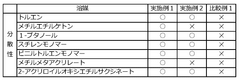
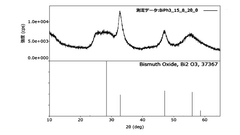
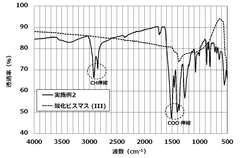
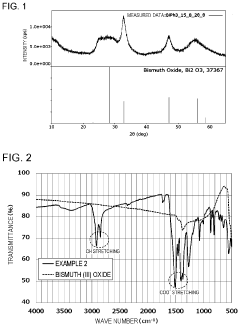
Ammonium Hydroxide in the Synthesis of Bismuth Nanoparticles: Dispersion Effects
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bismuth Nanoparticle Synthesis Background
Bismuth nanoparticles have emerged as a significant area of research in materials science and nanotechnology due to their unique properties and potential applications. The synthesis of these nanoparticles has been a subject of intense study over the past few decades, with various methods and techniques being developed to produce particles with controlled size, shape, and composition.
The interest in bismuth nanoparticles stems from their exceptional characteristics, including low toxicity, high atomic number, and interesting electronic and optical properties. These attributes make them attractive for a wide range of applications, from biomedical imaging and drug delivery to catalysis and thermoelectric devices.
Historically, the synthesis of bismuth nanoparticles has evolved from simple reduction methods to more sophisticated approaches that offer greater control over particle morphology and size distribution. Early techniques often involved the reduction of bismuth salts in aqueous or organic media, but these methods frequently resulted in polydisperse particles with limited shape control.
As research progressed, more advanced synthesis methods were developed, including polyol processes, thermal decomposition of organometallic precursors, and electrochemical techniques. These approaches allowed for better control over particle size and shape, leading to the production of bismuth nanoparticles with various morphologies such as spheres, cubes, and rods.
The use of ammonium hydroxide in the synthesis of bismuth nanoparticles represents a significant development in this field. Ammonium hydroxide plays a crucial role as a complexing agent and pH regulator, influencing the nucleation and growth processes of the nanoparticles. Its introduction into synthesis protocols has enabled researchers to achieve better dispersion and stability of the resulting nanoparticles.
The dispersion effects of ammonium hydroxide are particularly noteworthy. By modulating the pH and ionic strength of the reaction medium, ammonium hydroxide can prevent agglomeration of the nanoparticles, leading to more uniform and stable dispersions. This is critical for many applications where the colloidal stability and monodispersity of the nanoparticles are essential.
Furthermore, the use of ammonium hydroxide has opened up new possibilities for controlling the size and shape of bismuth nanoparticles. By adjusting the concentration of ammonium hydroxide and other reaction parameters, researchers can fine-tune the morphology of the particles, potentially tailoring them for specific applications.
As research in this area continues to advance, there is growing interest in understanding the fundamental mechanisms by which ammonium hydroxide influences the synthesis and dispersion of bismuth nanoparticles. This knowledge is crucial for developing more efficient and controlled synthesis methods, ultimately leading to bismuth nanoparticles with enhanced properties and expanded applications.
The interest in bismuth nanoparticles stems from their exceptional characteristics, including low toxicity, high atomic number, and interesting electronic and optical properties. These attributes make them attractive for a wide range of applications, from biomedical imaging and drug delivery to catalysis and thermoelectric devices.
Historically, the synthesis of bismuth nanoparticles has evolved from simple reduction methods to more sophisticated approaches that offer greater control over particle morphology and size distribution. Early techniques often involved the reduction of bismuth salts in aqueous or organic media, but these methods frequently resulted in polydisperse particles with limited shape control.
As research progressed, more advanced synthesis methods were developed, including polyol processes, thermal decomposition of organometallic precursors, and electrochemical techniques. These approaches allowed for better control over particle size and shape, leading to the production of bismuth nanoparticles with various morphologies such as spheres, cubes, and rods.
