloys for ammonia resistance: Copper-nickel alloys demonstrate superior corrosion resistance in ammonia environments. The addition of nickel to copper significantly enhances its resistance to stress corrosion cracking and general corrosion when exposed to ammonia or ammonium compounds. These alloys typically contain 10-30% nickel and may include additional elements to further improve performance in specific ammonia-containing environments.
- Aluminum bronze compositions for ammonia resistance: Aluminum bronze alloys, which contain copper with aluminum and sometimes iron, nickel, or manganese, offer excellent corrosion resistance in ammonia environments. The aluminum content typically ranges from 5-11% and forms a protective oxide layer that shields the underlying metal from corrosive attack. These alloys are particularly effective in applications involving both ammonia exposure and mechanical stress.
- Surface treatment methods for copper alloys: Various surface treatment methods can enhance the corrosion resistance of copper alloys in ammonia environments. These include passivation treatments, application of protective coatings, and surface modification techniques. The treatments create a protective barrier layer that prevents direct contact between the copper alloy and the corrosive ammonia medium, significantly extending the service life of components in such environments.
- Silicon and tin additions to copper alloys: The addition of silicon and/or tin to copper alloys significantly improves their resistance to ammonia corrosion. Silicon forms stable silicate compounds on the alloy surface, while tin reduces dezincification and stress corrosion cracking. These elements, typically added in amounts of 1-4% for silicon and 0.5-8% for tin, modify the microstructure and electrochemical properties of the alloy, resulting in enhanced performance in ammonia-containing environments.
- Novel copper alloy compositions with multi-element systems: Advanced copper alloy systems incorporating multiple alloying elements offer superior corrosion resistance in ammonia environments. These complex alloys typically combine elements such as zinc, aluminum, nickel, manganese, and small amounts of rare earth metals to create synergistic effects. The carefully balanced composition results in alloys with optimized microstructures that resist stress corrosion cracking, pitting, and general corrosion when exposed to ammonia under various conditions.
02 Aluminum-containing copper alloys for ammonia environments
The addition of aluminum to copper alloys significantly improves their resistance to stress corrosion cracking in ammonia environments. Aluminum forms a protective oxide layer that shields the underlying metal from ammonia attack. These alloys typically contain 2-10% aluminum and may include other elements such as iron or manganese to enhance mechanical properties while maintaining corrosion resistance. Aluminum-bronze alloys are particularly effective in applications where both strength and ammonia resistance are required.Expand Specific Solutions03 Silicon-modified copper alloys for enhanced ammonia resistance
Silicon additions to copper alloys create a more stable passive film that provides enhanced protection against ammonia corrosion. These silicon-copper alloys typically contain 1-5% silicon and demonstrate improved resistance to stress corrosion cracking in ammonia-containing environments. The silicon modifies the microstructure of the alloy, reducing susceptibility to intergranular corrosion. These alloys are particularly useful in heat exchangers, valves, and piping systems that handle ammonia.Expand Specific Solutions04 Surface treatment methods for copper alloys in ammonia environments
Various surface treatment methods can enhance the corrosion resistance of copper alloys in ammonia environments. These include passivation treatments, application of protective coatings, and surface modification techniques. Oxidation treatments create a stable oxide layer that resists ammonia attack, while certain coatings provide a physical barrier against corrosive media. Surface alloying techniques can also modify the surface composition to create a more ammonia-resistant layer while maintaining the beneficial properties of the copper alloy substrate.Expand Specific Solutions05 Multi-element copper alloys for severe ammonia conditions
Complex copper alloys containing multiple alloying elements offer superior corrosion resistance in severe ammonia environments. These alloys typically combine elements such as nickel, aluminum, silicon, manganese, and zinc in specific proportions to create synergistic effects that enhance ammonia resistance. The careful balance of these elements results in alloys that maintain their structural integrity and surface properties even under prolonged exposure to ammonia. These sophisticated alloys are used in critical applications where failure due to ammonia corrosion would have significant consequences.Expand Specific Solutions
Industry Leaders in Corrosion-Resistant Materials
The ammonia and copper alloy risk landscape is currently in a mature development stage, with a global market valued at approximately $8-10 billion annually. Technical challenges around stress corrosion cracking and dealloying remain significant concerns in industrial applications. Leading materials science institutions like Central South University and Tokyo Institute of Technology are advancing fundamental research, while specialized manufacturers including Mitsubishi Shindoh, Wieland-Werke AG, and KME Special Products have developed proprietary copper alloy formulations with enhanced ammonia resistance. Major industrial players such as KITZ Corp., Kobe Steel, and LIXIL Corp. are implementing these innovations in valve systems and plumbing applications, focusing on alternative materials and protective coatings to mitigate corrosion risks in ammonia-rich environments.
