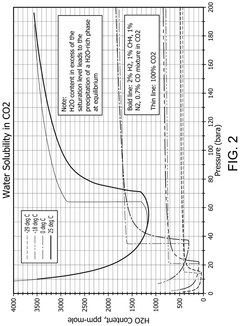
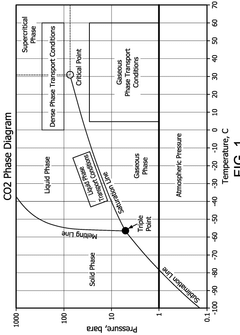
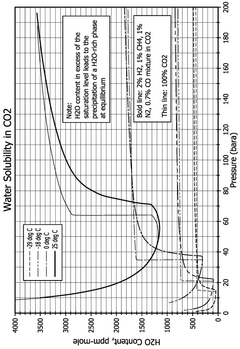
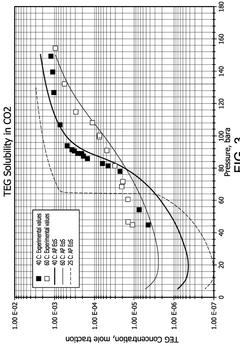
CO₂-Rich Streams: Carbonic Acid Corrosion, Material Selection And Duty Stability
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Corrosion Background and Objectives
Carbon dioxide corrosion, often referred to as sweet corrosion in the oil and gas industry, has been a significant challenge since the early days of hydrocarbon production. The phenomenon was first documented in the 1940s when CO₂-containing natural gas fields were developed. Over subsequent decades, as exploration moved into deeper reservoirs with higher CO₂ concentrations, the severity and economic impact of this corrosion mechanism became increasingly apparent.
The corrosion process occurs when CO₂ dissolves in water to form carbonic acid (H₂CO₃), which subsequently dissociates into bicarbonate and hydrogen ions, creating an acidic environment that attacks metal surfaces. This electrochemical process is particularly aggressive on carbon steel, which has historically been the material of choice for most oil and gas infrastructure due to its cost-effectiveness and mechanical properties.
The evolution of CO₂ corrosion understanding has progressed through several distinct phases. Initial empirical observations gave way to laboratory studies in the 1970s and 1980s, followed by the development of mechanistic models in the 1990s. The 21st century has seen the integration of computational fluid dynamics with electrochemical models, allowing for more accurate prediction of corrosion rates under complex flow conditions.
Current technological trends in this field include the development of advanced corrosion-resistant alloys, innovative coating technologies, and real-time corrosion monitoring systems. The push toward more sustainable energy solutions has also sparked interest in understanding CO₂ corrosion in carbon capture and storage (CCS) infrastructure, where concentrated CO₂ streams present unique challenges.
The primary objectives of current research and development efforts in CO₂ corrosion include: establishing reliable prediction models for diverse operating conditions; developing cost-effective materials and protection strategies for high-CO₂ environments; understanding the influence of various parameters such as temperature, pressure, flow regime, and solution chemistry on corrosion mechanisms; and creating standardized testing methodologies that accurately reflect field conditions.
Additionally, there is growing interest in understanding the long-term integrity of infrastructure exposed to CO₂-rich environments, particularly in the context of the energy transition. This includes evaluating the repurposing of existing oil and gas assets for hydrogen transport or CO₂ sequestration, where material compatibility becomes a critical consideration.
The ultimate goal is to develop a comprehensive framework that enables engineers to make informed decisions regarding material selection, corrosion allowance, inhibitor application, and monitoring strategies for systems handling CO₂-rich streams, thereby ensuring operational safety, environmental protection, and economic viability across various industrial applications.
The corrosion process occurs when CO₂ dissolves in water to form carbonic acid (H₂CO₃), which subsequently dissociates into bicarbonate and hydrogen ions, creating an acidic environment that attacks metal surfaces. This electrochemical process is particularly aggressive on carbon steel, which has historically been the material of choice for most oil and gas infrastructure due to its cost-effectiveness and mechanical properties.
The evolution of CO₂ corrosion understanding has progressed through several distinct phases. Initial empirical observations gave way to laboratory studies in the 1970s and 1980s, followed by the development of mechanistic models in the 1990s. The 21st century has seen the integration of computational fluid dynamics with electrochemical models, allowing for more accurate prediction of corrosion rates under complex flow conditions.