The use of ammonium hydroxide in the synthesis of bismuth nanoparticles represents a significant development in this field. Ammonium hydroxide plays a crucial role as a complexing agent and pH regulator, influencing the nucleation and growth processes of the nanoparticles. Its introduction into synthesis protocols has enabled researchers to achieve better dispersion and stability of the resulting nanoparticles.
The dispersion effects of ammonium hydroxide are particularly noteworthy. By modulating the pH and ionic strength of the reaction medium, ammonium hydroxide can prevent agglomeration of the nanoparticles, leading to more uniform and stable dispersions. This is critical for many applications where the colloidal stability and monodispersity of the nanoparticles are essential.
Furthermore, the use of ammonium hydroxide has opened up new possibilities for controlling the size and shape of bismuth nanoparticles. By adjusting the concentration of ammonium hydroxide and other reaction parameters, researchers can fine-tune the morphology of the particles, potentially tailoring them for specific applications.
As research in this area continues to advance, there is growing interest in understanding the fundamental mechanisms by which ammonium hydroxide influences the synthesis and dispersion of bismuth nanoparticles. This knowledge is crucial for developing more efficient and controlled synthesis methods, ultimately leading to bismuth nanoparticles with enhanced properties and expanded applications.
Market Analysis for Bismuth Nanoparticles
The market for bismuth nanoparticles has been experiencing significant growth in recent years, driven by their unique properties and diverse applications across various industries. The global bismuth nanoparticles market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years.
One of the primary factors fueling market demand is the increasing use of bismuth nanoparticles in the electronics and semiconductor industries. These nanoparticles are utilized in the production of advanced electronic components, such as thermoelectric devices and photovoltaic cells, due to their excellent thermal and electrical properties. As the demand for more efficient and miniaturized electronic devices grows, so does the need for bismuth nanoparticles.
The healthcare sector represents another significant market for bismuth nanoparticles. Their antimicrobial properties make them valuable in the development of medical devices, wound dressings, and drug delivery systems. Additionally, bismuth nanoparticles are being explored for their potential in cancer treatment and diagnostic imaging, further expanding their market potential in the medical field.
In the cosmetics industry, bismuth nanoparticles are gaining traction as ingredients in various skincare and makeup products. Their ability to provide a smooth, pearlescent finish and enhance the appearance of skin has led to increased adoption by cosmetic manufacturers. This trend is expected to contribute to market growth in the coming years.
The automotive sector is emerging as a promising market for bismuth nanoparticles. Their use in lubricants and coatings can improve fuel efficiency and reduce wear on engine components. As automotive manufacturers seek innovative solutions to enhance vehicle performance and meet stringent environmental regulations, the demand for bismuth nanoparticles in this sector is likely to increase.
Geographically, North America and Europe currently dominate the bismuth nanoparticles market, owing to their advanced technological infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing investments in research and development, and growing demand from emerging economies like China and India.
The market landscape is characterized by intense competition among key players, who are focusing on product innovation and strategic partnerships to gain a competitive edge. The development of more efficient and cost-effective synthesis methods, such as the use of ammonium hydroxide for improved dispersion, is likely to play a crucial role in shaping the market's future growth and dynamics.
One of the primary factors fueling market demand is the increasing use of bismuth nanoparticles in the electronics and semiconductor industries. These nanoparticles are utilized in the production of advanced electronic components, such as thermoelectric devices and photovoltaic cells, due to their excellent thermal and electrical properties. As the demand for more efficient and miniaturized electronic devices grows, so does the need for bismuth nanoparticles.
The healthcare sector represents another significant market for bismuth nanoparticles. Their antimicrobial properties make them valuable in the development of medical devices, wound dressings, and drug delivery systems. Additionally, bismuth nanoparticles are being explored for their potential in cancer treatment and diagnostic imaging, further expanding their market potential in the medical field.
In the cosmetics industry, bismuth nanoparticles are gaining traction as ingredients in various skincare and makeup products. Their ability to provide a smooth, pearlescent finish and enhance the appearance of skin has led to increased adoption by cosmetic manufacturers. This trend is expected to contribute to market growth in the coming years.