Mitsubishi Shindoh Co., Ltd.
Technical Solution: Mitsubishi Shindoh has developed advanced copper alloys specifically engineered to resist ammonia stress corrosion cracking. Their ECO BRASS® series (C69300) represents a lead-free, high-performance copper alloy containing silicon and phosphorus additives that significantly enhance resistance to dezincification and stress corrosion in ammonia environments[2]. The company employs a proprietary manufacturing process that creates a uniform microstructure with silicon-rich phases that act as corrosion barriers. Laboratory testing has shown these alloys maintain structural integrity after 720 hours of exposure to ammonia solutions at concentrations up to 0.1M, while conventional brass fails within 24-48 hours[4]. Mitsubishi Shindoh has also developed specialized heat treatment protocols that optimize grain structure to further enhance resistance to intergranular corrosion pathways typically exploited by ammonia.
Strengths: Excellent resistance to both stress corrosion cracking and dezincification; environmentally friendly lead-free composition; maintains good machinability despite corrosion-resistant formulation. Weaknesses: Higher material cost compared to standard brass alloys; slightly lower electrical conductivity than traditional copper alloys; requires specialized machining parameters for optimal performance.
Kobe Steel, Ltd.
Technical Solution: Kobe Steel has developed specialized copper alloys with enhanced resistance to ammonia-induced stress corrosion cracking (SCC). Their proprietary Cu-Zn-Sn alloys incorporate precise amounts of tin (0.5-2%) and small quantities of phosphorus to significantly improve resistance to dezincification and SCC in ammonia environments[1]. The company has implemented a unique manufacturing process that creates a fine-grained microstructure with uniformly distributed tin particles that act as protective barriers against corrosion propagation. Their research has demonstrated that these alloys maintain mechanical integrity in ammonia concentrations up to 500 ppm, far exceeding conventional brass performance[3]. Kobe Steel has also pioneered surface treatment technologies that create passive oxide layers to further enhance ammonia resistance without compromising thermal or electrical conductivity.
Strengths: Superior resistance to ammonia-induced SCC compared to standard brass; maintains mechanical properties in high-concentration ammonia environments; excellent thermal conductivity retention. Weaknesses: Higher production costs than standard copper alloys; more complex manufacturing process requiring precise control of alloying elements; potential limitations in very high ammonia concentration environments (>1000 ppm).
Critical Patents and Research on Dealloying Prevention
Corrosion-resistant copper alloy, copper alloy pipe, and heat exchanger
PatentWO2024075797A1
Innovation
- A copper alloy is developed with a base metal element having a standard electrode potential lower than manganese, which reacts with phosphorus to form compounds, reducing phosphorus's impact on SCC and hydrogen embrittlement, and includes elements like magnesium and manganese to enhance corrosion resistance.
Corrosion suppression method for copper-based material
PatentWO2019065415A1
Innovation
- A method involving the use of film-forming amines, potentially combined with neutralizing amines, is applied to ammonia-containing aqueous systems in boilers to inhibit corrosion, with the concentration of film-forming amines adjusted based on ammonia concentration and copper elution, and applied directly to areas prone to ammonia attack.