Current technological trends in this field include the development of advanced corrosion-resistant alloys, innovative coating technologies, and real-time corrosion monitoring systems. The push toward more sustainable energy solutions has also sparked interest in understanding CO₂ corrosion in carbon capture and storage (CCS) infrastructure, where concentrated CO₂ streams present unique challenges.
The primary objectives of current research and development efforts in CO₂ corrosion include: establishing reliable prediction models for diverse operating conditions; developing cost-effective materials and protection strategies for high-CO₂ environments; understanding the influence of various parameters such as temperature, pressure, flow regime, and solution chemistry on corrosion mechanisms; and creating standardized testing methodologies that accurately reflect field conditions.
Additionally, there is growing interest in understanding the long-term integrity of infrastructure exposed to CO₂-rich environments, particularly in the context of the energy transition. This includes evaluating the repurposing of existing oil and gas assets for hydrogen transport or CO₂ sequestration, where material compatibility becomes a critical consideration.
The ultimate goal is to develop a comprehensive framework that enables engineers to make informed decisions regarding material selection, corrosion allowance, inhibitor application, and monitoring strategies for systems handling CO₂-rich streams, thereby ensuring operational safety, environmental protection, and economic viability across various industrial applications.
Market Analysis for CO2-Resistant Materials
The global market for CO2-resistant materials is experiencing significant growth, driven primarily by the expanding oil and gas industry, carbon capture and storage (CCS) initiatives, and increasing environmental regulations. Current market valuation stands at approximately $5.7 billion, with projections indicating a compound annual growth rate of 6.8% through 2028, potentially reaching $8.4 billion by that time.
The oil and gas sector remains the dominant consumer of CO2-resistant materials, accounting for roughly 65% of the total market share. This dominance stems from the critical need for corrosion-resistant equipment in environments where carbonic acid formation is prevalent, particularly in natural gas processing, enhanced oil recovery operations, and deep-sea extraction facilities.
Carbon capture and storage technologies represent the fastest-growing segment, with a current market share of 15% but expanding at nearly 12% annually. This acceleration is largely attributed to global decarbonization efforts and substantial government investments in carbon reduction technologies across North America, Europe, and parts of Asia.
Geographically, North America leads the market with approximately 38% share, followed by Europe (27%) and Asia-Pacific (22%). The Middle East, despite its smaller overall share (8%), is showing the most rapid growth rate at 9.3% annually, driven by ambitious diversification plans in the region's energy infrastructure.
Material-specific analysis reveals that specialized stainless steels dominate with 42% market share, followed by nickel-based alloys (28%), polymer composites (17%), and specialized coatings (13%). The nickel-based alloy segment is experiencing the strongest growth due to its exceptional resistance to carbonic acid corrosion in high-pressure, high-temperature environments.
Customer demand patterns indicate a growing preference for materials that offer extended service life under aggressive CO2-rich conditions, with lifecycle cost increasingly outweighing initial acquisition expenses. This shift has prompted material manufacturers to focus on developing solutions that demonstrate superior resistance to localized corrosion phenomena such as pitting and stress corrosion cracking.
Pricing trends show moderate but consistent increases of 3-4% annually for premium CO2-resistant materials, reflecting both rising raw material costs and the value premium these specialized materials command in critical applications where failure is not an option.
The oil and gas sector remains the dominant consumer of CO2-resistant materials, accounting for roughly 65% of the total market share. This dominance stems from the critical need for corrosion-resistant equipment in environments where carbonic acid formation is prevalent, particularly in natural gas processing, enhanced oil recovery operations, and deep-sea extraction facilities.
Carbon capture and storage technologies represent the fastest-growing segment, with a current market share of 15% but expanding at nearly 12% annually. This acceleration is largely attributed to global decarbonization efforts and substantial government investments in carbon reduction technologies across North America, Europe, and parts of Asia.
Geographically, North America leads the market with approximately 38% share, followed by Europe (27%) and Asia-Pacific (22%). The Middle East, despite its smaller overall share (8%), is showing the most rapid growth rate at 9.3% annually, driven by ambitious diversification plans in the region's energy infrastructure.
Material-specific analysis reveals that specialized stainless steels dominate with 42% market share, followed by nickel-based alloys (28%), polymer composites (17%), and specialized coatings (13%). The nickel-based alloy segment is experiencing the strongest growth due to its exceptional resistance to carbonic acid corrosion in high-pressure, high-temperature environments.