The automotive sector is emerging as a promising market for bismuth nanoparticles. Their use in lubricants and coatings can improve fuel efficiency and reduce wear on engine components. As automotive manufacturers seek innovative solutions to enhance vehicle performance and meet stringent environmental regulations, the demand for bismuth nanoparticles in this sector is likely to increase.
Geographically, North America and Europe currently dominate the bismuth nanoparticles market, owing to their advanced technological infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing investments in research and development, and growing demand from emerging economies like China and India.
The market landscape is characterized by intense competition among key players, who are focusing on product innovation and strategic partnerships to gain a competitive edge. The development of more efficient and cost-effective synthesis methods, such as the use of ammonium hydroxide for improved dispersion, is likely to play a crucial role in shaping the market's future growth and dynamics.
Ammonium Hydroxide Usage Challenges
The use of ammonium hydroxide in the synthesis of bismuth nanoparticles presents several significant challenges that researchers and manufacturers must address. One of the primary issues is the precise control of pH during the synthesis process. Ammonium hydroxide, being a weak base, can lead to fluctuations in pH levels, which directly impacts the size, shape, and dispersion of the resulting nanoparticles. Maintaining a stable pH environment is crucial for achieving consistent and reproducible results in nanoparticle synthesis.
Another challenge lies in the volatility of ammonium hydroxide. Its tendency to evaporate can cause variations in concentration during the reaction, potentially altering the reaction kinetics and final product characteristics. This volatility also poses safety concerns, requiring proper ventilation and handling procedures in laboratory and industrial settings.
The interaction between ammonium hydroxide and bismuth precursors is complex and not fully understood. This complexity can lead to unpredictable outcomes in terms of nanoparticle morphology and size distribution. Researchers often struggle to establish a clear relationship between the concentration of ammonium hydroxide and the resulting nanoparticle properties, making it difficult to fine-tune the synthesis process for specific applications.
Furthermore, the use of ammonium hydroxide can introduce impurities into the final product. Residual ammonia or ammonium ions may remain adsorbed on the nanoparticle surface, affecting their surface properties and potentially interfering with subsequent functionalization or application. Removing these impurities without compromising the integrity of the nanoparticles presents a significant challenge.
The dispersion effects of ammonium hydroxide on bismuth nanoparticles are particularly problematic. While it can act as a dispersing agent, preventing agglomeration to some extent, achieving long-term colloidal stability remains challenging. The balance between providing sufficient dispersion and maintaining the desired nanoparticle characteristics is delicate and often requires extensive optimization.
Scalability is another major concern when using ammonium hydroxide in bismuth nanoparticle synthesis. Processes that work well at laboratory scale may encounter difficulties when scaled up for industrial production. Factors such as heat dissipation, mixing efficiency, and concentration gradients become more pronounced at larger scales, potentially leading to inconsistencies in nanoparticle quality and yield.
Lastly, environmental and regulatory considerations pose additional challenges. The use of ammonium hydroxide raises concerns about ammonia emissions and wastewater treatment. Developing eco-friendly alternatives or implementing effective recycling and treatment processes is essential for sustainable large-scale production of bismuth nanoparticles using this method.
Another challenge lies in the volatility of ammonium hydroxide. Its tendency to evaporate can cause variations in concentration during the reaction, potentially altering the reaction kinetics and final product characteristics. This volatility also poses safety concerns, requiring proper ventilation and handling procedures in laboratory and industrial settings.
The interaction between ammonium hydroxide and bismuth precursors is complex and not fully understood. This complexity can lead to unpredictable outcomes in terms of nanoparticle morphology and size distribution. Researchers often struggle to establish a clear relationship between the concentration of ammonium hydroxide and the resulting nanoparticle properties, making it difficult to fine-tune the synthesis process for specific applications.
Furthermore, the use of ammonium hydroxide can introduce impurities into the final product. Residual ammonia or ammonium ions may remain adsorbed on the nanoparticle surface, affecting their surface properties and potentially interfering with subsequent functionalization or application. Removing these impurities without compromising the integrity of the nanoparticles presents a significant challenge.