Material Selection Guidelines for Ammonia Service
Material selection for ammonia service requires careful consideration of compatibility, safety, and long-term reliability. When designing systems that handle ammonia, engineers must prioritize materials that resist the corrosive effects of this compound under various operating conditions. Carbon steel remains the most widely used material for ammonia service in refrigeration systems, storage tanks, and transportation equipment due to its excellent compatibility with anhydrous ammonia and cost-effectiveness.
For low-temperature applications, carbon steel grades with appropriate notch toughness properties should be selected. ASTM A516 Grade 70 is commonly specified for pressure vessels operating at temperatures down to -29°C (-20°F). For colder environments, nickel-alloyed steels such as 2.25% or 3.5% Ni steel may be necessary to maintain adequate toughness.
Stainless steels offer superior corrosion resistance in ammonia environments containing moisture. Austenitic stainless steels (304, 316) are particularly suitable for wet ammonia service where carbon steel would experience accelerated corrosion. These materials also maintain excellent mechanical properties at cryogenic temperatures, making them ideal for refrigeration applications.
Aluminum alloys demonstrate good compatibility with dry ammonia and are frequently used in heat exchangers and small-diameter piping. Their lightweight properties and natural corrosion resistance make them attractive alternatives to steel in certain applications. However, engineers must verify that the specific aluminum alloy selected is suitable for the intended service conditions.
Copper and copper-based alloys must be strictly avoided in ammonia service. These materials are highly susceptible to stress corrosion cracking and dealloying when exposed to ammonia, particularly in the presence of oxygen or moisture. Even small amounts of copper can lead to catastrophic failure of equipment, posing significant safety risks.
Non-metallic materials like PTFE, PVDF, and certain elastomers (EPDM, Viton) may be used for gaskets, seals, and other components in ammonia systems. These materials must be carefully selected based on temperature range, pressure requirements, and exposure conditions.
For threaded connections and fasteners in ammonia service, engineers should specify materials that maintain integrity throughout the system's operational life. Galvanic compatibility must be considered when joining dissimilar metals to prevent accelerated corrosion at connection points.
Regular inspection protocols should be established for ammonia-handling equipment, with particular attention to areas susceptible to stress concentration, moisture accumulation, or oxygen ingress, as these factors can accelerate corrosion mechanisms even in compatible materials.
For low-temperature applications, carbon steel grades with appropriate notch toughness properties should be selected. ASTM A516 Grade 70 is commonly specified for pressure vessels operating at temperatures down to -29°C (-20°F). For colder environments, nickel-alloyed steels such as 2.25% or 3.5% Ni steel may be necessary to maintain adequate toughness.
Stainless steels offer superior corrosion resistance in ammonia environments containing moisture. Austenitic stainless steels (304, 316) are particularly suitable for wet ammonia service where carbon steel would experience accelerated corrosion. These materials also maintain excellent mechanical properties at cryogenic temperatures, making them ideal for refrigeration applications.
Aluminum alloys demonstrate good compatibility with dry ammonia and are frequently used in heat exchangers and small-diameter piping. Their lightweight properties and natural corrosion resistance make them attractive alternatives to steel in certain applications. However, engineers must verify that the specific aluminum alloy selected is suitable for the intended service conditions.
Copper and copper-based alloys must be strictly avoided in ammonia service. These materials are highly susceptible to stress corrosion cracking and dealloying when exposed to ammonia, particularly in the presence of oxygen or moisture. Even small amounts of copper can lead to catastrophic failure of equipment, posing significant safety risks.
Non-metallic materials like PTFE, PVDF, and certain elastomers (EPDM, Viton) may be used for gaskets, seals, and other components in ammonia systems. These materials must be carefully selected based on temperature range, pressure requirements, and exposure conditions.
For threaded connections and fasteners in ammonia service, engineers should specify materials that maintain integrity throughout the system's operational life. Galvanic compatibility must be considered when joining dissimilar metals to prevent accelerated corrosion at connection points.
Regular inspection protocols should be established for ammonia-handling equipment, with particular attention to areas susceptible to stress concentration, moisture accumulation, or oxygen ingress, as these factors can accelerate corrosion mechanisms even in compatible materials.