Customer demand patterns indicate a growing preference for materials that offer extended service life under aggressive CO2-rich conditions, with lifecycle cost increasingly outweighing initial acquisition expenses. This shift has prompted material manufacturers to focus on developing solutions that demonstrate superior resistance to localized corrosion phenomena such as pitting and stress corrosion cracking.
Pricing trends show moderate but consistent increases of 3-4% annually for premium CO2-resistant materials, reflecting both rising raw material costs and the value premium these specialized materials command in critical applications where failure is not an option.
Carbonic Acid Corrosion Challenges
Carbonic acid corrosion represents one of the most significant challenges in CO₂-rich environments, particularly affecting industries such as oil and gas production, carbon capture and storage (CCS), and geothermal energy systems. When carbon dioxide dissolves in water, it forms carbonic acid (H₂CO₃), which dissociates into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺), creating an acidic environment that accelerates corrosion processes on metal surfaces.
The severity of carbonic acid corrosion is influenced by multiple factors, including CO₂ partial pressure, temperature, flow conditions, and solution chemistry. At higher CO₂ partial pressures, more carbonic acid forms, intensifying the corrosion rate. Temperature effects are complex, with corrosion rates generally increasing with temperature up to approximately 80°C, after which protective scale formation may reduce corrosion rates under certain conditions.
Flow dynamics significantly impact corrosion mechanisms, with turbulent flow potentially removing protective scales and accelerating metal degradation through flow-accelerated corrosion (FAC). This is particularly problematic in pipeline bends, restrictions, and areas with high fluid velocities where protective films cannot establish or maintain integrity.
The presence of other species in CO₂-rich streams compounds corrosion challenges. Hydrogen sulfide (H₂S), even in small concentrations, can lead to sulfide stress cracking and hydrogen-induced cracking. Oxygen contamination, often introduced during operational activities, can disrupt protective film formation and dramatically increase corrosion rates through synergistic effects with CO₂.
Localized corrosion presents a particularly insidious challenge, manifesting as pitting, crevice corrosion, or mesa attack. These forms of corrosion are difficult to detect and predict but can lead to catastrophic failures with minimal warning. The formation and breakdown of iron carbonate (FeCO₃) scales play a crucial role in determining whether uniform or localized corrosion predominates.
Material selection becomes increasingly complex in environments with fluctuating conditions. Systems experiencing temperature cycling, pressure variations, or changing fluid compositions face accelerated degradation as protective scales repeatedly form and break down. This cycling effect can reduce the effectiveness of otherwise suitable materials and necessitates more conservative design approaches.
Traditional monitoring techniques often prove inadequate for accurately assessing carbonic acid corrosion rates in real-time, particularly in remote or subsea applications. The limitations of current monitoring technologies create significant uncertainty in predicting asset integrity and determining appropriate maintenance intervals.
The severity of carbonic acid corrosion is influenced by multiple factors, including CO₂ partial pressure, temperature, flow conditions, and solution chemistry. At higher CO₂ partial pressures, more carbonic acid forms, intensifying the corrosion rate. Temperature effects are complex, with corrosion rates generally increasing with temperature up to approximately 80°C, after which protective scale formation may reduce corrosion rates under certain conditions.
Flow dynamics significantly impact corrosion mechanisms, with turbulent flow potentially removing protective scales and accelerating metal degradation through flow-accelerated corrosion (FAC). This is particularly problematic in pipeline bends, restrictions, and areas with high fluid velocities where protective films cannot establish or maintain integrity.
The presence of other species in CO₂-rich streams compounds corrosion challenges. Hydrogen sulfide (H₂S), even in small concentrations, can lead to sulfide stress cracking and hydrogen-induced cracking. Oxygen contamination, often introduced during operational activities, can disrupt protective film formation and dramatically increase corrosion rates through synergistic effects with CO₂.
Localized corrosion presents a particularly insidious challenge, manifesting as pitting, crevice corrosion, or mesa attack. These forms of corrosion are difficult to detect and predict but can lead to catastrophic failures with minimal warning. The formation and breakdown of iron carbonate (FeCO₃) scales play a crucial role in determining whether uniform or localized corrosion predominates.
Material selection becomes increasingly complex in environments with fluctuating conditions. Systems experiencing temperature cycling, pressure variations, or changing fluid compositions face accelerated degradation as protective scales repeatedly form and break down. This cycling effect can reduce the effectiveness of otherwise suitable materials and necessitates more conservative design approaches.