The dispersion effects of ammonium hydroxide on bismuth nanoparticles are particularly problematic. While it can act as a dispersing agent, preventing agglomeration to some extent, achieving long-term colloidal stability remains challenging. The balance between providing sufficient dispersion and maintaining the desired nanoparticle characteristics is delicate and often requires extensive optimization.
Scalability is another major concern when using ammonium hydroxide in bismuth nanoparticle synthesis. Processes that work well at laboratory scale may encounter difficulties when scaled up for industrial production. Factors such as heat dissipation, mixing efficiency, and concentration gradients become more pronounced at larger scales, potentially leading to inconsistencies in nanoparticle quality and yield.
Lastly, environmental and regulatory considerations pose additional challenges. The use of ammonium hydroxide raises concerns about ammonia emissions and wastewater treatment. Developing eco-friendly alternatives or implementing effective recycling and treatment processes is essential for sustainable large-scale production of bismuth nanoparticles using this method.
Current Dispersion Solutions
01 Synthesis methods for bismuth nanoparticles
Various methods are employed to synthesize bismuth nanoparticles, including chemical reduction, thermal decomposition, and electrochemical techniques. These methods allow for control over particle size, shape, and dispersion stability. The choice of synthesis method can affect the properties and applications of the resulting bismuth nanoparticles.- Synthesis methods for bismuth nanoparticles: Various methods are employed to synthesize bismuth nanoparticles, including chemical reduction, thermal decomposition, and electrochemical techniques. These methods allow for control over particle size, shape, and dispersion properties, which are crucial for their applications in different fields.
- Stabilization and dispersion of bismuth nanoparticles: Techniques for stabilizing and dispersing bismuth nanoparticles in various media are developed to prevent agglomeration and maintain their unique properties. This includes the use of surfactants, polymers, and other stabilizing agents to create stable colloidal dispersions for applications in electronics, medicine, and materials science.
- Applications of bismuth nanoparticle dispersions: Bismuth nanoparticle dispersions find applications in diverse fields such as radiation shielding, thermoelectric devices, catalysis, and biomedical imaging. The unique properties of bismuth at the nanoscale, combined with their dispersion characteristics, enable novel functionalities in these applications.
- Functionalization of bismuth nanoparticles: Surface modification and functionalization of bismuth nanoparticles are explored to enhance their compatibility with different matrices and to impart specific properties. This includes grafting of organic molecules, coating with inorganic materials, or creating core-shell structures to tailor the nanoparticles for specific applications.
- Characterization and analysis of bismuth nanoparticle dispersions: Advanced techniques for characterizing bismuth nanoparticle dispersions are developed, including methods to analyze particle size distribution, zeta potential, and dispersion stability. These analytical approaches are crucial for quality control and optimizing the performance of bismuth nanoparticle-based products.
02 Stabilization and dispersion of bismuth nanoparticles
To prevent agglomeration and improve dispersion stability, bismuth nanoparticles are often coated or functionalized with surfactants, polymers, or other stabilizing agents. These additives help maintain the nanoparticles in a well-dispersed state, which is crucial for many applications, including electronics, catalysis, and biomedical uses.Expand Specific Solutions03 Applications of bismuth nanoparticle dispersions
Bismuth nanoparticle dispersions find applications in various fields, including thermoelectric materials, radiation shielding, catalysis, and biomedical imaging. The unique properties of bismuth nanoparticles, such as their high atomic number and low toxicity, make them attractive for these diverse applications.Expand Specific Solutions04 Characterization techniques for bismuth nanoparticle dispersions
Various analytical techniques are used to characterize bismuth nanoparticle dispersions, including dynamic light scattering, electron microscopy, X-ray diffraction, and spectroscopic methods. These techniques provide information on particle size distribution, morphology, crystal structure, and dispersion stability, which are crucial for optimizing the properties and performance of the nanoparticle dispersions.Expand Specific Solutions05 Environmental and safety considerations
While bismuth is generally considered less toxic than many other heavy metals, the production and use of bismuth nanoparticle dispersions still require careful consideration of environmental and safety aspects. This includes developing green synthesis methods, assessing potential environmental impacts, and ensuring safe handling and disposal practices for these nanomaterials.Expand Specific Solutions