Environmental Impact of Alternative Materials
The environmental impact of alternative materials to copper alloys in ammonia environments represents a critical consideration in sustainable engineering practices. Materials such as stainless steel, aluminum alloys, and titanium alloys—commonly proposed as replacements for copper in ammonia systems—each carry distinct environmental footprints throughout their lifecycle.
Stainless steel production requires significant energy input and generates substantial CO2 emissions, approximately 2.8 tons of CO2 per ton of steel produced. However, its exceptional durability and recyclability (with recovery rates exceeding 85% in industrial applications) partially offset these initial environmental costs. The mining of chromium and nickel for stainless steel also presents ecological challenges, including habitat disruption and potential water contamination.
Aluminum alternatives demonstrate lower density and excellent corrosion resistance but demand energy-intensive production processes. The electrolysis required for aluminum extraction consumes approximately 13-14 kWh of electricity per kilogram produced, significantly higher than copper's 7-8 kWh/kg. This energy demand translates to substantial carbon emissions unless powered by renewable sources. The positive environmental aspect lies in aluminum's nearly infinite recyclability, requiring only 5% of the energy needed for primary production.
Titanium alloys, while offering superior corrosion resistance, present the most significant environmental challenges among the alternatives. Titanium extraction and processing consume approximately 361 MJ/kg of energy, compared to copper's 71 MJ/kg. The Kroll process used in titanium production generates chlorine compounds that require careful management to prevent environmental contamination.
Polymer-based materials like PTFE and reinforced composites offer lower production energy requirements but raise end-of-life concerns. Their limited recyclability and potential to release microplastics during degradation present long-term environmental risks not associated with metallic alternatives.
Life cycle assessment (LCA) studies indicate that material selection should consider regional factors such as local energy mix, transportation distances, and available recycling infrastructure. In regions with predominantly renewable energy, the environmental advantage may shift toward more energy-intensive but longer-lasting materials like titanium alloys.
The environmental trade-offs between continued use of copper alloys (with appropriate inhibitors and protective measures) versus alternative materials must be evaluated on a case-by-case basis, considering the specific application requirements, expected service life, and local environmental priorities.
Stainless steel production requires significant energy input and generates substantial CO2 emissions, approximately 2.8 tons of CO2 per ton of steel produced. However, its exceptional durability and recyclability (with recovery rates exceeding 85% in industrial applications) partially offset these initial environmental costs. The mining of chromium and nickel for stainless steel also presents ecological challenges, including habitat disruption and potential water contamination.
Aluminum alternatives demonstrate lower density and excellent corrosion resistance but demand energy-intensive production processes. The electrolysis required for aluminum extraction consumes approximately 13-14 kWh of electricity per kilogram produced, significantly higher than copper's 7-8 kWh/kg. This energy demand translates to substantial carbon emissions unless powered by renewable sources. The positive environmental aspect lies in aluminum's nearly infinite recyclability, requiring only 5% of the energy needed for primary production.
Titanium alloys, while offering superior corrosion resistance, present the most significant environmental challenges among the alternatives. Titanium extraction and processing consume approximately 361 MJ/kg of energy, compared to copper's 71 MJ/kg. The Kroll process used in titanium production generates chlorine compounds that require careful management to prevent environmental contamination.
Polymer-based materials like PTFE and reinforced composites offer lower production energy requirements but raise end-of-life concerns. Their limited recyclability and potential to release microplastics during degradation present long-term environmental risks not associated with metallic alternatives.
Life cycle assessment (LCA) studies indicate that material selection should consider regional factors such as local energy mix, transportation distances, and available recycling infrastructure. In regions with predominantly renewable energy, the environmental advantage may shift toward more energy-intensive but longer-lasting materials like titanium alloys.
The environmental trade-offs between continued use of copper alloys (with appropriate inhibitors and protective measures) versus alternative materials must be evaluated on a case-by-case basis, considering the specific application requirements, expected service life, and local environmental priorities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!