Traditional monitoring techniques often prove inadequate for accurately assessing carbonic acid corrosion rates in real-time, particularly in remote or subsea applications. The limitations of current monitoring technologies create significant uncertainty in predicting asset integrity and determining appropriate maintenance intervals.
Current Solutions for CO2-Rich Environments
01 Corrosion inhibitors for CO₂-rich environments
Various chemical compounds can be used as corrosion inhibitors specifically designed for CO₂-rich streams. These inhibitors form protective films on metal surfaces, preventing direct contact with corrosive CO₂ and carbonic acid. Formulations may include organic amines, imidazolines, quaternary ammonium compounds, and phosphate esters that adsorb onto metal surfaces to create a barrier against corrosive species. These inhibitors are particularly effective in pipelines, processing equipment, and storage vessels handling CO₂-rich fluids.- Corrosion inhibitors for CO₂-rich environments: Various chemical compounds can be used as corrosion inhibitors specifically designed for CO₂-rich streams. These inhibitors form protective films on metal surfaces, preventing direct contact with corrosive CO₂ and carbonic acid. Formulations may include organic amines, imidazolines, quaternary ammonium compounds, and other specialized chemicals that adsorb onto metal surfaces to provide a barrier against corrosive species in CO₂-rich environments.
- Materials selection for CO₂ corrosion resistance: Selecting appropriate materials of construction is crucial for handling CO₂-rich streams. Corrosion-resistant alloys such as stainless steels, nickel-based alloys, and specialized carbon steels with chromium content can significantly reduce corrosion rates. Surface treatments and coatings can also enhance the corrosion resistance of conventional materials when exposed to CO₂-rich environments, extending equipment life and reducing maintenance costs.
- Process control strategies to mitigate CO₂ corrosion: Implementing specific process control strategies can help mitigate corrosion in systems handling CO₂-rich streams. These include controlling temperature, pressure, flow rates, and pH levels to minimize corrosion potential. Removing water or maintaining water content below critical levels can prevent formation of carbonic acid. Proper monitoring systems and regular inspection protocols are essential components of effective corrosion management programs for CO₂-rich environments.
- CO₂ capture and processing technologies with corrosion considerations: Technologies for capturing, processing, and transporting CO₂-rich streams incorporate specific design features to address corrosion challenges. These include specialized equipment designs, dehydration systems to remove water, and materials selection optimized for corrosive conditions. Advanced separation techniques and process configurations help minimize corrosion while maintaining operational efficiency in carbon capture and storage applications, as well as in natural gas processing where CO₂ is a common contaminant.
- Monitoring and prediction of CO₂ corrosion: Advanced monitoring techniques and predictive models help assess and forecast corrosion rates in CO₂-rich environments. These include electrochemical monitoring systems, corrosion coupons, ultrasonic thickness measurements, and computational models that account for various parameters affecting CO₂ corrosion. Real-time monitoring combined with predictive analytics enables proactive maintenance strategies and optimization of corrosion inhibitor programs, reducing the risk of unexpected failures in systems handling CO₂-rich streams.
02 Materials selection for CO₂ corrosion resistance
Selecting appropriate materials is crucial for equipment handling CO₂-rich streams. Corrosion-resistant alloys such as stainless steels, duplex steels, and nickel-based alloys offer superior performance compared to carbon steel in CO₂-rich environments. These materials form stable passive films that resist carbonic acid attack. For severe service conditions, special coatings or linings may be applied to enhance corrosion resistance. Material selection considerations include CO₂ partial pressure, temperature, flow conditions, and the presence of other corrosive species.Expand Specific Solutions03 CO₂ capture and processing technologies with corrosion mitigation
Advanced CO₂ capture and processing technologies incorporate specific design features to mitigate corrosion risks. These include optimized process conditions, specialized equipment designs, and integrated corrosion management systems. Technologies such as amine-based absorption, membrane separation, and cryogenic distillation are engineered with materials and operating parameters that minimize corrosion damage. Process modifications like dehydration, temperature control, and pH adjustment help reduce the corrosivity of CO₂-rich streams during capture, transport, and storage operations.Expand Specific Solutions04 Monitoring and detection systems for CO₂ corrosion
Specialized monitoring systems are employed to detect and track corrosion in CO₂-rich environments. These include electrochemical probes, ultrasonic thickness measurements, electrical resistance techniques, and real-time corrosion rate monitors. Advanced systems may incorporate fiber optic sensors, acoustic emission detection, and wireless monitoring capabilities. Data analytics and predictive modeling help identify corrosion trends and optimize maintenance schedules. Early detection allows for timely intervention before critical equipment damage occurs.Expand Specific Solutions05 CO₂ stream purification to reduce corrosivity