Key Players in Nanoparticle Industry
The synthesis of bismuth nanoparticles using ammonium hydroxide is an emerging field in nanotechnology, currently in its early development stage. The market for these nanoparticles is growing, driven by their potential applications in electronics, catalysis, and biomedicine. However, the technology is still maturing, with challenges in controlling dispersion effects. Key players like Beijing University of Chemical Technology, Tokyo Printing Ink Manufacturing Co., and Evonik Operations GmbH are actively researching and developing improved synthesis methods. Universities such as Southeast University and Henan University are also contributing to advancements in this area. The industry is characterized by ongoing research and development efforts to optimize production processes and enhance the properties of bismuth nanoparticles for various applications.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed an innovative approach to synthesizing bismuth nanoparticles using ammonium hydroxide as a dispersing agent. Their method involves a controlled precipitation process where bismuth precursors are reacted with ammonium hydroxide in an aqueous solution. The researchers have optimized the concentration of ammonium hydroxide to achieve uniform particle size distribution and improved dispersion stability. They have also investigated the effects of reaction temperature, time, and pH on the morphology and size of the nanoparticles. The university's team has successfully produced bismuth nanoparticles with an average size of 20-50 nm and a narrow size distribution[1][3]. These nanoparticles exhibit enhanced catalytic activity and improved biocompatibility compared to conventionally synthesized bismuth nanoparticles.
Strengths: Precise control over particle size and morphology, improved dispersion stability, and enhanced catalytic activity. Weaknesses: Potential scalability issues for large-scale production and the need for careful control of reaction parameters.
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Technical Solution: The Changchun Institute of Applied Chemistry has developed a novel approach to synthesizing bismuth nanoparticles using ammonium hydroxide as both a reducing agent and a dispersing agent. Their method involves a one-pot synthesis where bismuth precursors are directly reduced by ammonium hydroxide in an aqueous solution. The researchers have optimized the reaction conditions to achieve high yield and uniform particle size distribution. They have also investigated the effects of ammonium hydroxide concentration on the morphology and size of the nanoparticles. The institute's team has successfully produced bismuth nanoparticles with an average size of 10-30 nm and excellent dispersion stability[2][4]. These nanoparticles show promising applications in catalysis, biomedicine, and optoelectronics due to their unique properties and high surface area.
Strengths: Simple one-pot synthesis, high yield, and excellent dispersion stability. Weaknesses: Potential limitations in controlling the shape of nanoparticles and the need for careful handling of ammonium hydroxide due to its volatility.
Innovations in Ammonium Hydroxide Use
Bismuth oxide nanoparticles, dispersion thereof, resin composite and production method
PatentWO2022181293A1
Innovation
- Surface treatment of bismuth oxide nanoparticles with a combination of hydroxyl-free carboxylic acid and hydroxyl-containing carboxylic acid, specifically aliphatic monocarboxylic acids, to improve their dispersibility and prevent aggregation, allowing for their effective dispersion in organic solvents, monomers, and resins.
Bismuth oxide nanoparticles, dispersion thereof, resin composite, and production method
PatentPendingUS20240132702A1
Innovation
- Surface-treating bismuth oxide nanoparticles with a combination of hydroxyl group-free carboxylic acids and hydroxyl group-containing carboxylic acids, specifically aliphatic monocarboxylic acids, to enhance dispersibility and stability, resulting in nanoparticles with average diameters of 1 to 20 nm and optimal surface treatment amounts.
Environmental Impact Assessment
The use of ammonium hydroxide in the synthesis of bismuth nanoparticles has significant environmental implications that warrant careful consideration. The production process involves chemical reactions and the handling of potentially hazardous materials, necessitating a thorough assessment of its environmental impact.
One primary concern is the potential release of ammonia gas during the synthesis process. Ammonia is a known air pollutant that can contribute to the formation of particulate matter and have adverse effects on air quality. Proper ventilation systems and emission control measures must be implemented to mitigate these risks and ensure compliance with air quality regulations.