Purification processes can significantly reduce the corrosivity of CO₂-rich streams by removing contaminants that exacerbate corrosion. These processes target the removal of water, oxygen, hydrogen sulfide, and other impurities that contribute to corrosion mechanisms. Technologies include dehydration systems, chemical scavengers, molecular sieves, and membrane separators. Maintaining low moisture content is particularly important as water combines with CO₂ to form corrosive carbonic acid. Purification not only extends equipment life but also improves process efficiency and product quality.Expand Specific Solutions
Leading Companies in Corrosion-Resistant Materials
The CO₂-rich streams carbonic acid corrosion market is in a growth phase, with increasing focus on material selection and duty stability solutions as energy transition accelerates. The market is expanding due to rising carbon capture projects and aging oil and gas infrastructure concerns. Technologically, the field shows varying maturity levels across different applications. Leading players include established steel manufacturers like JFE Steel, NIPPON STEEL, and Baoshan Iron & Steel, who focus on corrosion-resistant alloys, alongside energy giants such as Saudi Aramco, ExxonMobil, and PetroChina developing proprietary solutions. Service providers like Halliburton and Baker Hughes offer specialized field implementation expertise, while research institutions including KFUPM and Southwest Petroleum University drive fundamental innovation. The competitive landscape features increasing collaboration between materials science experts and energy operators to address this critical challenge.
JFE Steel Corp.
Technical Solution: JFE Steel has pioneered advanced metallurgical solutions for CO₂-rich environments, focusing on developing specialized steel grades with enhanced resistance to carbonic acid corrosion. Their technology includes proprietary alloying techniques that incorporate optimized chromium, molybdenum, and nitrogen content to create microstructures highly resistant to carbonic acid attack. The company has developed a unique heat treatment process that creates a passive oxide layer with superior stability in CO₂-rich environments compared to conventional stainless steels. JFE's research has resulted in specialized martensitic stainless steel grades that maintain mechanical properties while offering corrosion resistance comparable to much more expensive nickel-based alloys in certain CO₂-rich applications. Their materials undergo extensive testing in simulated environments that replicate actual service conditions, including fluctuating temperatures and pressures typical in carbon capture and storage systems.
Strengths: Industry-leading metallurgical expertise specifically focused on CO₂ corrosion resistance; cost-effective alternatives to expensive high-nickel alloys; materials optimized for specific operating conditions. Weaknesses: Limited experience in chemical treatment approaches to complement material solutions; some specialized grades may have restricted availability or longer lead times; solutions primarily focused on metallic materials rather than comprehensive system approaches.
NIPPON STEEL CORP.
Technical Solution: Nippon Steel has developed the "CO₂-Shield" technology platform specifically addressing carbonic acid corrosion in industrial applications. Their approach focuses on metallurgical innovations that enhance steel's inherent resistance to CO₂-rich environments. The company has pioneered microalloying techniques that incorporate precise amounts of chromium, molybdenum, and copper to create steel microstructures with enhanced resistance to carbonic acid attack. Their research has identified specific grain boundary engineering approaches that minimize preferential corrosion pathways common in CO₂-rich environments. Nippon Steel has also developed specialized surface treatment technologies that create protective oxide layers with superior stability in fluctuating CO₂ partial pressure conditions. Their materials undergo rigorous testing in simulated environments that replicate actual service conditions, including the effects of flow-induced corrosion acceleration common in CO₂ transport systems.
Strengths: Extensive metallurgical research specifically targeting CO₂ corrosion mechanisms; cost-effective solutions that balance corrosion resistance with mechanical properties; materials optimized for specific temperature and pressure ranges. Weaknesses: Limited integration with monitoring or chemical treatment approaches; solutions primarily focused on material selection rather than comprehensive corrosion management systems; some specialized grades may have availability constraints in certain markets.
Key Innovations in Material Science for Corrosion Resistance
Co2 pipeline corrosion
PatentPendingUS20250066664A1
Innovation
- The method involves transporting a carbon dioxide stream with controlled concentrations of H2S, oxygen, sulfur oxides, and water to prevent the formation of sulfuric acid, and avoiding glycol-based drying and amine-based capture processes to minimize corrosion risks.
Co2 pipeline corrosion
PatentWO2025042554A1
Innovation
- The method involves reducing the concentration of water and oxygen to prevent sulfuric acid formation, maintaining a specific ratio of H2S to oxidizing compounds, and avoiding glycol-based drying and amine-based CO2 capture during gas-phase transport to prevent corrosion in carbon steel pipelines.
Environmental Impact Assessment of Anti-Corrosion Technologies
The environmental implications of anti-corrosion technologies used in CO₂-rich environments are increasingly scrutinized as industries strive for sustainability. Traditional corrosion prevention methods often involve chemicals and materials that can pose significant environmental risks when improperly managed or disposed of. Chromate-based inhibitors, while effective against carbonic acid corrosion, contain hexavalent chromium compounds classified as carcinogenic and environmentally persistent pollutants, leading to their restriction under various international regulations.
Coating technologies utilizing heavy metals such as cadmium and lead present similar environmental concerns, with potential for soil and water contamination during application, maintenance, and disposal phases. These substances can bioaccumulate in ecosystems, causing long-term ecological damage that extends beyond the immediate industrial setting.
More environmentally conscious alternatives have emerged in recent years, including vapor phase inhibitors and green inhibitors derived from plant extracts. These solutions demonstrate reduced toxicity profiles while maintaining acceptable performance in mitigating carbonic acid corrosion. Life cycle assessments indicate that these bio-based alternatives typically generate 40-60% less environmental impact compared to conventional options.
Material selection also plays a crucial role in environmental impact considerations. The production of highly corrosion-resistant alloys often requires energy-intensive processes and rare earth elements with significant extraction footprints. However, the extended service life and reduced maintenance requirements of these materials can offset initial environmental costs over the operational lifetime of industrial systems exposed to CO₂-rich environments.
Carbon footprint analysis reveals that preventive anti-corrosion strategies generally result in lower overall emissions compared to reactive approaches requiring frequent component replacement. Studies indicate that effective corrosion management in CO₂ transport pipelines can reduce associated greenhouse gas emissions by approximately 15-20% through prevention of leakage and extended infrastructure lifespan.
Water consumption and contamination represent another significant environmental concern. Many traditional passivation processes require substantial water resources and generate hazardous wastewater streams containing heavy metals and chemical inhibitors. Advanced treatment technologies such as electrocoagulation and membrane filtration are increasingly implemented to mitigate these impacts, though they add complexity and cost to industrial operations.
Regulatory frameworks worldwide are evolving to address these environmental challenges, with increasing emphasis on life cycle impact assessment for anti-corrosion technologies. The European Union's REACH regulation and similar initiatives globally are driving innovation toward more sustainable corrosion management solutions specifically designed for CO₂-rich environments, balancing technical performance with reduced environmental footprint.
Coating technologies utilizing heavy metals such as cadmium and lead present similar environmental concerns, with potential for soil and water contamination during application, maintenance, and disposal phases. These substances can bioaccumulate in ecosystems, causing long-term ecological damage that extends beyond the immediate industrial setting.
More environmentally conscious alternatives have emerged in recent years, including vapor phase inhibitors and green inhibitors derived from plant extracts. These solutions demonstrate reduced toxicity profiles while maintaining acceptable performance in mitigating carbonic acid corrosion. Life cycle assessments indicate that these bio-based alternatives typically generate 40-60% less environmental impact compared to conventional options.
Material selection also plays a crucial role in environmental impact considerations. The production of highly corrosion-resistant alloys often requires energy-intensive processes and rare earth elements with significant extraction footprints. However, the extended service life and reduced maintenance requirements of these materials can offset initial environmental costs over the operational lifetime of industrial systems exposed to CO₂-rich environments.
Carbon footprint analysis reveals that preventive anti-corrosion strategies generally result in lower overall emissions compared to reactive approaches requiring frequent component replacement. Studies indicate that effective corrosion management in CO₂ transport pipelines can reduce associated greenhouse gas emissions by approximately 15-20% through prevention of leakage and extended infrastructure lifespan.
Water consumption and contamination represent another significant environmental concern. Many traditional passivation processes require substantial water resources and generate hazardous wastewater streams containing heavy metals and chemical inhibitors. Advanced treatment technologies such as electrocoagulation and membrane filtration are increasingly implemented to mitigate these impacts, though they add complexity and cost to industrial operations.