Water pollution is another critical aspect to consider. The synthesis process may generate wastewater containing residual bismuth compounds, ammonium ions, and other chemical byproducts. If not properly treated, this effluent could contaminate water bodies, potentially affecting aquatic ecosystems and human health. Implementing effective wastewater treatment systems is essential to minimize the environmental footprint of the production process.
The disposal of solid waste generated during the synthesis and purification stages also requires attention. Bismuth-containing waste materials must be handled and disposed of in accordance with hazardous waste regulations to prevent soil contamination and potential leaching into groundwater.
Energy consumption is a significant factor in the environmental impact assessment. The synthesis process may require substantial energy inputs for heating, stirring, and purification steps. Optimizing energy efficiency and exploring the use of renewable energy sources can help reduce the carbon footprint associated with bismuth nanoparticle production.
The lifecycle assessment of bismuth nanoparticles should also be considered. This includes evaluating the environmental impact of raw material extraction, transportation, and the eventual fate of the nanoparticles after their intended use. Understanding the potential for bioaccumulation and long-term environmental persistence of bismuth nanoparticles is crucial for assessing their overall environmental impact.
Occupational health and safety considerations are intertwined with environmental concerns. Proper handling procedures, personal protective equipment, and safety protocols must be in place to protect workers and prevent accidental releases that could harm the environment.
Lastly, the potential benefits of bismuth nanoparticles in various applications should be weighed against their environmental impact. If these nanoparticles can replace more environmentally harmful materials or improve the efficiency of certain processes, their net environmental impact may be positive when considered in a broader context.
One primary concern is the potential release of ammonia gas during the synthesis process. Ammonia is a known air pollutant that can contribute to the formation of particulate matter and have adverse effects on air quality. Proper ventilation systems and emission control measures must be implemented to mitigate these risks and ensure compliance with air quality regulations.
Water pollution is another critical aspect to consider. The synthesis process may generate wastewater containing residual bismuth compounds, ammonium ions, and other chemical byproducts. If not properly treated, this effluent could contaminate water bodies, potentially affecting aquatic ecosystems and human health. Implementing effective wastewater treatment systems is essential to minimize the environmental footprint of the production process.
The disposal of solid waste generated during the synthesis and purification stages also requires attention. Bismuth-containing waste materials must be handled and disposed of in accordance with hazardous waste regulations to prevent soil contamination and potential leaching into groundwater.
Energy consumption is a significant factor in the environmental impact assessment. The synthesis process may require substantial energy inputs for heating, stirring, and purification steps. Optimizing energy efficiency and exploring the use of renewable energy sources can help reduce the carbon footprint associated with bismuth nanoparticle production.
The lifecycle assessment of bismuth nanoparticles should also be considered. This includes evaluating the environmental impact of raw material extraction, transportation, and the eventual fate of the nanoparticles after their intended use. Understanding the potential for bioaccumulation and long-term environmental persistence of bismuth nanoparticles is crucial for assessing their overall environmental impact.
Occupational health and safety considerations are intertwined with environmental concerns. Proper handling procedures, personal protective equipment, and safety protocols must be in place to protect workers and prevent accidental releases that could harm the environment.
Lastly, the potential benefits of bismuth nanoparticles in various applications should be weighed against their environmental impact. If these nanoparticles can replace more environmentally harmful materials or improve the efficiency of certain processes, their net environmental impact may be positive when considered in a broader context.
Scale-up Considerations
When considering the scale-up of bismuth nanoparticle synthesis using ammonium hydroxide, several critical factors must be addressed to ensure successful production at larger scales. The dispersion effects observed in laboratory-scale experiments may be significantly altered during scale-up, necessitating careful optimization of process parameters.
One of the primary challenges in scaling up this synthesis process is maintaining uniform dispersion of the nanoparticles. As batch sizes increase, the mixing dynamics change, potentially leading to inhomogeneous distribution of ammonium hydroxide and bismuth precursors. This can result in inconsistent particle sizes and morphologies across the batch. To mitigate this issue, advanced mixing technologies such as high-shear mixers or ultrasonic devices may need to be employed to ensure adequate dispersion throughout the reaction volume.