Regulatory frameworks worldwide are evolving to address these environmental challenges, with increasing emphasis on life cycle impact assessment for anti-corrosion technologies. The European Union's REACH regulation and similar initiatives globally are driving innovation toward more sustainable corrosion management solutions specifically designed for CO₂-rich environments, balancing technical performance with reduced environmental footprint.
Lifecycle Cost Analysis of Material Selection
When evaluating material selection for CO₂-rich environments, lifecycle cost analysis (LCA) provides a comprehensive economic framework that extends beyond initial procurement costs. This analysis encompasses acquisition, installation, maintenance, replacement, and eventual disposal expenses over the operational lifespan of the equipment or system.
For carbon steel in CO₂-rich environments, the initial capital expenditure is significantly lower compared to corrosion-resistant alloys (CRAs). However, the accelerated corrosion rates in carbonic acid environments necessitate frequent inspections, maintenance interventions, and potentially premature replacements. These recurring costs, coupled with production downtime during maintenance activities, substantially increase the total ownership cost despite the lower initial investment.
Corrosion-resistant alloys such as 13Cr, duplex stainless steels, and nickel-based alloys present higher upfront costs but demonstrate superior resistance to carbonic acid corrosion. The extended service life and reduced maintenance requirements of these materials often result in lower long-term expenditures, particularly in aggressive environments with high CO₂ partial pressures, elevated temperatures, or when combined with H₂S.
Quantitative lifecycle cost modeling reveals that in moderate CO₂ environments (partial pressure <2 bar), properly inhibited carbon steel may present the most economical solution over a 15-20 year service life. Conversely, in severe conditions (high temperature, high CO₂ partial pressure, or presence of H₂S), CRAs typically demonstrate superior economic performance despite higher initial investment.
Risk assessment must also be incorporated into lifecycle cost calculations. The potential consequences of material failure—including environmental damage, safety incidents, and reputational harm—represent significant financial liabilities that may justify investment in more corrosion-resistant materials even when direct cost analysis suggests otherwise.
Emerging technologies such as internal cladding, composite materials, and advanced coating systems are altering the lifecycle cost equation by offering intermediate solutions between carbon steel and full CRA implementation. These hybrid approaches can provide optimized cost-performance ratios for specific operating conditions.
The time value of money, expressed through net present value (NPV) calculations, further influences material selection decisions. Materials requiring significant future expenditures may appear less economically attractive when future costs are appropriately discounted to present value, particularly in high-interest-rate environments or for projects with shorter expected operational durations.
For carbon steel in CO₂-rich environments, the initial capital expenditure is significantly lower compared to corrosion-resistant alloys (CRAs). However, the accelerated corrosion rates in carbonic acid environments necessitate frequent inspections, maintenance interventions, and potentially premature replacements. These recurring costs, coupled with production downtime during maintenance activities, substantially increase the total ownership cost despite the lower initial investment.
Corrosion-resistant alloys such as 13Cr, duplex stainless steels, and nickel-based alloys present higher upfront costs but demonstrate superior resistance to carbonic acid corrosion. The extended service life and reduced maintenance requirements of these materials often result in lower long-term expenditures, particularly in aggressive environments with high CO₂ partial pressures, elevated temperatures, or when combined with H₂S.
Quantitative lifecycle cost modeling reveals that in moderate CO₂ environments (partial pressure <2 bar), properly inhibited carbon steel may present the most economical solution over a 15-20 year service life. Conversely, in severe conditions (high temperature, high CO₂ partial pressure, or presence of H₂S), CRAs typically demonstrate superior economic performance despite higher initial investment.
Risk assessment must also be incorporated into lifecycle cost calculations. The potential consequences of material failure—including environmental damage, safety incidents, and reputational harm—represent significant financial liabilities that may justify investment in more corrosion-resistant materials even when direct cost analysis suggests otherwise.
Emerging technologies such as internal cladding, composite materials, and advanced coating systems are altering the lifecycle cost equation by offering intermediate solutions between carbon steel and full CRA implementation. These hybrid approaches can provide optimized cost-performance ratios for specific operating conditions.
The time value of money, expressed through net present value (NPV) calculations, further influences material selection decisions. Materials requiring significant future expenditures may appear less economically attractive when future costs are appropriately discounted to present value, particularly in high-interest-rate environments or for projects with shorter expected operational durations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!