Temperature control becomes increasingly critical at larger scales. The exothermic nature of the reaction between bismuth precursors and ammonium hydroxide can lead to localized hot spots in larger reactors, affecting the nucleation and growth kinetics of the nanoparticles. Implementation of sophisticated temperature control systems, such as jacketed reactors with precise coolant circulation, may be necessary to maintain uniform temperature profiles throughout the reaction mixture.
The concentration of ammonium hydroxide plays a crucial role in determining the dispersion and stability of bismuth nanoparticles. During scale-up, it is essential to carefully adjust the ammonium hydroxide concentration to account for changes in surface area-to-volume ratios. This may require a series of pilot-scale experiments to optimize the concentration for larger batch sizes while maintaining the desired nanoparticle characteristics.
Another important consideration is the potential for agglomeration of nanoparticles during synthesis and post-synthesis processing. As batch sizes increase, the probability of particle collisions and subsequent agglomeration rises. To address this, the introduction of stabilizing agents or surfactants may be necessary. These additives can help maintain nanoparticle dispersion and prevent unwanted aggregation, but their use must be carefully balanced to avoid interfering with the desired properties of the final product.
The choice of reactor design is crucial for successful scale-up. Continuous flow reactors may offer advantages over batch reactors for large-scale production, as they can provide better control over reaction conditions and reduce the risk of batch-to-batch variations. However, the transition from batch to continuous processing requires careful engineering and may necessitate modifications to the synthesis protocol.
Lastly, the scale-up process must consider downstream processing and purification steps. Larger batch sizes will require proportionally scaled separation and purification techniques, such as centrifugation or membrane filtration. The efficiency of these processes at larger scales may differ from laboratory-scale operations, potentially affecting the final yield and purity of the bismuth nanoparticles.
One of the primary challenges in scaling up this synthesis process is maintaining uniform dispersion of the nanoparticles. As batch sizes increase, the mixing dynamics change, potentially leading to inhomogeneous distribution of ammonium hydroxide and bismuth precursors. This can result in inconsistent particle sizes and morphologies across the batch. To mitigate this issue, advanced mixing technologies such as high-shear mixers or ultrasonic devices may need to be employed to ensure adequate dispersion throughout the reaction volume.
Temperature control becomes increasingly critical at larger scales. The exothermic nature of the reaction between bismuth precursors and ammonium hydroxide can lead to localized hot spots in larger reactors, affecting the nucleation and growth kinetics of the nanoparticles. Implementation of sophisticated temperature control systems, such as jacketed reactors with precise coolant circulation, may be necessary to maintain uniform temperature profiles throughout the reaction mixture.
The concentration of ammonium hydroxide plays a crucial role in determining the dispersion and stability of bismuth nanoparticles. During scale-up, it is essential to carefully adjust the ammonium hydroxide concentration to account for changes in surface area-to-volume ratios. This may require a series of pilot-scale experiments to optimize the concentration for larger batch sizes while maintaining the desired nanoparticle characteristics.
Another important consideration is the potential for agglomeration of nanoparticles during synthesis and post-synthesis processing. As batch sizes increase, the probability of particle collisions and subsequent agglomeration rises. To address this, the introduction of stabilizing agents or surfactants may be necessary. These additives can help maintain nanoparticle dispersion and prevent unwanted aggregation, but their use must be carefully balanced to avoid interfering with the desired properties of the final product.
The choice of reactor design is crucial for successful scale-up. Continuous flow reactors may offer advantages over batch reactors for large-scale production, as they can provide better control over reaction conditions and reduce the risk of batch-to-batch variations. However, the transition from batch to continuous processing requires careful engineering and may necessitate modifications to the synthesis protocol.
Lastly, the scale-up process must consider downstream processing and purification steps. Larger batch sizes will require proportionally scaled separation and purification techniques, such as centrifugation or membrane filtration. The efficiency of these processes at larger scales may differ from laboratory-scale operations, potentially affecting the final yield and purity of the bismuth nanoparticles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!